Abstract:
The current practice of perfusion in Australia and New Zealand continues to adopt new techniques and procedures into clinical practice. Our aims were to report current practice in 2003 and to compare and contrast current practice with historic practice. A total of 62 centers (40 perfusion groups) performing procedures using cardiopulmonary bypass (CPB) were identified and were e-mailed a detailed electronic survey. The survey was comprised of an excel worksheet that contained 233 single answer questions (either dropdown lists, yes/no, true/false, or numeric) and 12 questions that allowed the respondent to provide a commentary. Respondents were instructed to answer all questions based on what represented the predominant practice of perfusion in their institutions during 2003. We report an 89% response rate representing a caseload of 20,688 adult cases. These data allowed us to profile the following. A standard adult CPB setup in 2003 consisted of a membrane oxygenator (100% of cases), a roller pump (70%) as the main arterial pump, although a centrifugal pump would be considered for selected procedures (30%), a circuit incorporating a hard-shell venous reservoir (86%), and a mixture of biocompatible and nonbiocompatible circuit components (66%). The circuit would include a pre-bypass filter (88%), an arterial line filter (94%), and would allow monitoring of the following: hard-shell venous reservoir low level (100%) with servo-regulation of the arterial pump (85%), microbubble alarm (94%) with servo-regulation of the arterial pump (79.5%), arterial line pressures (100%) with servo-regulation of the arterial pump (79%), inline venous O2 saturation (100%), and inline hematocrit (58%). Perfusion practice in Australia and New Zealand has adopted changes over the last decade; however, some areas of practice show wide variation. This survey provides a baseline of contemporary practice for Australian and New Zealand perfusionists.
Keywords: survey, questionnaire, cardiopulmonary bypass
The current practice of perfusion in Australia and New Zealand continues to adopt new techniques and procedures into clinical practice. The rate of the introduction of new technology into practice varies from other parts of the world where different factors (financial, political, and clinical) may dictate when or if new technologies or practices are introduced. New technology is often available in the market place before there is extensive availability of reliable evidence-based literature, resulting in many unproven changes being incorporated into routine practice.
Perfusion practices have not been extensively surveyed and reported in Australia and New Zealand. The most recent publication was reflective of perfusion practice in Australia in 1992 (1), whereas Jenkins et al. (2) included practice aspects in their report on perfusion incidents in Australia and New Zealand in 1995. A number of international surveys of perfusion practice have been conducted on different aspects of both adult and pediatric populations (3–8).
The focus of previous papers has varied from primarily looking at the type of equipment used and general strategies of perfusion to papers looking at various aspects of perfusion management. There has been less scrutiny on some aspects of perfusion management, such as the use of monitoring strategies during bypass, with the exception of Charr iere et al. (7) who focused on monitoring systems.
With this background, we conducted a detailed survey to look at many aspects of perfusion practice in Australia and New Zealand. Our aims were to report current practice (equipment, monitoring, management, quality assurance) in 2003 and to compare and contrast current practice with historic practice. To enable us to compare current practice with previous practice in the region, we included questions in common with the previous Australian survey (1). In this, the first of two reports, we discuss equipment and monitoring aspects of perfusion practice.
MATERIALS AND METHODS
A total of 62 centers performing procedures using cardiopulmonary bypass (CPB), 56 adult only, 2 pediatric only, and 4 combined adult/pediatric centers, were identified. The senior perfusionist responsible for performing CPB or the person deemed to be in charge of perfusion at each institution was e-mailed a detailed electronic survey. Where a group of perfusionists serviced multiple institutions and the perfusion practice of each unit was deemed identical, units were amalgamated before the distribution of the questionnaire for ease of analysis. This resulted in 40 surveys (hospital groups) being identified, representing between one and seven institutions.
The survey was comprised of an excel worksheet that contained 233 single answer questions (either dropdown lists, yes/no, true/false or numeric) and 12 questions that allowed the respondent to provide a commentary. Questions covered both pediatric and adult CPB. Specific questions were designed to allow direct comparison with the previous survey of Australian CPB practice published in 1993 (1). The survey related to practice in the calendar year of 2003 and examined the number and types of procedures, the type of equipment used (oxygenator, type of circuit and pump system used), filtration, cardioplegia, monitoring systems used, temperatures (measured and recorded, strategies, rewarming), type of prime, drugs used during bypass, pump set-up and storage, coagulation management, glucose management, blood gas and acid base management, pressure and flow management, management of cardiotomy blood, risk stratification, specialized perfusion management (including deep hypothermic circulatory arrest), data management, education, and training (for full survey, see Appendix 1).
