Abstract:
Cardiopulmonary bypass (CPB) causes a systemic inflammatory response syndrome (SIRS), which can progress to an acute lung inflammation known as postperfusion syndrome. We developed a two-phase hypothesis: first, that SIRS, as indicated by elevated cytokines post-CPB, would be correlated with postoperative pulmonary dysfunction (Phase I), and second, that the cytokine interleukin-6 (IL-6) is predominantly released from the heart in CPB patients (Phase II). Blood samples were collected from patients undergoing CPB for elective cardiac surgery. In seven patients (Phase I), arterial samples were drawn before, during (5 minutes and 60 minutes), and after CPB. In 14 patients (Phase II), samples were collected from the coronary sinus, superior vena cava, and a systemic artery at the times indicated previously. Samples were analyzed with enzyme-linked immunosorbent assay: IL-1, IL-6, IL-8, IL-10, and tumor necrosis factor-α were assessed in Phase I and IL-6 assessed in Phase II. In Phase I, IL-6, IL-8, and IL-10 were elevated after CPB, but only IL-6 concentrations correlated with lung function. In summary, Phase I data demonstrate that increased IL-6 levels at the end of CPB correlate with reduced lung function postoperatively. In Phase II, IL-6 elevation was similar at all sample sites suggesting that the heart is not the major source of IL-6 production. We suggest that IL-6 be implemented as a prognostic measure in patient care, and that patients with elevated IL-6 after CPB be targeted for more aggressive anti-inflammatory therapy to protect lung function.
Keywords: cardiopulmonary bypass, cytokine, lung injury
Cardiovascular surgery with cardiopulmonary bypass (CPB) is associated with a transient inflammatory response known as the systemic inflammatory response syndrome (SIRS). SIRS involves the activation of chemotactic factors and oxygen-free radicals and the release of proinflammatory cytokines interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) and the anti-inflammatory cytokine interleukin-10 (IL-10) (1–7). Features of SIRS include a collection of cardiovascular and pulmonary pathophysiologies, including increased pulmonary resistance and capillary permeability, increased alveolar-arterial oxygen gradients, hypotension, coagulopathy, and decreased peripheral vascular resistance (6,8). Although the magnitude of proinflammatory cytokine release during CPB is directly related to the duration of ischemia (6,9,10), the source of these cytokines remains under study (11–15). The myocardium, lungs, brain, and body as a whole have been implicated as potential sources of cytokine release (11–15).
SIRS plays a key role in the development of acute respiratory distress syndrome (ARDS), known as postperfusion syndrome (PPS) in the CPB patient. According to the “two-hit” model of PPS, PPS results from two consecutive insults (16). CPB itself is the first insult and leads to the development of SIRS in virtually all CPB patients (8). When CPB patients with SIRS are subjected to a second hit such as infection, hypoxia, or ischemia, PPS ensues (16). Although PPS occurs in less than 2% of CPB patients (8), it has a mortality rate of more than 50% (17), making it a serious risk in CPB and therefore an important subject of future research. We studied the inflammatory mediators present during CPB that are involved in the development of SIRS and the initiation of PPS.
Phase I of this study examines the magnitude of cytokine release and the correlation between cytokinemia and pulmonary dysfunction in SIRS. We analyzed plasma levels of IL-1, IL-6, IL-8, IL-10, and TNF-α in CPB patients for potential correlation with measures of pulmonary dysfunction (alveolar-arterial PO2 gradient, respiratory index, P/F ratio and time to extubation).
Phase II of our study was undertaken to examine organ-specific cytokine release. We selected IL-6 for Phase II because it exhibited the greatest magnitude of release in Phase I. Also, IL-6 is more consistently elevated during cardiac surgery than other cytokines (2) and has been reported as a predictor of patient outcome in sepsis (18). We hypothesized that the myocardium is the predominant site of IL-6 release in CPB patients and compared IL-6 levels in the coronary sinus (CS), superior vena cava (SVC), and systemic arterial blood (A).
MATERIALS AND METHODS
The Institutional Review Board approved Phases I and II of the study on May 17, 2000, and April 13, 2002, respectively. Written, informed consent was obtained from each patient.
Patients
Patients 18 years of age or older undergoing elective cardiovascular surgery with CPB and with preserved left ventricular function and without pre-existing disease were considered eligible for this study.
