Abstract:
Acute preoperative plateletpheresis (APP), cell salvage (CS) technique, and the use of aprotinin have been individually reported to be effective in reducing blood loss and blood component transfusion while improving hematological profiles in patients undergoing cardiac surgery with cardiopulmonary bypass (CPB). In this prospective randomized clinical study, the efficacy of these combined approaches on reducing blood loss and transfusion requirements was evaluated. Seventy patients undergoing primary coronary artery bypass grafting (CABG) were randomly divided into four groups: a control group (group I, n = 10) did not receive any of the previously mentioned approaches. An APP and CS group (group II, n = 20) experienced APP in which preoperative platelet-rich plasma was collected and reinfused after reversal of heparin, along with the cell salvage technique throughout surgery. The third group (group III, n = 22) received aprotinin in which 5,000,000 KIU Trasylol was applied during surgery, and a combination group (group IV, n = 18) was treated with all three approaches, i.e., APP, CS, and aprotinin. Compared with group I (896 ± 278 mL), the postoperative total blood loss was significantly reduced in groups II, III, and IV (468 ± 136,388 ± 122,202 ± 81 mL, respectively, p < 0.05). The requirements of packed red blood cells in the three approached groups (153 ± 63,105 ± 178,0 ± 0 mL, respectively) also were reduced when compared with group I (343 ± 118 mL, p < 0.05). In group I, six patients (6/10) received fresh-frozen plasma and three patients (3/10) received platelet transfusion, whereas no patients in the other three groups required fresh-frozen plasma and platelet. In conclusion, both plateletpheresis concomitant with cell salvage and aprotinin contribute to the improvement of postoperative hemostasis, and the combination of these two approaches could minimize postoperative blood loss and requirement.
Keywords: Cardiopulmonary bypass (CPB), acute preoperative plateletpheresis (APP), cell salvage (CS), aprotinin, cardiac surgery
Cardiovascular surgery with cardiopulmonary bypass (CPB) often is associated with the loss of large quantities of blood. Because massive blood loss and blood components transfusion are always associated with increased morbidity and mortality, and homologous blood products transfusion also exposes the patients to the risks of transfusion reaction and viral infection, it is critical to minimize the blood loss and transfusion requirement during cardiovascular surgery (1).
Multiple approaches, both physical (mechanical sequestration) and chemical (drugs), have been applied in clinical situations to improve hemostasis during cardiopulmonary bypass. Acute preoperative plateletpheresis (APP) or the separation of platelet-rich plasma (PRP) at induction of anesthesia with the return of PRP after neutralization of heparin, cell salvage (CS) technique, and the use of aprotinin have been individually reported to be effective in reducing blood loss and blood component transfusion while improving hematological profiles in patients undergoing cardiac surgery (1–7). In addition, most manufacturers design disposables together to achieve patient’s APP and CS in the same machine and decrease the medical costs. However, very few studies have been undertaken on the combination of these approaches on clinical hemostasis effect.
In this prospective randomized clinical study, we evaluated the efficacy of combining these approaches on reducing blood loss and transfusion requirement. We hypothesized that, in patients undergoing elective coronary artery bypass grafting (CABG), either acute preoperative plateletpheresis concomitant with cell salvage or aprotinin could improve postoperative hemostasis and that the combination of these two approaches could minimize blood loss and transfusion requirement.
MATERIALS AND METHODS
The study protocol was approved by the Fu Wai Cardiovascular Hospital Institutional Review Board. All patients gave informed consent to participate in the study. Patients who had preoperative known bleeding or coagulation disorders were excluded. Randomized patients also were excluded from further statistic analysis if, during the surgery, they experienced unexpected surgical hemorrhage with hemodynamic instability.