Respondents were instructed to answer all questions based on what represented the predominant practice of perfusion in their institutions during 2003.
RESULTS
Thirty-four of 40 possible respondents completed the survey, covering 54 centers identified as performing adult bypass surgery (90%), and 5 respondents, covering 5 centers (83%) identified as performing pediatric bypass surgery, resulting in an overall 89% response rate, representing a caseload of 20,688 adult and 1282 pediatric cases. Because of the wide variation in pediatric practice encountered, we report only results relating to adult perfusion from our survey. These data are reported in detail in Tables 1–4, and Figures 1–4.
Table 1.
Demography.
| Total | n |
|---|---|
| Total number of adult cardiopulmonary bypass cases | 20,688 |
| Total number coronary artery bypass cases | 12,876 |
| Percentage off-pump coronary artery bypass cases (%) | 19 |
Table 2.
Equipment.
| Type | Percentage |
|---|---|
| Oxygenator type | |
| Membrane | 100 |
| Bubble | 0 |
| Open or closed system | |
| Open | 86.1 |
| Closed | 13.95 |
| Use of biocompatible circuit | |
| All | 10.4 |
| Part | 63.3 |
| None | 26.3 |
| Biocompatible component (partial use only) | |
| Oxygenator | 65.4 |
| Reservoir | 33.6 |
| Arterial filter | 41.2 |
| Cardioplegia circuit | 15.8 |
| Tubing/connectors | 23.7 |
| Type of arterial pump-head (routine CPB) | |
| Roller | 70.1 |
| Centrifugal | 13.6 |
| Either | 16.3 |
| Is occlusion reset each case | 100 |
| Is vacuum assisted venous drainage used | |
| Always | 0 |
| Nearly always | 0 |
| Sometimes | 38 |
| Never | 62 |
| Is arterial line filter used | |
| Always | 94.1 |
| Sometimes | 3.6 |
| Never | 2.3 |
| Is arterial line leukocyte filter used | |
| Sometimes | 21.7 |
| Never | 78.3 |
| Is pre-CPB filter used | |
| Always | 87.9 |
| Nearly always | 8.2 |
| Never | 3.9 |
| Pore size of pre-CPB filter | |
| 0.2 | 67.2 |
| Other | 32.8 |
| Is cardioplegia filter used | |
| Always | 16.7 |
| Nearly always | 2.3 |
| Sometimes | 4.7 |
| Never | 72.2 |
Table 3.
Monitoring.
| Site | Percentage |
|---|---|
| Level alarm (always) | 100 |
| Level alarm + pump stop | |
| Always | 84.6 |
| Never | 15.4 |
| Microbubble (always) | |
| Microbubble + pump stop | |
| Always | 79.5 |
| Nearly always | 0 |
| Never | 20.5 |
| Arterial line pressure | 100 |
| Arterial line pressure + pump stop | |
| Always | 79 |
| Nearly always | 4.2 |
| Sometimes | 1.4 |
| Never | 15.4 |
| In-line arterial saturation | |
| Always | 7.1 |
| Nearly always | 0 |
| Sometimes | 13.3 |
| Never | 79.7 |
| In-line venous saturation (always) | 100 |
| In-line hematocrit | |
| Always | 57.9 |
| Nearly always | 0 |
| Sometimes | 8.1 |
| Never | 34 |
| In-line blood gas monitoring | |
| Always | 5.2 |
| Nearly always | 2 |
| Sometimes | 20 |
| Never | 71.5 |
Table 4.
Monitoring.
| Site | Percentage |
|---|---|
| Dedicated patient monitor (slave screen) | |
| Always | 73.1 |
| Nearly always | 11.9 |
| Sometimes | 3.9 |
| Never | 11.1 |
| Closed circuit television/mirror for surgical field vision | |
| Always | 17.1 |
| Nearly always | 6.9 |
| Sometimes | 19.8 |
| Never | 56.2 |
| Trans-cranial doppler | |
| Sometimes | 9.1 |
| Never | 90.9 |
| Bispectral index monitor | |
| Always | 12 |
| Nearly always | 24.9 |
| Sometimes | 17.6 |
| Never | 45.2 |
| Regional cerebral oximetry | |
| Sometimes | 25.7 |
| Never | 74.3 |
| Activated clotting time | |
| Always | 100 |
| Heparin concentration | |
| Always | 5.3 |
| Nearly always | 0 |
| Sometimes | 9.5 |
| Never | 85.2 |
| Thromboelastograph | |
| Always | 2.3 |
| Nearly always | 1.4 |
| Sometimes | 16.1 |
| Never | 80.2 |
| Superior vena cava/jugular pressure | |
| Always | 70 |
| Nearly always | 9.4 |
| Sometimes | 15.6 |
| Never | 5.1 |
| Left atrial pressure/pulmonary artery pressure | |
| Always | 39.2 |
| Nearly always | 26.6 |
| Sometimes | 32.9 |
| Never | 1.3 |
| Electroencephalogram | |
| Always | 2 |
| Nearly always | 0 |
| Sometimes | 2.3 |
| Never | 95.7 |
Figure 1.