Phase I:
Seven eligible patients, ranging from 44–84 years of age, were enrolled in the study; six patients underwent coronary artery bypass grafting (CABG) and one patient had an atrial septal defect (ASD) repaired. CPB time ranged from 83 to 481 minutes. Analysis of cytokine release and pulmonary function demonstrated that the patient with the exceptionally long CPB run (481 minutes) did not have an order of magnitude different response in either cytokine release or pulmonary dysfunction so there was no reason to exclude this patient from the database.
Phase II:
Fourteen patients, ranging from 49 to 82 years of age, were enrolled in the study; eight patients underwent CABG, two underwent valve repair, and four underwent both procedures. CPB time ranged from 108 to 245 minutes.
Operative Technique
All procedures were performed at SUNY Upstate Medical University Hospital. Patients underwent a median sternotomy and heparinization (300 IU/kg of body weight) to maintain an activated clotting time of greater than 400 seconds. The CPB circuit was completed by cannulating the ascending aorta and right atrium according to standard techniques. A retrograde catheter was placed in the CS, the aorta was cross-clamped and CPB was performed with standard extracorporeal circulation using a standard membrane oxygenator (Optima, Cobe Cardiovascular, Arvada, CO) and centrifugal pump (Bio-Pump, Medtronic, Parker, CO). The circuit was primed with lactated Ringers, 5000 IU of heparin, 5 g Amicar, 50 Meq sodium bicarbonate, and 12.5 g mannitol. Patients were maintained at moderate hypothermia with systemic temperatures between 30 and 32°C. Nonpulsatile pump flow was adjusted to maintain SvO2 greater than 70% and mean arterial pressure between 50 and 90 mmHg.
Myocardial protection was afforded exclusively with a modified Buckberg cardioplegia: patients were induced with1Lof warm solution (500 mL antegrade, 500 mL retrograde) and1Lof cold solution (500 mL antegrade, 500 mL retrograde), followed by intermittent cold retrograde cardioplegia (300 mL/20 min).
After cardiac arrest, distal anastomoses were performed first, followed by the proximal and left anterior descending (LAD) anastomoses. A pericardial patch was used at this stage in the patient who underwent ASD repair. The patients were rewarmed, and a hot shot of substrate-rich warm blood cardioplegia with potassium was infused retrograde. The aortic cross clamp was removed, and the patients were weaned from CPB. After the operation, patients were transported to the ICU and ventilated in a volume-controlled mode until extubation.
Sample Collection
Phase I:
Fifteen-milliliter samples of systemic arterial blood were collected in heparinized tubes at four time points. The first blood sample (baseline/pre-CPB) was obtained from the patients immediately before the initiation of CPB and before the administration of heparin intraoperatively. Blood samples were also collected 5 minutes after the initiation of CPB, 1 hour after the initiation of CPB, and at the termination of CPB before protamine administration. Blood was immediately centrifuged at 3100 rpm for 5 minutes, and plasma samples were stored at −70°C until assayed.
Phase II:
Fifteen milliliter samples were collected from the CS, SVC, and A at each of the four time points indicated above (a total of 12 samples per patient) and prepared as outlined in Phase I.
Cytokine Concentration (ELISA Assay)
Plasma cytokine levels were measured with enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s recommendations (Genzyme, Cambridge, MA). Samples from Phase I were analyzed for IL-1, IL-6, IL-8, IL-10, and TNF-α, and samples from Phase II were analyzed for IL-6.
Pulmonary Function
In Phase I, the alveolar-arterial PO2 gradient (AaDO2), the respiratory index (RI), and the P/F ratio were used as a measure of pulmonary dysfunction and were determined immediately postoperatively and at time of extubation. AaDO2 was calculated with ([FiO2 × 713] − [5/4 × PaCO2]) − PaO2), and this value was used to calculate RI (AaDO2/PaO2). P/F ratio was calculated according to the formula PaO2/FiO2. In addition, time to extubation was recorded and used as a measure of pulmonary dysfunction.
Statistics
Data are reported as the mean ± standard deviation (SD). Analysis of variance (ANOVA) of Phase I cytokine concentration versus CPB time was performed with Tukey-Kramer post hoc analysis using JMP Software, 5.0.1 (SAS, Cary, NC). Fisher’s r to z test was used to examine the correlation between cytokine levels and pulmonary parameters. ANOVA of Phase II cytokine concentrations versus CPB time was performed with Newman-Keuls posthoc analysis using StatView Software, 5.0.1 (SAS, Cary, NC). A p value of <.05 was accepted as statistically significant.