All operations were performed at Fu Wai Cardiovascular Hospital, Beijing, China. All procedures were conducted with general anesthesia, with a balanced technique of moderate-dose fentanyl and low-dose isoflurane. Pulmonary capillary wedge pressure (PCWP) and radial artery pressure were monitored for all patients. A 9-French sheath was inserted into a central vein for blood withdrawal and fluid infusion. During the operation, nitroglycerine was used to treat elevations in PCWP, and nitroprusside was used to treat increases in afterload. Inotropic and chronotropic drugs were administered as needed to optimize systolic and diastolic function. Systemic heparin of 400 unit/kg was administered to all patients before CPB and was reversed with protamine after CPB. All patients received CABG under CPB with a nonpulsatile roller pump (Stockert-II, Munich, Germany), a membrane oxygenator (Capiox SX-18, Terumo, Tokyo, Japan), and 4:1 cold blood cardioplegia (Perfect, Beijing, China). The pump circuits were primed with 1000 mL of colloid solution (Gelofusine, B. Braun, Melsungen, Germany) and 750 mL of crystalloid solution (Lactate Ringer Injection, Baxter, Deerfield, IL). After CPB, all remained pump blood was returned to the patient through the aortic cannula or intravenously by infusion bag without hemoconcentration. The established criteria for transfusion of Fu Wai Cardiovascular Hospital were used. Red blood cells were transfused to maintain the hematocrit (Hct) ≥30% before CPB, ≥21% during CPB, ≥24% after CPB, and ≥27% in the surgical intensive care unit (ICU).
Patients undergoing elective primary CABG were randomly divided into four groups: a control group (group I) without any approach performed; an APP and CS group (group II) in which preoperative PRP was collected and reinfused after reversal of heparin and cell salvage was performed throughout surgery; an aprotinin group (group III) in which 5,000,000 KIU aprotinin was applied during surgery; and a combination group (group IV) in which APP, CS, and aprotinin all were used.
In group II (APP + CS group), shortly after the induction of anesthesia, blood was withdrawn via the 9-French central venous catheter at a rate of 35–45 mL/min and collected in the 125-mL centrifugal bowel of an autotransfusion unit (Cell Saver 5, Haemonetics Corp., Braintree, MA). No systemic heparin was administered at this time, but calcium in the blood was sequestered with citrate by mixing the blood and ACD (adenosine, citrate, dextrose) agent (Perfect, Beijing, China) at a volume ratio of 8:1. The withdrawn blood volume was replaced with a mixing (1:2) of plasma substitute (Gelofusion) and crystalloid (Lactate Ringer Injection) at a volume ratio of 1:2.5–3to maintain a steady PCWP. The withdrawn blood was centrifuged at 2400 rpm to separate the red blood cells (RBCs) from the plasma and platelets. After removing the RBCs, we continually centrifuged the plasma at 2400 rpm to separate the PRP from the platelet-poor plasma (PPP). An average of 30 minutes and three to four passes were required to complete blood withdrawal and separation of the PRP and PPP. A volume of blood was withdrawn to obtain approximately 300 mL of PRP from each study patient. If the Hct after separation was <30%, the RBCs were immediately returned to the patient; otherwise, the RBCs were saved at room temperature and reinfused as needed during the course of the operation.
In addition, in group II, the autotranfusion device at the same machine (Cell Saver 5, Haemonetics Corp., Brain-tree, MA) also was used to retrieve RBCs that were lost throughout the course of the operation. Saline (0.9% NaCl) was used to irrigate all the sponges with blood in the surgical field and then suctioned to the cell saver for further wash. Autologous RBCs, both obtained during initial blood withdrawal and obtained via the autotransfusion device during the operation, were reinfused as necessary for Hct < 30% or hemodynamic instability (MAP < 60 mmHg and/or CVP < 8 mmHg) before CPB, and Hct < 21% during CPB. After reversal of heparin, the autologous PPP and PRP were reinfused back to the patients, as were any remaining autologous RBCs.
In group III (aprotinin group), a high-dose Trasylol (Bayer, Leverkusen, Germany) was administered to all patients. The protocol is as previous described (8). A loading dose of 2.0 × 106 KIU was given before CPB. An additional dose of 2.0 × 106 KIU was dropped into the circuit during CPB, and after CPB continuous infusion of 1.0 × 106 KIU/hour was given until skin closure or until a total dose of 5.0 × 106 KIU aprotinin was achieved. In group IV (APP + CS + aprotinin group), all patients received the combining treatment of group II and group III, which was described previously.