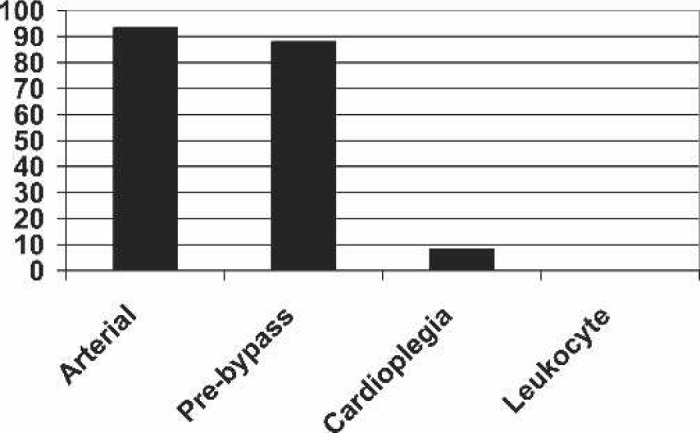
Use of filters (always).
Figure 2.
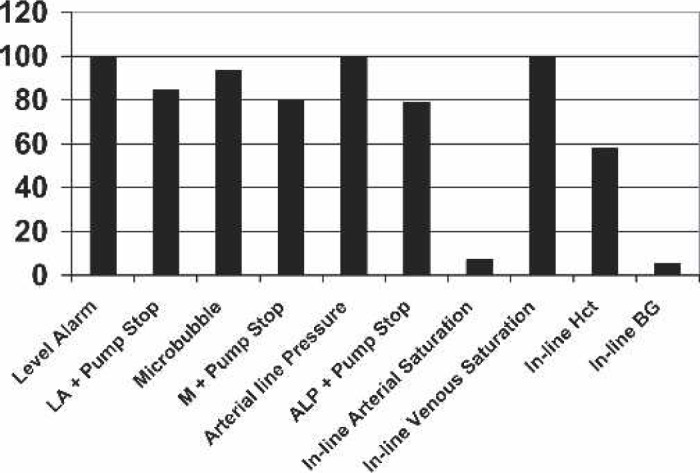
Use of monitoring apparatus (always).
Figure 3.
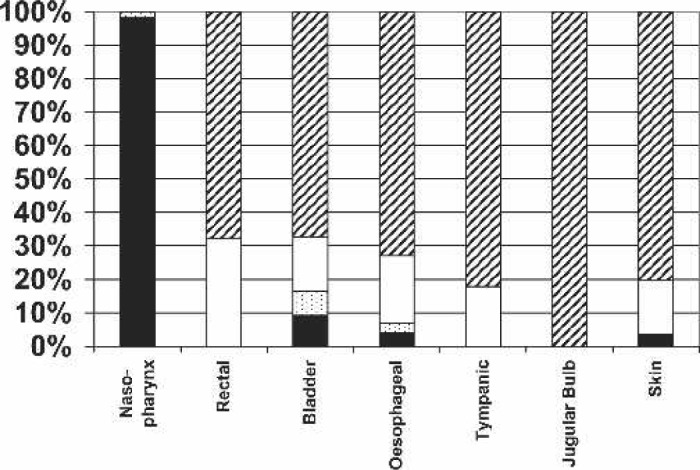
Temperature monitoring sites: never, cross-hatch; sometimes, white; nearly always, speckled; always, black.
Figure 4.
