Abstract:
A clinically relevant rat cardiopulmonary bypass (CPB) model would be a valuable tool for investigating pathophysiological and therapeutic strategies on bypass. Previous rat CPB models have been described in the literature; however, they have many limitations, including large circuit surface area, the inability to achieve full bypass, and donor blood requirements for prime. Therefore, we have established a rat CPB model designed to overcome these limitations. The miniature circuit consisted of a filtered reservoir, heat exchanger, membrane oxygenator (surface area = 0.02 m2) with a static priming volume of 2.8 mL, and an inline blood gas monitor. The circuit was primed with 9.5 ± 0.5 mL of crystalloid solution and CPB was established on male Sprague–Dawley rats (430–475 g, n = 5) by cannulating the left common carotid artery and the right external jugular vein. The animals were placed on CPB at full flow (111 ± 13 mL/kg/min) for 1 hour and were monitored for and additional 2 hours after the CPB procedure. Hemodynamics, hemoglobin concentration (Hb), and blood gases were analyzed at three time intervals: before, during, and after CPB. The circuit performance was evaluated according to prime volume, compliance, hemodynamic parameters, and gas and heat exchange as described by modified AMMI standards. Data are expressed as mean ± SD and a repeated-measures analysis of variance with post-Hoc test was used for data comparison between the three time intervals. The ratio of oxygenator surface area to subject body weight for this model is comparable with that of current human adult CPB practice (0.05 m2/kg vs 0.057 m2/kg) Full CPB was achieved and we observed clinically acceptable PaO2, PaCO2, and SvO2 values (209 ± 86 mmHg, 25 ± 2 mmHg, 78 ± 8%, respectively) while on CPB. The use of asanguinous prime did produce statistically significant Hg reduction (15.7 ± 0.76 vs. 9.2 ± 0.59 g/dL) comparable with clinical practice. No statistically significant differences between pre-and post-CPB hemodynamics and blood gases were found in our study. We have established a miniature circuit consisting of asanquineous prime for a rat CPB model that maintains clinically acceptable results regarding hemodynamic parameters, blood gases, and hemodilution. This model would be valuable for further use in clinically relevant research studies.
Keywords: rat, cardiopulmonary bypass, asanguinous prime
Cardiopulmonary bypass (CPB) is an essential component of conventional cardiac surgery that enables the surgeon to operate in a bloodless, motionless, field. Although the morbidity and mortality rate has decreased during the last few years, bypass continues to be associated with significant mortality (1). To further reduce mortality, researchers need to investigate pathophysiology and novel therapeutic strategies. A specific preclinical bypass model enables the researcher to do so.
Small-animal CPB is desirable because experimental work with large animals has become increasingly expensive. The main advantages of a small animal model include the reduced cost of animals and supplies and the fact it does not require a full-scale operating environment. Rats are ideal for use in a CPB model because they have been extensively studied in other physiological models and many commercial tests and assays have been defined for these species (2).
Since 1970, researchers have attempted to develop rat CPB models; however, they have failed to establish a research model that closely mimics our clinical versions (3–5). In short, these rat models have a number of limitations, including large circuit surface area, the inability to achieve full CPB, and donor blood requirements for priming purposes (4–6). The purpose of this study is to overcome these limitations by designing a rat CPB model using a miniature extracorporeal circuit with asanguinous prime.
MATERIALS AND METHODS
Circuit Design
Oxygenator:
A flat plate membrane oxygenator with a static prime volume of 2.8 ± 0.1 mL was designed and built for this study. The device uses the same microporous polypropylene membrane material (surface area = 199.75 cm2) used clinically on humans and has a maximum flow rate of 90–100 ml/min. The oxygenator was tested inhouse according to modified AAMI standards.
Reservoir/Heat Exchanger:
A 30-mL plastic syringe barrel was equipped with a custom-designed stainless-steel heat exchanger device, and 1 cm2 of filter material. The reservoir was sealed and vacuum was applied (−20 mmHg). The volume displaced by the heat exchanger reduced the maximum volume of the syringe to 6 mL. Approximately 1–2 mL of prime remained in the reservoir during CPB.
Arterial Pump:
A roller pump (Masterflex Inc. Barrington, IL) equipped with a piece of 8 cm of PVC tubing (size #16) was used to remove blood from the reservoir and pump it into the oxygenator.