RESULTS
The demographics, CPB time, number of coronary bypasses performed, and length of stay for all patients are shown in Table 1.
Table 1.
Clinical characteristics of CPB patients (n = 21).
| Phase I | Phase II | |
|---|---|---|
| Mean age (yrs) | 70.0 ± 14 | 67.4 ± 10 |
| Males | 3/7 | 9/14 |
| CPB time (min) | 312 ± 133 | 174 ± 47 |
| No. of bypasses | 4.2 ± 0.98 | 2.4 ± 1.3 |
| Length of stay (days) | 11 ± 4.3 | 8.5 ± 3.7 |
Phase I
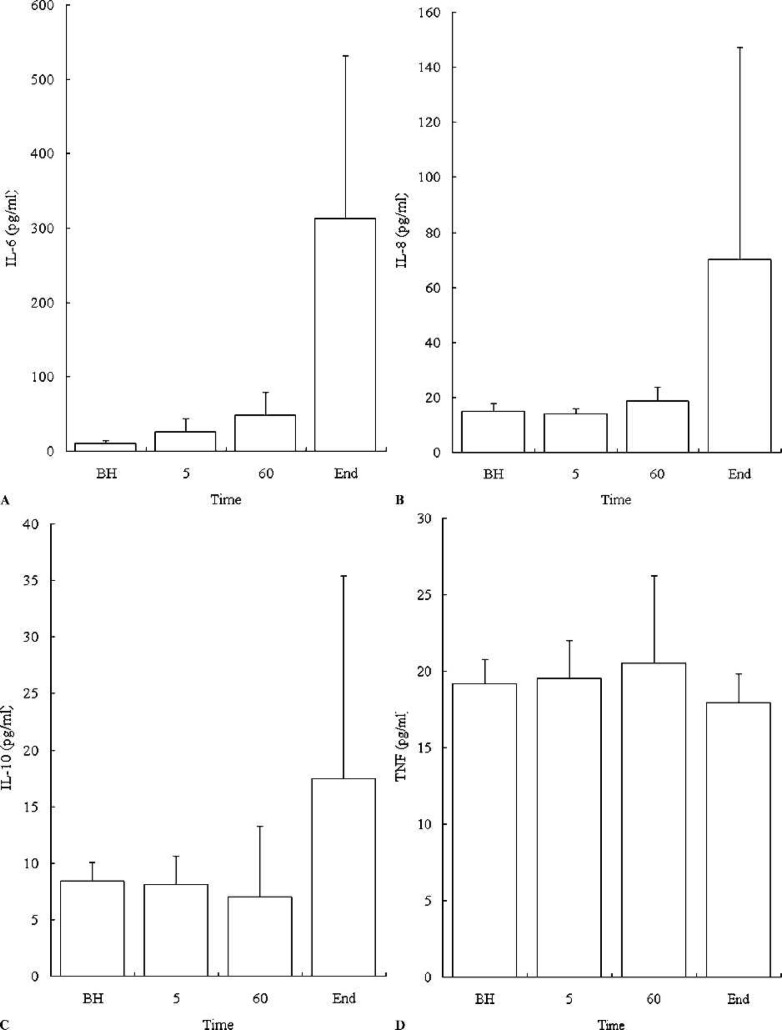
Figure 1A–D presents plasma levels of IL-6, IL-8, IL-10, and TNF-α before and after CPB. At the termination of CPB, a statistically significant increase in the level of IL-6 was observed when compared to baseline values. An increase in IL-8 and IL-10 levels was observed during CPB with a trend toward significance (p = .0563 and .1036, respectively). The IL-1 levels were negligible during CPB. There was no significant increase in TNF-α during CPB. The alveolar-arterial PO2 gradients, respiratory indices, P/F ratios, and times to extubation of all patients are reported in Table 2.
Figure 1.
Cytokine plasma concentration by ELISA. Plasma levels of IL-6* (A), IL-8* (B), IL-10 (C), and TNF-α (D) in seven patients undergoing CPB. Data are mean ± SD (error bars). BH, before heparinization; 5 minutes after crossclamping; 60 minutes after crossclamping; End, at the end of CPB, prior to reperfusion. *The level of IL-6 and IL-8 was significantly increased after CPB (p < .05).
Table 2.