Postoperatively, all patients were monitored in the cardiovascular surgical ICU. Clinical hemostasis was evaluated by measurement of 24-hour blood loss, blood requirement, and total number of homologous blood products transfused. RBC units were transfused to keep Hct ≥ 27%. Homologous FFP and platelet transfusions were administered only to the patients with active bleeding (>2 mL · kg−1 · h−1 chest tube drainage) and a platelet count <80 × 103/mm3. The ICU physicians and nurses were blinded regarding the groups to which the patients belonged.
The χ2 test was used to compare discrete (categorical) variables, and unpaired Student’s t tests were used to compare continuous variables. Data were expressed as means ± standard deviation, and statistical significance was accepted at p < 0.05.
RESULTS
A total of 70 patients signed the consents, were enrolled in the study, and were randomized into the four groups: 10 patients in group I, 20 patients in group II, 22 patients in group III, and 18 patients in group IV. No patient was excluded because of unexpected hemorrhage with hemodynamic instability. The four groups were not statistically different with respect to preoperative demographic characteristics and laboratory values (Table 1).
Table 1.
Preoperative demographic characteristics and laboratory values.
| Group I (Control) n = 10 | Group II (APP + CS) n = 20 | Group III (Aprotinin) n = 22 | Group IV (APP + CS + Aprotinin)n = 18 | |
|---|---|---|---|---|
| Age (yrs) | 59 ± 9 | 59 ± 6 | 61 ± 7 | 62 ± 8 |
| Gender (male and percentage) | 9 (90%) | 17 (85%) | 19 (86%) | 16 (89%) |
| Weight (kg) | 75 ± 7 | 78 ± 17 | 72 ± 14 | 79 ± 13 |
| No. Stenosed coronary vessels | 3.0 ± 0.7 | 3.1 ± 0.5 | 2.9 ± 0.4 | 3.2 ± 0.5 |
| LV ejection fraction | 58 ± 9 | 57 ± 14 | 61 ± 13 | 60 ± 17 |
| Diabetes mellitus | 2 (20%) | 5 (25%) | 4 (18%) | 4 (22%) |
| Hematocrit | 40.2 ± 3.6 | 42.6 ± 5.5 | 40.5 ± 4.5 | 41.5 ± 6.5 |
| Platelet (×1000/mm3) | 241 ± 71 | 224 ± 52 | 209 ± 59 | 236 ± 63 |
Values are mean ± standard deviation or n (%).
There was no significant difference in the demographic characteristics and laboratory values among the four groups.
APP, acute preoperative plateletpheresis; CS, cell salvage; No., number; LV, left ventricular.
In the 38 patients receiving APP enrolled in group II (n = 20) and group IV (n = 18), an average of 1037 ± 184 mL of whole blood was withdrawn for preoperative plateletpheresis. This yielded an average of 270 ± 124 mL of autologous packed red blood cells (PRBCs), 312 ± 48 mL of PRP, and 449 ± 67 mL of PPP for each patient. In the PRP, the platelet count was 989 ± 173 × 103/mm3, equivalent to 28% ± 8% of the circulating platelet mass after calculation. All the autologous PRP and PPP were reinfused after heparin reversal.
Intraoperatively, no significant differences were found regarding duration of operation, CPB time, aortic cross-clamp time, and lowest nasopharyngeal temperature among the four groups. However, in group I, 4 patients received homologous PRBC and 2 of them received homologous FFP as well, while no patient in the other three groups required any homologous blood products (Table 2).
Table 2.