Inline Blood Gas Monitoring:
100% of postoxygenator blood flow was directed through an inline blood gas monitoring cell (CDI 500, Terumo, Ann Arbor, MI) providing a blood gas panel including PO2, PCO2, pH, temperature, and K+. The units were calibrated upon initiation of CPB and recalibrated at 30 minutes of CPB.
Arterial and Venous Tubing:
With the exception of the pump boot, a total length of 15 cm high-pressure tubing with inner diameter of 2.4 mm was used for venous and arterial lines.
Circuit Preparation
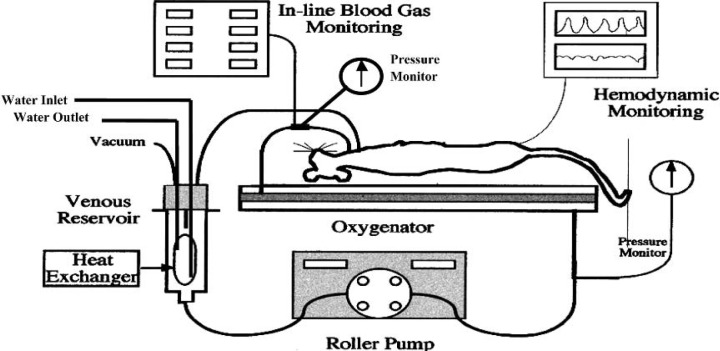
The miniature extracorporeal circuit (Figure 1) was prepared and primed with Plasmalyte A solution (Table 1) composed of final concentrations of Na+ = 159, K+ = 4.8 and iCa2+ = 1.89. Total prime volume of the circuit was 9.5 mL. The oxygenator contained 2.8 mL, and 1 mL remained in the reservoir.
Figure 1.
Schematic representation of the miniature rat CPB circuit.
Table 1.
Composition of the stock solution used for circuit priming and volume replacement.
| Stock | Amount (mL) |
|---|---|
| Plasmalyte A | 25 |
| 6% Hetastarch | 22.7 |
| NaHCO3 (1 m Eq/mL) | 1.5 |
| Mannitol (1.25/mL) | 0.5 |
| KCl (2 m Eq/mL) | 0.05 |
| CaCl (100 μ/mL) | 0.15 |
| Heparin (1000 μg/mL) | 0.1 |
| Total | 50 |
Surgical Procedure
The experimental protocol was approved by the Upstate Medical University Animal Care and Use Committee. Five male Sprague–Dawley rats (430–475 g) were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (9 mg/kg). Anesthesia was maintained throughout the experiment with additional intraperitoneal injections of ketamine and xylazine. The right femoral artery was cannulated with PE 50 polypropylene tubing (Becton-Dickson, Spark, MD) to monitor systemic blood pressure (ADInstruments Ltd, Castle Hill, Australia). After giving a full dose of heparin (500 IU/kg), the left common carotid artery was exposed and cannulated with an 18-G plastic needle (Johnson & Johnson, Arlington, TX). The right external jugular vein was isolated and cannulated with a 14-G modified venous cannula (Johnson & Johnson), which was advanced into the right ventricle. Venous cannula placement in the right ventricle was confirmed by observing the pressure waveform.
CPB Management
CPB was initiated at a flow of 40 mL/min and then the vacuum was applied at a pressure of −10 mmHg and maintained between −20 to −25 mmHg. Within 3–5 minutes, pump flow was slowly increased to 55–60 mL/min in response to the increase in venous return. Full-flow CPB (111 ± 13 mL/kg/min) was achieved when the systemic arterial pressure waveform became a flat line. Constant inline arterial blood gas was monitored, and blood and rectal temperature were maintained at 32–35°C. Animals were uneventfully weaned from CPB at 60 minutes. The venous cannula was pulled back to the right atrium for continuous central venous pressure (CVP) monitoring and the animal was intubated via tracheotomy for assisted ventilation (Harvard Apparatus, Holliston, MA) with a tidal volume of 5 mL/breath and 60 breaths/min. Animals were monitored for 180 minutes after CPB. Within 30 minutes after CPB, blood remaining in the circuit was transfused while monitoring arterial blood pressure and CVP. Artificial ventilation was discontinued 60 min after CPB. Animals were sacrificed 180 minutes after CPB with an overdose injection of ketamine.
Data Collection
Baseline blood samples were taken before CPB; at 10, 30, and 60 minutes during CPB; and at 60 and 120 minutes after CPB. Hemodynamics, blood gases, and hemoglobin concentrations were obtained from these samples. Oxygenator pressures were monitored before and after the procedure and recorded every 10 minutes while on CPB.