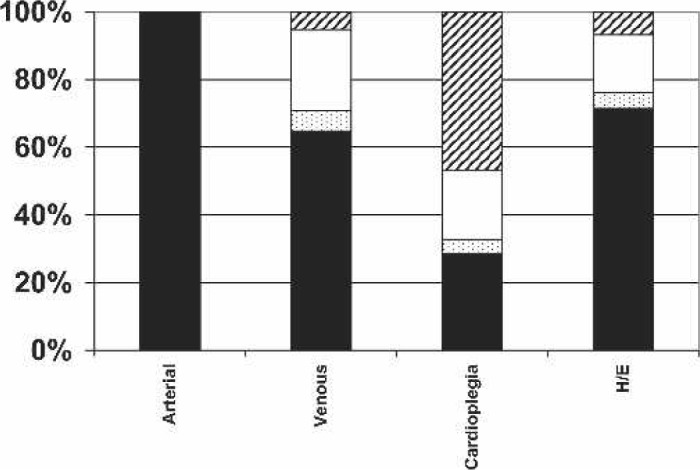
Circuit temperature monitoring sites: never, cross-hatch; sometimes, white; nearly always, speckled; always, black.
These data allowed us to profile the following. A standard CPB set-up in 2003 consisted of: a membrane oxygenator (100% of cases), a roller pump (70%) as the main arterial pump, although a centrifugal pump would be considered for selected procedures (30%), a circuit incorporating a hard-shell venous reservoir (86%), and a mixture of biopassive and non-biopassive circuit components (66%), with only 10.4% having a tip to tip biocompatible circuit (Table 2). The circuit would include a pre-bypass filter (88%) and an arterial line filter (94%) and would allow the monitoring of the following: hard-shell venous reservoir low level (100%) with servo-regulation of the arterial pump (85%), microbubble alarm (94%) with servo-regulation of the arterial pump (79.5%), arterial line pressures (100%) with servo-regulation of the arterial pump (79%), inline venous O2 saturation (100%), and inline hematocrit (58%). In addition, a dedicated patient slave monitor would be positioned specifically for the perfusionist (73%), but few perfusionists had direct vision of the surgical field by closed circuit television monitor or mirror (17%).
DISCUSSION
Perfusionists are required to perform complex procedures in a professional and timely manner, at a cost that is considered reasonable either by hospital budget controllers or third party payers and in a manner that adopts the most appropriate and current methodology and techniques. The importance of this has now been widely recognized with a growing interest in evidence-based “best practice” in perfusion and in the assessment and initiation of quality improvement initiatives. Continual evaluation and monitoring of current perfusion practice are an important adjuncts to these endeavors, allowing perfusionists to understand, evaluate, and compare their own practices with contemporary perfusion practice both within their own region and internationally. This may be achieved by a number of different avenues, including discussion with colleagues, attending perfusion meetings, and by reviewing current perfusion techniques reported in the scientific literature.
This survey has enabled us to describe practices common throughout the Australasian perfusion community and to identify some divergent practices. This report will allow all Australasian perfusion units to compare themselves to contemporary practice (calendar year 2003) in Australia and New Zealand. In addition, we are able to see what major changes have occurred both in the equipment used and the techniques of CPB over the last decade.
A number of questions highlighted quite divergent clinical practice. This is evident in the adoption of monitoring techniques and the use of servo-regulation to control the arterial pump (Table 3). We report that 100% of cases performed had low-level alarms activated for all cases; however, only 85.8% of the time was this coupled with servo-regulation of the arterial pump. Similarly, 100% of cases were performed with arterial line pressure monitoring, whereas only 79% were servo-regulated; whereas 96% of cases were performed with microbubble detection, only 80% were accompanied by servo-regulation. This interesting observation seems at odds with the American experience as reported by Stammers et al. (4), who reported 98.5% use of arterial line pump manometers but only a 35.1% adoption of servo-regulation of the arterial pump. This difference may be attributable to the comparative widespread use of centrifugal pumps in the United States. In the survey of Stammers et al., 258 of the 524 (49%) respondents reported using centrifugal pumps for the arterial pump compared with a 13.6% exclusive use of a centrifugal pump and a 16.3% occasional use in this Australasian report. From closer scrutiny of the data of Stammers et al., it is plausible that, for those using roller pumps, the rate of servo regulation would be 70%–80%. However, of those who had changed practice to stop using servo-regulation, 7 of 14 reported that the main reason for this was the ineffectiveness of automated pump shut-down devices. This practice of non-use of servo-regulation is divergent from the guidelines of the Australasian Society of Cardiovascular Perfusionists (ASCVP), which state that such servo-regulation of the arterial pump should occur (ASCVP guidelines 1.4.1, 1.4.2, 1.4.3, and 1.4.4) (9).