Intraoperative details.
| Group I (Control) n = 10 | Group II (APP + CS) n = 20 | Group III (Aprotinin) n = 22 | Group IV (APP + CS + Aprotinin) n = 18 | |
|---|---|---|---|---|
| Duration of operation (min) | 237 ± 28 | 253 ± 61 | 248 ± 52 | 224 ± 81 |
| Pre-CPB fluid volume (mL) | 1239 ± 218 | 2955 ± 484* | 1083 ± 301 | 2792 ± 512* |
| CPB time (min) | 105 ± 16 | 98 ± 18 | 104 ± 30 | 96 ± 23 |
| Aortic cross-clamp time (min) | 66 ± 11 | 65 ± 22 | 64 ± 15 | 61 ± 14 |
| Lowest nasopharyngeal temp. (°C) | 30.9 ± 0.8 | 31.2 ± 0.5 | 30.8 ± 0.6 | 31.1 ± 0.5 |
| No. received homologous PRBCs | 4 (40%) | 0 (0%)* | 0 (0%)* | 0 (0%)* |
| No. received homologous FFP | 2 (20%) | 0 (0%)* | 0 (0%)* | 0 (0%)* |
There was no significant difference in the duration of operation, CPB time, aortic cross-clamp time, and lowest nasopharyngeal temperature among the four groups.
APP, acute preoperative plateletpheresis; CPB, cardiopulmonary bypass; Temp., temperature; PRBC, packed red blood cell; FFP, fresh-frozen plasma.
Compared with group I, p < 0.05.
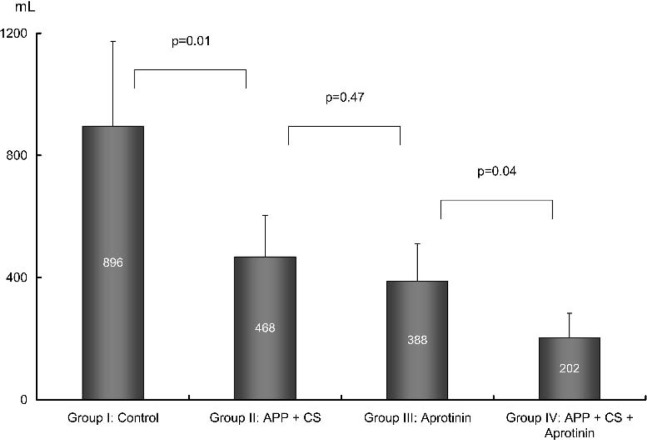
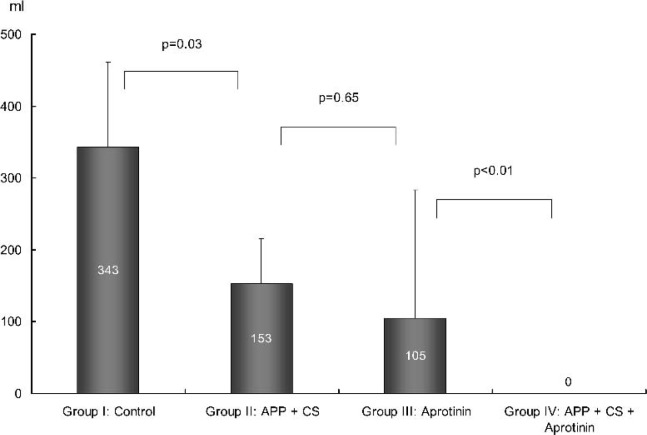
Postoperative ICU data are provided in Table 3. Compared with group I, the postoperative total cumulative blood loss was significantly reduced in group II, group III, and group IV, respectively. The requirements of packed red blood cells also were reduced in the three approached groups (Figures 1 and 2). In group I, six patients (6/10) received homologous FFP and three patients (3/10) received platelet transfusion, whereas no patients in the other three groups required FFP and platelets.
Table 3.