Phase I pulmonary parameters.
| Immediately Post-Op | Extubation | Time to Extubation (h) | |||||
|---|---|---|---|---|---|---|---|
| A-a Gradient (mmHg) | Respiratory Index | P/F Ratio | A-a Gradient (mmHg) | Respiratory Index | P/F Ratio | ||
| Patient 1 | 263 | 2.2 | 206 | 107 | 0.78 | 210 | 8 |
| Patient 2 | 230 | 1.5 | 304 | 129 | 1.1 | 363 | 15 |
| Patient 3 | 250 | 2.3 | 425 | 70 | 0.35 | 433 | 12 |
| Patient 4 | 154 | 0.69 | 300 | 110 | 0.82 | 420 | 17 |
| Patient 5 | 182 | 0.88 | 275 | 105 | 0.81 | 303 | 12 |
| Patient 6 | 230 | 1.6 | 253 | 94 | 0.71 | 295 | 144 |
| Patient 7 | 274 | 2.6 | 370 | 139 | 1.4 | 400 | 12 |
A-a gradient, alveolar-arterial PO2 gradient
Fisher’s r to z test revealed a correlation between IL-6 concentration at the end of CPB and postoperative RI (r2 = .653, p = .0249). There were no other statistically significant correlations between cytokine levels and any of the other pulmonary parameters.
Phase II
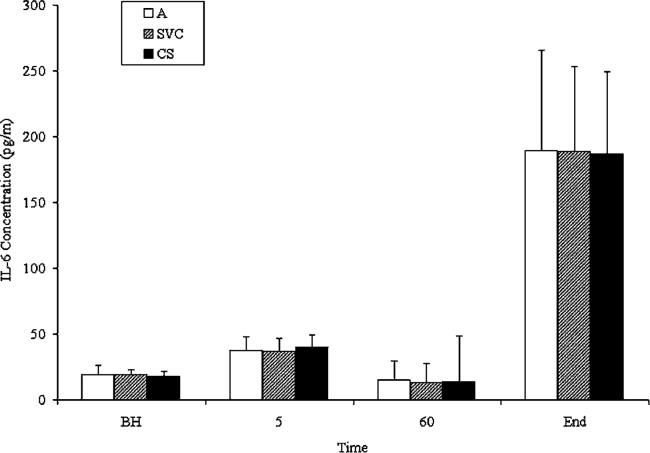
The plasma concentration of IL-6 after 5 minutes of CPB was not significantly different from the baseline value (Figure 2). At the termination of CPB, a statistically significant increase in IL-6 concentration was observed when compared to baseline at all three locations (CS, SVC, and A). However, there was no significant difference between the IL-6 levels in the CS, SVC, and A at the end of CPB (Figure 2).
Figure 2.
IL-6 plasma concentration by ELISA. Plasma levels of IL-6 in peripheral artery* (A), coronary sinus* (CS), and superior vena cava* (SVC) in 14 patients undergoing uncomplicated CPB. Data are mean ± SD (error bars). BH, before heparinization; 5 minutes after crossclamping; 60 minutes after crossclamping; End, at the end of CPB, prior to reperfusion. *The level of IL-6 was significantly increased after CPB (p < .05).
DISCUSSION
In Phase I, we examined the relationship between cytokine levels during CPB and respiratory dysfunction postoperatively. Clinical studies have shown significant elevations of plasma cytokine levels in patients who undergo CPB (1–7). This response may be initiated by the blood’s contact with the artificial surfaces of extracorporeal oxygenation, reperfusion or endotoxin (2). It is postulated that increased cytokine levels correlate with and are largely responsible for lung injury after CPB (2). We demonstrated, however, that IL-8, IL-10, and TNF-α levels during CPB did not correlate with respiratory distress as measured by the AaDO2, RI, P/F ratio, and time to extubation. This is not an unexpected finding because IL-8, IL-10, and TNF-α levels were not significantly elevated at the end of CPB in the patients studied. Conversely, IL-6 levels were elevated at the end of CPB and were found to be significantly correlated to the postoperative RI. We postulate that the failure to find a correlation between IL-6 levels and other measures of respiratory function (AaDO2, P/F ratio, and time to extubation) resulted from the absence of respiratory distress in the patients studied as well as from the small study size. Therefore, because of the strong correlation between elevated IL-6 levels and depressed RI scores, we suggest that clinically significant pulmonary dysfunction is preceded by elevated IL-6 levels. Thus, IL-6 at the end of CPB may be an important prognostic marker for postoperative respiratory complications. This corresponds to a similar finding by Groeneveld et al., who reported that elevated IL-6 levels are predictive of poor outcomes in septic patients (18). Thus, we suggest that IL-6 measurements at the end of CPB may be of prognostic value to clinicians, allowing them to target patients with higher IL-6 values at the end of CPB for more aggressive anti-inflammatory therapy to prevent pulmonary dysfunction.