Cockroft (10) and Feneck (11), reporting on monitoring during CPB in the United Kingdom and Ireland in 1994, suggested that there were “Few established standards for monitoring for perfusionists” and that “One other striking feature … is the paucity of monitoring during CPB … suggests that a large portion of patients undergoing CPB are inadequately monitored during the bypass period.” Subsequent establishment of guidelines and recommendations for patient monitoring (12) are likely to have impacted on these findings. Indeed, our survey showed high rates of adoption of monitoring devices such as level, microbubble, and pressure alarms, in-line venous oxygen saturation, and dedicated patient monitors, whereas other new technologies such as the bispectral index monitor (BIS) and regional cerebral oximetry are adopted on a more constrained basis.
There have been some dramatic changes in perfusion practice in the interval between 1991 (1) and 2003, for example, the complete adoption of membrane oxygenators (cf. 87% in 1992) and the reduction in the sole use of roller pumps from 97% to 67%, with a concomitant increase in the use of centrifugal pumps (14%) or a combination of both (16%). Similarly the adoption of new technologies, specifically biocompatible components, with 72.2% of cases being performed using some biocompatible component, and 42% of all cases being performed with biocompatible oxygenators, has been dramatic. It would be interesting to see whether this trend has continued as rapidly over the last 2 years. Other techniques have shown a far more restrained adoption into practice, such as the use of vacuum-assisted venous drainage (VAVD). Other changes that we observed over this period were an increase in the use of pre-bypass (58%–97%), arterial line filters (66%–94%), CO2 flushing (62%–85%), and bubble detectors (30%–90%). Geographical difference in practice is shown by almost universal adoption of arterial line filtration in Australasia that mirrors the practice reported in pediatric and adult perfusion in the United States (4,6) but contrasts with their use in the United Kingdom (T.J. Jones, personal communication).
There has been an enormous focus on monitoring for CPB over the last decade, with manufacturers providing an apparent never-ending array of monitoring devices that may be used during surgery. We found that, whereas some centers have adopted a large number of different monitoring devices, including transcranial Doppler, BIS, and thromboelastogram (TEG), most centers relied on the provision of a dedicated patient monitor for the perfusionists (73%), along with a number of physiological parameter monitors [in-line venous saturation (100%) and hematocrit (58%), superior vena cava/jugular pressure (70%), left atrial pressure (LAP)/pulmonary artery pressure (PAP)] and an activated clotting time monitor (87%). The adoption of in-line blood gas monitoring has not been rapid, with only 5.2% of centers routinely using such devices. This may reflect the relative high disposable cost associated with these products and the relative lack of evidence showing a clinical benefit.
The importance of temperature monitoring is also evident in this survey, with all respondents reporting that arterial outlet temperatures were monitored, which is of interest given increasing evidence that this is a better surrogate of brain temperature than nasopharyngeal and esophageal temperature (13–15). In excess of 60% of all cases were performed with monitoring of venous inlet and heat exchanger temperatures. Interestingly, in only 28.3% of cases was cardioplegia delivery temperature monitored. The predominant patient monitoring site was overwhelmingly nasopharyngeal.
Like previous surveys, this survey was subject to a number of limitations including representing a snapshot of activity rather than a continuous sampling of activity. However, the ability to distribute the survey electronically, to have e-mail feedback for problematic issues, and to follow-up electronically resulted in the high response rate for the survey. One area that would require further clarification in a follow-up survey would be the definition of what the terms “nearly always” and “sometimes” mean when they were part of four choices. Another limitation is that we surveyed the “person identified” as being responsible for perfusion, usually the chief or head of perfusion unit. This may have resulted in responses representing unit protocol rather than practice. Furthermore, as highlighted by Cecere et al. (6), it is impossible to determine whether all questions were interpreted correctly. When an obvious interpretation error occurred, the respondent was contacted and the data were checked; however, clearly this is a limited process.
There have been significant changes in perfusion practice throughout Australia and New Zealand over that last decade. Given the paucity of perfusion-focused research, it is questionable whether the driving force in change has been evidence based; however, future change and development will be heavily focused on improving the outcomes for patients undergoing cardiac surgery with CPB. While there has been some erosion of the procedure base with increasing cardiological interventions and alternative surgical strategies, the focus on conventional CPB will remain important and will rely on the adoption of evidence-based best practice and the adoption of tailored perfusion strategies in high-risk groups.