Postoperative ICU data.
| Group I (Control) n = 10 | Group II (APP + CS) n = 20 | Group III (Aprotinin) n = 22 | Group IV (APP + CS + Aprotinin) n = 18 | |
|---|---|---|---|---|
| Hematocrit (%) | ||||
| ICU admission | 30.3 ± 1.8 | 31.5 ± 3.6 | 32.1 ± 4.2 | 32.4 ± 4.5 |
| ICU discharge | 32.1 ± 1.5 | 32.1 ± 3.3 | 35.1 ± 3.6 | 32.3 ± 2.7 |
| Platelet count (×103/mm3) | ||||
| ICU admission | 105 ± 33 | 131 ± 27 | 118 ± 28 | 131 ± 23 |
| ICU discharge | 126 ± 42 | 151 ± 33 | 137 ± 32 | 164 ± 47 |
| Total cumulative blood loss (mL) | 896 ± 278 | 468 ± 136* | 388 ± 122* | 202 ± 81*† |
| Homologous PRBC transfused (mL) | 343 ± 118 | 153 ± 63* | 105 ± 178* | 0 ± 0*† |
| No. received homologous PRBC | 8 (80%) | 4 (20%)* | 5 (23%)* | 0 (0%)*† |
| No. received homologous FFP | 6 (60%) | 0 (0%)* | 0 (0%)* | 0 (0%)* |
| No. received homologous platelet | 3 (30%) | 0 (0%)* | 0 (0%)* | 0 (0%)* |
Note: There was no significant difference in hematocrit and platelet count.
APP, acute preoperative plateletpheresis; CS, cell salvage; No., number; ICU, intensive care unit; PRBC, packed red blood cell; FFP, fresh-frozen plasma.
Compared with Group I, p < 0.05.
Compared with group II and group III, p < 0.05.
Figure 1.
Postoperative blood loss. APP, acute preoperative plateletpheresis; CS, cell salvage.
Figure 2.
Postoperative packed red blood cell requirement. APP, acute preoperative plateletpheresis; CS, cell salvage.
DISCUSSION
The major causes of postoperative bleeding of cardiovascular surgery are the platelet dysfunction and depletion, the lower plasma level of clotting factors, and the activation of fibrinolysis (9). All of these are directly related to the nonphysiological change of cardiopulmonary bypass, i.e., the activation of contact system and coagulation cascade (10–14), direct and indirect activation of platelets and leukocytes (15–19), and even the systemic inflammatory response (20,21). Therefore, it is critical for the improvement of hemostasis to find the optimal approach for preventing platelet and clotting factors activation and depletion, and inhibiting fibrinolysis.
As demonstrated by the previous studies, cardiopulmonary bypass has a deleterious effect on platelets, with a decrease on platelet number and agreeability, a decrease in thrombin and fibrinogen binding sites on the platelet surface, and a decrease in glycoproteins IIb/IIIa on the platelet surface (22–24). Preoperative sequestration and preservation of autologous RBCs, platelets, and plasma with a tolerable volume replacement would have several advantages: decreased loss of RBCs, platelets, and clotting factors during bleeding; lower activation and depletion of these portions of whole blood; and the ability to return them to increase oxygen content and assist hemostasis after completion of cardiopulmonary bypass (3–5). In addition, the concomitant cell salvage technique would retrieve most surviving RBCs in the blood lost during the course of surgery (2). The combination of plateletpheresis and cell salvage performed with the same device would decrease the cost to a reasonable level. In this study, APP and CS resulted in significant lower blood loss and obviously reduced use of homologous PRBCs in group II patients. This finding is in agreement with the above mentioned trials.
In addition, a number of studies have demonstrated the use of aprotinin could improve postoperative hemostasis by inhibiting fibrinolysis and protecting platelets (25–29). High doses of this serine proteinase inhibitor dose not only inhibit fibrinolysis and some clotting factors but also temporarily inhibit the activation of platelets during bypass and, therefore, allow the inhibited platelets to assist clot formation after bypass. Again, results of the current study showed a significant lower amount of blood loss and requirement in the aprotinin treated patients (group III) than those for control (group I).
Moreover, we noticed that in the current study, the patients in the combining treatment group (group IV), in which preoperative plateletpheresis, cell salvage device, and aprotinin were all used, had the best clinical outcomes on postoperative hemostasis. In this group, the cumulative amount of mediastinal chest tube drainage is surprisingly low as 202 ± 81 mL, and therefore, no blood products were required. This suggests an exciting prospect on surgical hemostasis, and the realization of bloodless cardiovascular surgery.