Statistical Analysis
Data are expressed as mean ± SD and a repeated-measures analysis of variance and Post-Hoc test were used to identify the statistical differences among time intervals. Statistical significance was determined by a p value less than 0.05.
RESULTS
Oxygenator Performance
Oxygenator performance was tested on three newly assembled oxygenators. Blood gas exchange was evaluated using deoxygenated porcine blood according to the Modified AMMI Standards. The blood gas outlet was measured at flows of 30, 60, and 75 mL/min and is presented in Table 2.
Table 2.
In vitro analysis of oxygenator performance.
| Modified AAMI Standards | Inlet Preoxygenation | Postoxygenator (30 mL/min) | Postoxygenator (60 mL/min) | Postoxygenator (75 mL/min) | |
|---|---|---|---|---|---|
| Hb (g/dL) | 12 ± 1 | 12 ± 1 | 12 ± 1 | 12 ± 1 | 12 ± 1 |
| PO2 (mmHg) | 35 ± 2 | 550 ± 114 | 339 ± 34 | 225 ± 48 | |
| PCO2 (mm Hg) | 45 ± 2 | 45 ± 1 | 17 ± 1 | 17 ± 2 | 21 ± 3 |
| SatO2 (%) | 65 ± 1 | 61 ± 4 | 100 | 100 | 100 |
| BE | 0 ± 2 | 0 ± 1 | 0 ± 2 | 0 ± 2 | 0 ± 2 |
Rat CPB Hemodynamics
Figure 2 is a representative example of the animal’s hemodynamic trace before CPB and after initiation. The absence of pulsatile arterial pressure after initiation was evidence of full support. Blood pressure, during and after CPB, is shown in Table 3.
Figure 2.
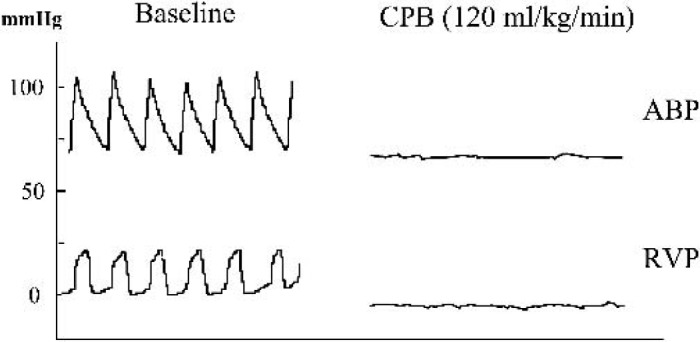
Representative hemodynamic waveforms from animals before and after initiation of CPB. ABP, arterial blood pressure; RVP, right ventricle pressure.
Table 3.
Arterial blood pressures before, during, and after CPB.
| Pre-CPB | 5 Min CPB | 30 Min CPB | 60 Min CPB | 1 Hour Post-CPB | 2 Hours Post-CPB | |
|---|---|---|---|---|---|---|
| ABPs | 101 ± 5 | N/A | N/A | N/A | 101 ± 4 | 104 ± 4 |
| ABPd | 68 ± 5 | N/A | N/A | N/A | 63 ± 6 | 68 ± 3 |
| MAP | 79 ± 5 | 44 ± 2* | 47 ± 4* | 53 ± 5* | 76 ± 3 | 80 ± 4 |
ABP, systolic arterial blood pressure; ABPd, diastolic arterial blood pressure; MAP, mean arterial pressure.
Hemodilution and Gas Exchange
Hemoglobin concentration and blood gas analyses are shown in Table 4. The rats on bypass had lower Hb concentrations compared to baseline (p < 0.05). Hemoglobin concentration increased 1 to 2 hours after CPB, yet it remained below baseline. The PaO2 and PCO2 valves at times 5 and 30 minutes represent oxygenator performance. Values at 60 minutes were collected during artificial ventilation and values at 120 and 180 reflect the unsupported cardiopulmonary function of the animal. Svo2 was maintained in the normal range during CPB.
Table 4.