In Phase II, we examined the hypothesis that the myocardium is the major site of IL-6 release during CPB. We found that IL-6 levels in the CS, SVC and A were significantly elevated from baseline after CPB. However, at each sampling time there was no significant difference between the IL-6 levels in the CS, SVC and A. Because IL-6 levels in the CS and SVC were not significantly elevated above systemic arterial IL-6 levels, we conclude that the myocardium and brain were not primary sources of IL-6 in CPB patients. Instead, IL-6 appears to be produced in comparable levels throughout the body, through a combination of cardiogenic and systemic processes that most likely involves plasma monocytes and vascular endothelial cells. A recent study by Karube et al. measured IL-6 levels in the coronary sinus and radial artery and reported no significant difference between the two locations after CPB (13). This dual myocardial and systemic inflammatory response is corroborated by our study. Karube et al. also suggested that systemic IL-6 release is more pronounced than myocardial release, a conclusion that is again corroborated by our data. However, Wan et al. suggested that the myocardium is the major source of IL-6 after CPB, and further suggested that the lungs may consume inflammatory cytokines (11).
The different times during CPB that investigators measured IL-6 may explain the discrepancy between the sources of cytokine release. The varying results may reflect different mechanisms of injury in SIRS. We speculate that the inflammatory response during CPB may be physiologically different from the inflammatory process that occurs after aortic declamping/reperfusion. We and Karube et al. (13) compared IL-6 levels before CPB to those immediately before the termination of CPB (essentially zero off-pump time); both studies demonstrated a systemic and cardiogenic inflammatory response consistent with injury caused by the blood’s contact with the extracorporeal surfaces of the oxygenator. However, Wan et al. and Massoudy et al. compared IL-6 levels before CPB to those after various reperfusion times (from 1 minute to 2 hours of reperfusion) (11,12). They demonstrated that the heart and lungs are key players in cytokine production, a conclusion consistent with reperfusion injury.
There are multiple techniques that can potentially reduce the inflammation associated with CPB (19–22). To optimize the reduction in inflammation associated with CPB, an integrated approach must be implemented (22). Using the currently available biocompatible surfaces on perfusion circuitry can reduce inflammation (22). A more aggressive approach would be to use a technique that actively filtered out inflammatory mediators from the blood, such as zero balanced ultrafiltration (19). Potent anti-inflammatory pharmacological compounds such as the modified tetracycline, COL-3, which inhibits neutrophil released proteases, has been shown to prevent postpump-induced acute lung injury (20). Finally, elimination of CPB entirely by performing coronary artery bypass grafting surgery on the beating heart (OPCAB) will obviously eliminate all of the inflammation associated with the CPB circuit (21). Much more study is necessary until we finally understand the key inflammatory mediators that actually cause pathology and how best to prevent the release and/ or inhibit these mediators during CPB.
In conclusion, we have demonstrated increased plasma levels of IL-6, IL-8, and IL-10 in patients undergoing CPB. IL-6 was the only cytokine whose level correlated with measures of pulmonary dysfunction. A study that involves a larger patient population, including patients experiencing serious respiratory dysfunction, is necessary to further document the correlation between IL-6 levels at the end of CPB and postoperative respiratory distress. We also have observed that neither the heart, brain, nor the systemic circuit alone causes localized increases in plasma IL-6 levels during CPB. We theorize that increased cytokine levels immediately after CPB represent a systemic response initiated by the blood’s contact with the extracorporeal oxygenator.