We wish to mention that in group II and group IV, in which autologous blood was withdrawn and plateletpheresis was proceeded, patients received more fluid volume than the other two groups (group I and group III) before cardiopulmonary bypass, to maintain a stable hemodynamic status. Although this is reasonable because of the large volume replacement, we suggest careful operation of blood withdrawal and strict observance of patient hemodynamic monitoring.
In summary, in this prospective randomized clinical study, both acute preoperative plateletpheresis concomitant with cell salvage and aprotinin contributed to the improvement of postoperative hemostasis. The combination of these two approaches could minimize blood loss and transfusion requirement after cardiovascular surgery.
The authors also wish to emphasize that this is a pilot study because the eight surgeons and nine anesthesiologists involved were not evenly assigned to each group. Our findings may differ from some previous comparable trials. However, the reasons for this cannot be clearly addressed because of methodological difference. In consideration of the variation among institutions and even surgical groups, further larger sample trial should be done.
REFERENCES
- 1.Chavez AM, Cosgrove DM.. Blood conservation. Semin Thorac Cardiovasc Surg. 1990;2:358–63. [PubMed] [Google Scholar]
- 2.Breyer RH, Engelman RM, Rousou JA, Lemeshow SA.. A comparison of cell saver versus ultrafilter during coronary artery bypass operations. J Thorac Cardiovasc Surg. 1985;90:736–40. [PubMed] [Google Scholar]
- 3.Hall RI, Schweiger IM, Finlayson DC.. The benefit of the Hemonetics cell saver apparatus during cardiac surgery. Can J Anaesth. 1990;37:618–23. [DOI] [PubMed] [Google Scholar]
- 4.Boldt J, Kling D, Zickmann B, Jacobi M, Dapper F, Hempelmann G.. Acute preoperative plasmapheresis and established blood conservation techniques. Ann Thorac Surg. 1990;50:62–8. [DOI] [PubMed] [Google Scholar]
- 5.Gravlee GP.. Autologous platelet-rich plasma in cardiac surgery: aesthetics versus virtue. J Cardiothorac Vasc Anesth. 1993;7:1–3. [DOI] [PubMed] [Google Scholar]
- 6.Boldt J, Zickmann B, Ballesteros M, Oehmke S, Stertmann F, Hempelmann G.. Influence of acute preoperative plasmapheresis on platelet function in cardiac surgery. J Cardiothorac Vasc Anesth. 1993;7:4–9. [DOI] [PubMed] [Google Scholar]
- 7.Smith PK, Datta SK, Muhlbaier LH, Samsa G, Nadel A, Lipscomb J.. Cost analysis of aprotinin for coronary artery bypass patients: analysis of the randomized trials. Ann Thorac Surg. 2004;77:635–42. [DOI] [PubMed] [Google Scholar]
- 8.Hu XQ, Liu MZ, Wu LM, et al. Application of different dose aprotinin on open-heart surgery. Chin Circ J. 1995;10:110–2. [Google Scholar]
- 9.Woodman RC, Harker LA.. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76:1680–97. [PubMed] [Google Scholar]
- 10.Spanier TB, Chen JM, Oz MC, et al. Selective anticoagulation with active site-blocked factor IXA suggests separate roles for intrinsic and extrinsic coagulation pathways in cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1998;116:860–9. [DOI] [PubMed] [Google Scholar]
- 11.Gil W.. Inflammo-coagulatory response, extrinsic pathway thrombin generation and a new theory of activated clotting time interpretation. Perfusion. 2001;16:27–35. [DOI] [PubMed] [Google Scholar]
- 12.Gu YJ, Huyzen RJ, van Oeveren W.. Extrinsic pathway–associated activated clotting time for anticoagulation monitoring during cardiopulmonary bypass. Anesth Analg. 1996;83:433. [DOI] [PubMed] [Google Scholar]