The results of hemoglobin (Hb) and arterial and venous blood gases before, during, and after CPB.
| Pre-CPB | 5 Min CPB | 30 Min CPB | 60 Min CPB | 1 Hour Post-CPB | 2 Hours Post-CPB | |
|---|---|---|---|---|---|---|
| PaO2 (mmHg) | 109 ± 15 | 211 ± 78* | 209 ± 86* | 165 ± 41* | 188 ± 55* | 108 ± 38 |
| PaCO2 (mmHg) | 42 ± 5 | 26 ± 1* | 25 ± 2* | 23 ± 3* | 40 ± 4 | 38 ± 4 |
| BE | −1.6 ± 1.4 | −2.8 ± 1.5 | −3.0 ± 0.6 | −2.0 ± 0.5 | −3.0 ± 0.7 | −4.0 ± 0.7 |
| Svo2 (%) | 68 ± 5 | 66 ± 6 | 78 ± 5 | 72 ± 2 | 72 ± 5 | 64 ± 4 |
| Hb (g/dl) | 15.7 ± 0.8 | 10.2 ± 0.8* | 9.4 ± 0.3* | 9.2 ± 0.6* | 10.3 ± 0.6* | 11.6 ± 0.4* |
DISCUSSION
Although the mortality of open-heart surgery has decreased, CPB continues to be associated with significant mortality (2). Neurological injuries are a major complication and occur in as many as 80% of patients after cardiac surgery (11). The first rat CPB model was published by Popovic in 1968 (7); since then, nine other groups have reported their rat CPB models. However, most models have never been used for clinically relevant research studies which can be attributed to a number of limitations introduced earlier.
Ballaux et al. (2) described an ideal rat CPB model as having to the ability to eliminate the use of donor blood for priming, small prime volume (about 15 mL), venous cannulation in the right atrium and arterial return to the aorta, an oxygenator that is capable of a more than adequate oxygenation at the average cardiac output, full-flow bypass, and inline blood gas monitoring Our model meets these parameters. Table 5 compares our rat CPB model to previous models.
Table 5.
Comparison of previously published rat CPB models.
| Authors | Year | Type of Oxygenator | Surface Area of Oxygenator (m2) | Priming Vol. of Oxygenator (mL) | Extracorporeal Volume (mL) | Priming Solution | Flow Rate |
|---|---|---|---|---|---|---|---|
| Popovic et al. (7) | 1968 | Membrane | 11.3 | 13.7 | Donor | 32 mL/min | |
| Subramanian et al. (3) | 1968 | Bubble | 120 | >120 | Donor + RG | 150 mL/min/300g | |
| Triggiani et al. | 1970 | Bubble | ? | Donor | 15–20 mL/min to 50% CO | ||
| Proctor (4) | 1978 | Bubble | 20–30 | 25 | Donor + colloid | 200–280 mL/kg/min | |
| Alexander & Al Ani (5) | 1983 | Membrane | 0.017 | 4.4 | 12 | Hartmann’s solution | 12–20 mL/min to 50% of cardiac output |
| Wehberg et al. | 1996 | Membrane | ? | 46 | Donor | 125 mL/kg/min | |
| Sasaki et al. (6) | 1996 | Membrane | 0.05 | ? | 23 | Ringer’s | 50 mL/kg/min |
| Grocott et al. (10) | 2001 | Membrane | 0.33 | 27 | 40 | Donor | 160 mL/kg/min |
| Fabre et al. (8) | 2001 | Membrane | 15 | 35 | Donor | 100 mL/kg/min | |
| Present study | 2005 | Membrane | 0.02 | 2.8 | 9.5 | Plasmalyte A + Hetastarch | 120 mL/kg/min |
Figures 3 and 4 illustrates the prime volume required by our circuit. Miniaturization of the extracorporeal circuit represents the major limiting factor for rat CPB models historically. Popovic et al. (7) reported the first rat CPB model using bubble oxygenation. Although the prime volume was 13.7 mL, the flow rate was only 32 mL/min. Subramanian et al. (3) published a rat CPB model using a bubble oxygenator with a prime volume of 120 mL. Proctor (4) developed an experimental bubble oxygenator with a prime volume of 25 mL that was able to achieve adequate tissue oxygenation (200–280 mL/kg/min). The most recent models have used commercially available pediatric membrane oxygenators (8,10), which have a total prime volume of 35–42 mL. Although improvements have been made, these models still have a large prime volume which requires donor blood from one to two rats (8,10). These large blood transfusions are a concern as they increase the likelihood of post-CPB myocardial injury and lung edema (8,9). Our miniature circuit required a prime volume of 9.5 mL, which is approximately 32% of the total blood volume of a rat. This proportion is more clinically relevant than other models. Despite the use of asanguious prime, we were enable to maintain the hemoglobin concentration at clinically relevant values without sacrificing more than one animal per study.