REFERENCES
- 1.Sablotzki A, Dehne M, Welters I, et al. Alterations of the cytokine network in patients undergoing cardiopulmonary bypass. Perfusion. 1997;12:393–403. [DOI] [PubMed] [Google Scholar]
- 2.Hall R, Smith M, Rocker G.. The systemic inflammatory response to cardiopulmonary bypass: Pathophysiological, therapeutic, and pharmacological considerations. Anesthesia. 1997;85:766–82. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Pillai R, Rocker G, et al. Effect of CPB on systemic release of neutrophil elastase and tumore necrosis factor. J Thorac Cardiovasc Surg. 1993;105:25–30. [PubMed] [Google Scholar]
- 4.Casey LC.. Role of cytokines in the pathogenesis of cardiopulmonary-induced multisystem organ failure. Ann Thorac Surg. 1993;56:92–6. [DOI] [PubMed] [Google Scholar]
- 5.Sablotzki A, Friedrich I, Muhling J, et al. The systemic inflammatory response syndrome folowing cardiac surgery: Different expression of proinflammatory cytokines and procalcitonin in patients with and without multiorgan dysfunctions. Perfusion. 2002;17:103–9. [DOI] [PubMed] [Google Scholar]
- 6.Khabar K, ElBarbary MA, Khouqeer F, et al. Circulating endotoxin and cytokines after cardiopulmonary bypass: Differential correlation with duration of bypass and systemic inflammatory response/multiple organ dysfunction syndromes. Clin Immunol Immunopathol. 1997;85:97–103. [DOI] [PubMed] [Google Scholar]
- 7.Casey LC.. Role of cytokines in the pathogenesis of cardiopulmonary-induced multisystem organ failure. Ann Thorac Surg. 1993;56:92–6. [DOI] [PubMed] [Google Scholar]
- 8.Conti V.. Pulmonary injury after cardiopulmonary bypass. Chest. 2001;119:2–4. [DOI] [PubMed] [Google Scholar]
- 9.Hennein HA, Ebba H, Rodriguez JL, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108:626–35. [PubMed] [Google Scholar]
- 10.Wan S, Marchant A, DeSmet JM, et al. Human cytokine responses to cardiac transplantation and coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1996;111:469–77. [DOI] [PubMed] [Google Scholar]
- 11.Wan S, DeSmet JM, Barvais L, et al. Myocardium is a major source of proinflammatory cytokines in patients undergoing cardiopulmonary bypass. J Thor Cardiovasc Surg. 1996;112:806–11. [DOI] [PubMed] [Google Scholar]
- 12.Kotani N, Hashimoto H, Sessler D, et al. Cardiopulmonary bypass produces greater pulmonary than systemic proinflammatory cytokines. Anesthesia and Analgesia. 2000;90:1039–45. [DOI] [PubMed] [Google Scholar]
- 13.Karube N, Adachi R, Ichikawa Y, et al. Measurement of cytokine levels by coronary sinus blood sampling during cardiac surgery with cardiopulmonary bypass. ASAIO. 1996;42:787–91. [DOI] [PubMed] [Google Scholar]
- 14.Massoudy P, Zahler S, Becker B, et al. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest. 2001;119:31–6. [DOI] [PubMed] [Google Scholar]
- 15.Nandate K, Vuylsyeke A, Crosbie A, et al. Cerebrovascular cytokine responses during coronary artery bypass surgery: specific production of interlukin-8 and its attenuation by hypothermic cardiopulmonary bypass. Anesthesia. 1999;89:823–8. [DOI] [PubMed] [Google Scholar]
- 16.Picone AL, Lutz CJ, Finck C, et al. Multiple sequential insults cause post-pump syndrome. Ann Thorac Surg. 1999;67:978–85. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg J, Fink G, Picone A, et al. Evidence of increased metalloproteinase-9 concentration in patients following cardiopulmonary bypass. J Extra Corpor Technol. 2001;33:218–22. [PubMed] [Google Scholar]
- 18.Groeneveld ABJ, Tacx AN, Bossink AWJ, et al. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin Immunol. 2003;106:106–15. [DOI] [PubMed] [Google Scholar]
- 19.Darling E, Searles B, Nasrallah F, et al. High-volume, zero balanced ultrafiltration improves pulmonary function in a model of post-pump syndrome. J Extra Corpor Technol. 2002;34:254–9. [PubMed] [Google Scholar]
- 20.Carney DE, Lutz CJ, Picone AL, et al. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation. 1999;100:400–6. [DOI] [PubMed] [Google Scholar]
- 21.Larmann J, Theilmeier G.. Inflammatory response to cardiac surgery: Cardiopulmonary bypass versus non-cardiopulmonary bypass surgery. Best Pract Res Clin Anaesthesiol. 2004;18:425–38. [DOI] [PubMed] [Google Scholar]
- 22.Rubens FD, Mesana T.. The inflammatory response to cardiopulmonary bypass: A therapeutic overview. Perfusion. 2004;19(Suppl 1):S5–12. [DOI] [PubMed] [Google Scholar]