- 13.Sonntag J, Dahnert I, Stiller B, Hetzer R, Lange PE.. Complement and contact activation during cardiovascular operations in infants. Ann Thorac Surg. 1998;65:525–31. [DOI] [PubMed] [Google Scholar]
- 14.Bertolino G, Locatelli A, Noris P, et al. Platelet composition and function in patients undergoing cardiopulmonary bypass for heart surgery. Haematologica. 1996;81:116–20. [PubMed] [Google Scholar]
- 15.Levy JH, Cormack JG, Morales A.. Heparin neutralization by recombinant platelet factor 4 and protamine. Anesth Analg. 1995;81:35–7. [DOI] [PubMed] [Google Scholar]
- 16.Schlame M, Schmid AB, Haupt R, Rustow B, Kox WJ.. Study of platelet-activating factor acetylhydrolase in the perioperative period of patients undergoing cardiac surgery. Shock. 1998;9:313–9. [DOI] [PubMed] [Google Scholar]
- 17.Rinder C, Fitch J.. Amplification of the inflammatory response: adhesion molecules associated with platelet/white cell responses. J Cardiovasc Pharmacol. 1996;27:S6–12. [DOI] [PubMed] [Google Scholar]
- 18.Kawahito K, Kobayashi E, Ohmori M, et al. Enhanced responsiveness of circulatory neutrophils after cardiopulmonary bypass: increased aggregability and superoxide producing capacity. Artif Organs. 2000;24:37–42. [DOI] [PubMed] [Google Scholar]
- 19.Ilton MK, Langton PE, Taylor ML, et al. Differential expression of neutrophil adhesion molecules during coronary artery surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1999;118:930–37. [DOI] [PubMed] [Google Scholar]
- 20.Baufreton C, Intrator L, Jansen PG, et al. Inflammatory response to cardiopulmonary bypass using roller or centrifugal pumps. Ann Thorac Surg. 1999;67:972–7. [DOI] [PubMed] [Google Scholar]
- 21.Pintar T, Collard CD.. The systemic inflammatory response to cardiopulmonary bypass. Anesthesiol Clin North America. 2003;21:453–64. [DOI] [PubMed] [Google Scholar]
- 22.Holloway DS, Summaria L, Sandesara J, Vagher Jp, Alexander JC, Caprini JA.. Decreased platelet number and function and increased fibrinolysis contribute to postoperative bleeding in cardiopulmonary bypass patients. Thromb Haemost. 1988;59:62–7. [PubMed] [Google Scholar]
- 23.Wenger RK, Lukasiewicz H, Mikuta BS, Niewiarowski S, Edmunds LH.. Loss of platelet fibrinogen receptors during clinical cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1989;97:235–9. [PubMed] [Google Scholar]
- 24.Rinder CS, Mathew JP, Rinder HM, Bonan J, Ault KA, Simth BR.. Modulation of platelet surface adhesion receptors during cardiopulmonary bypass. Anesthesiology. 1991;75:563–70. [DOI] [PubMed] [Google Scholar]
- 25.Mohr R, Goor DA, Lusky A, Lavee J.. Aprotinin prevents cardiopulmonary bypass–induced platelet dysfuanction. Circulation. 1992;86(Suppl 2):405–9. [PubMed] [Google Scholar]
- 26.Blauhut B, Gross C, Necek S, Doran JE, Spath P, Hansen PL.. Effects of high-dose aprotinin on blood loss, platelet function, fibrinolysis, complement, and renal function after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1991;101:958–67. [PubMed] [Google Scholar]
- 27.Lass M, Welz A, Kochs M, Mayer G, Schwandt M, Hannekum A.. Aprotinin in elective primary bypass surgery. Graft patency and clinical efficacy. Eur J Cardiothorac Surg. 1995;9:206–10. [DOI] [PubMed] [Google Scholar]
- 28.Royston D.. Blood-sparing drugs: aprotinin, tranexamic acid, and epsilon-aminocaproic acid. Int Anesthesiol Clin. 1995;33:155–79. [PubMed] [Google Scholar]
- 29.Shinfeld A, Zippel D, Lavee J, et al. Aprotinin improves hemostasis after cardiopulmonary bypass better than single-donor platelet concentrate. Ann Thorac Surg. 1995;59:872–6. [DOI] [PubMed] [Google Scholar]