Figure 3.
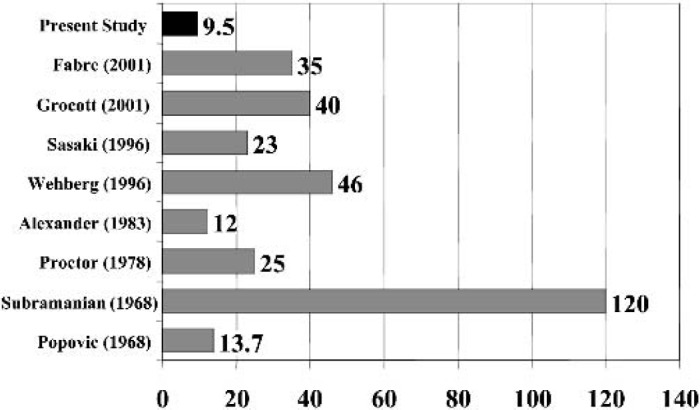
Circuit priming volumes (in mL) of previously published rat CPB models.
Figure 4.
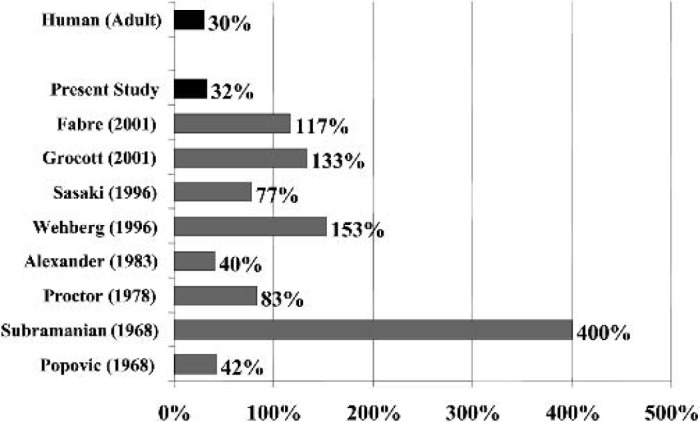
The proportions of the priming volume in body blood volume are calculated using the formula: proportion (%) = blood volume (mL)/priming volume (mL). We assume that the blood volume in an adult is 5000 mL and the priming volume is 1500 mL and the blood volume in a rat is 30 mL.
The oxygenator in our model has a much smaller surface area than previous models (Figure 5). The oxygenator used by Grocott et al. (10) used the Cobe Micro infant oxygenator has a surface area of 0.33 m2. Fabre et al. (8) used a modified a Capiox 308 membrane using two silicon “cones” to reduce approximately 75% of the membrane surface, but it continues to have a large surface area (0.2 m2).
Figure 5.
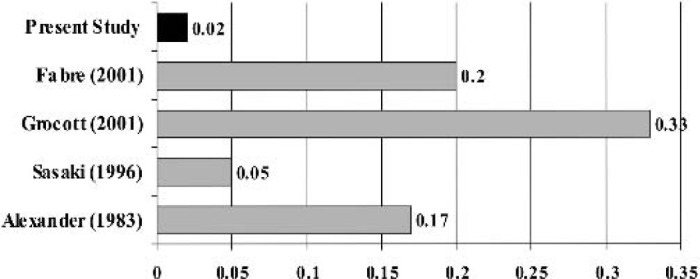
Surface area of previously published rat CPB models.
Increased surface area leads to activation of blood components and has the potential to induce a strong inflammatory response. Figure 6 illustrates the ratio between foreign surface area to the weight of a rat.
Figure 6.
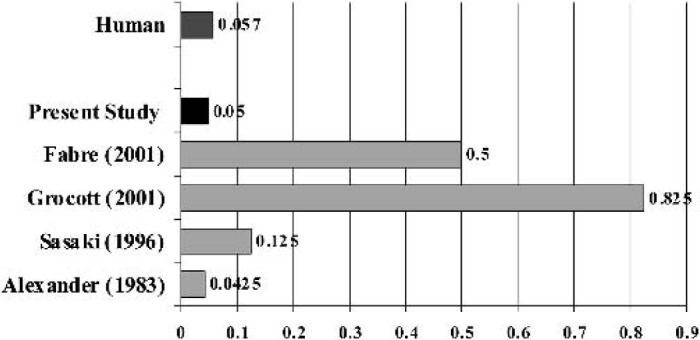
The surface areas (m2/kg) are calculated. A surface area of 4 m2 is assumed for an adult membrane oxygenator, and the average weight of the adult is 70 kg. We assume that the rat body weight is 0.4 kg.
The only oxygenator that was specifically designed for the rat was reported by Alexander and Al Ani (5) in 1983. This membrane oxygenator consisted of 5 hollow Silastic membrane tubing (diameter of 1.0–1.5 mm, 0.5–1 m in length of each tubing), and had an average surface area of 0.015 m2. Although it was much smaller than average pediatric oxygenators there were a few limitations. Silicone is not a clinically relevant material for CPB oxygenators and flow rates only reached 12–20/kg/min, which is half of the cardiac output of a rat. In summary, we have demonstrated the performance of custom designed rat CPB model which can be used to collect clinically relevant data regarding the techniques and the pathophysiology of CPB.
ADDENDUM
This work was originally presented at the American Society of ExtraCorporeal Technology Conference, New Orleans, Louisiana, in March of 2001. Since that time several researchers have made significant contributions towards the development of miniaturized rodent CPB models with clinically relevant materials and prime volumes. Most notably, Hamamoto et al. (12) and Gourlay et al. (13) have published works, which utilize circuits that prim with 9 and 12 ml of fluid, respectively.
REFERENCES
- 1.Kirklin JW. Barratt-Boyes BG eds. Hypothermia, circulatory arrest, and cardiopulmonary bypass. In Cardiac Surgery. 2nd ed. New York: Churchill Livingstone; 1993; 61–128 [Google Scholar]
- 2.Ballaux PK, Gourlay T, Ratnatunga CP, Taylor KM.. A literature review of cardiopulmonary bypass models for rats. Perfusion. 1999;14:411–7. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian V, McLeod J, Gans H.. Effect of extracorporeal circulation on reticuloendothelial function. I. Experimental evidence for impaired reticuloendothelial function following cardiopulmonary bypass in rats. Surgery. 1968;64:775–84. [PubMed] [Google Scholar]
- 4.Proctor E.. An oxygenator for cardiopulmonary bypass in the rat. J Surg Res. 1976;22:124–7. [DOI] [PubMed] [Google Scholar]
- 5.Alexander B, Al Ani HR.. Prolonged partial cardiopulmonary bypass in rats. J Surg Res. 1983;35:28–34. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki S, Takigami K, Shiiya N, Yasuda K.. Partial cardiopulmonary bypass in rats for evaluating ischemia-reperfusion injury. ASAIO J. 1996;42:1027–30. [DOI] [PubMed] [Google Scholar]
- 7.Popovic P, Horecky J, Popovic VP.. Hypothermic cardiopulmonary bypass in white rats. Ann Surg. 1968;168:298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabre O, Zegdi R, Vincentelli A, et al. A recovery model of partial cardiopulmonary bypass in the rat. Perfusion. 2001;16:215–20. [DOI] [PubMed] [Google Scholar]
- 9.Alexander B, Al Ani HR.. Prolonged partial cardiopulmonary bypass in rats. J Surg Res. 1983;35:28–34. [DOI] [PubMed] [Google Scholar]
- 10.Grocott HP Mackensen GB. Newman MF, Warner DS.. Neurological injury during cardiopulmonary bypass in the rat. Perfusion. 2001;16:75–81. [DOI] [PubMed] [Google Scholar]
- 11.Fearn SJ, Pole R, Burgess M, Ray SG, Hooper TL, McCollum CN.. Cerebral embolisation during modern cardiopulmonary bypass. Eur J Cardiothoracic Surg. 2001;20:1163–7. [DOI] [PubMed] [Google Scholar]
- 12.Hamamoto M, Suga M, Nakatani T, et al. Phosphodiesterase type 4 inhibitor prevents acute lung injury induced by cardiopulmonary bypass in a rat model. Eur J of Cardio-Thorac Surg. 2004;25:833–8. [DOI] [PubMed] [Google Scholar]
- 13.Gourlay T, Ballaux PK, Draper ER, Taylor KM.. Early experience with a new technique and technology designed for the study of pulsatile cardiopulmonary bypass in the rat. Perfusion. 2002;17:191–8. [DOI] [PubMed] [Google Scholar]