We were able to quantify current practice in Australia and New Zealand. This does not purport to be best practice. While being intuitively beneficial to define best practice for perfusion, the reality of the process is not necessarily simple. Clearly defined practices are evident, for example, the universal use of membrane oxygenators, a practice that would not raise much question. However, why the inclusion of other practices or their lack of adoption has occurred is not so easily evident. The low use of a total biocompatible-coated circuit in routine practice is also contrary to much of the evidence (16); however, the influence of a number of factors, including price, is difficult to take into account. The Australasian market has seen a dramatic increase in the availability of relatively affordable biocompatible products from a variety of manufacturers since the time of this survey.
CONCLUSION
Perfusion practice in Australia and New Zealand is contemporary and has adopted changes in practice over the last decade; however, some areas of practice show wide variation. This survey provides a baseline of contemporary practice for Australian and New Zealand perfusionists, allowing them to compare and contrast themselves with fellow practitioners.
ACKNOWLEDGMENTS
To all perfusionists who provided the data for this report and specifically the perfusion colleagues we work with. Special acknowledgment to Teena West, Statistician, Auckland City Hospital, for helping with the design of the survey and the collation of the data and Rebecca Stanley, Manager, Information Technology, Cardiac Surgery Research, Flinders Medical Centre.
APPENDIX 1. NEW ZEALAND AND AUSTRALIA PERFUSION PRACTICE SURVEY 2004 QUESTIONNAIRE
The actual survey was conducted using a purpose designed excel spreadsheet.
PLEASE ANSWER FOR THE PREDOMINANT PRACTICE IN YOUR UNIT FOR THE 2003 CALENDAR YEAR.
PLEASE USE A SEPARATE SPREADSHEET FOR EACH HOSPITAL (OR GROUP OF HOSPITALS), IF YOU ARE RESPONDING FOR MORE THAN ONE.
Step 1: SAVE AS 2004SURVEY_hospital name
Step 2: complete survey
Step 3: e-mail to timw@adhb.govt.nz
1.01, List hospital(s) that this survey form covers? text
1.02, How many Adult CPB cases does this form cover for 2003 (do not include OPCAB)? text
2, How many Pediatric CPB cases does this form cover for 2003? text
3.01, How many CABG (including OPCAB) were done in 2003? text
3.02, What percentage of CABG in 2003 were OPCAB? text
EQUIPMENT
4, Oxygenators used (brand and model)
4.1, text, 4.2, text, 4.3, text, 4.4, text, 4.5, text, 4.6, text,,4.7, text
5, Is an open or closed system (hard-shell/venous bag) used? text
6.01, What part of the CPB perfusion circuit has biocompatible coating? all, part, none
6.02, If 6.01 not all then check coated components
Oxygenator true/false,
Reservoir true/false,
Arterial filter true/false,
Cardioplegia circuit true/false,
Tubing/connectors true/false,
7.01, What type of arterial pump head is used for routine CPB? roller /centrifugal / both
7.02, Is pump occlusion reset for each pump boot use? always, nearly always, sometimes, never
8, Please comment on your indications for use of each type of pump head. text
9.01, Is an arterial line filter used? always, nearly always, sometimes, never
9.02, Comment why? if answer 9.01 = sometimes or never, text
9.03, Is arterial line leukocyte filtration used? always, nearly always, sometimes, never
10, Is VAVD used? always, nearly always, sometimes, never
11, Is a pre-CPB filter used? always, nearly always, sometimes, never
12, If 11 = always or sometimes please state brand and pore size (microns)
12.1 text,12.2 text
13, Is a cardioplegia filter used? always, nearly always, sometimes, never
14, Monitoring used?
14.01 Level Alarm always, nearly always, sometimes, never
14.02 Level Alarm + pump stop always, nearly always, sometimes, never
14.03 Microbubble Alarm always, nearly always, sometimes, never
14.04 Microbubble Alarm+ pump stop always, nearly always, sometimes, never
14.05 Art Line Pressure Monitor always, nearly always, sometimes, never
14.06 Art Line Pressure Monitor + pump stop always, nearly always, sometimes, never
14.07 Inline O2 Sat–Arterial always, nearly always, sometimes, never
14.08 Inline O2 Sat–Venous always, nearly always, sometimes, never
14.09 Inline HCT Monitor always, nearly always, sometimes, never
14.10 In line ABGs always, nearly always, sometimes, never
14.11 Dedicated patient monitor (slave screen) always, nearly always, sometimes, never
14.12 CCTV/mirror for surgical field vision always, nearly always, sometimes, never
14.13 Trans-cranial Doppler always, nearly always, sometimes, never
14.14 BIS monitor always, nearly always, sometimes, never
14.15 Regional Cerebral Oximetry always, nearly always, sometimes, never
14.16 ACT always, nearly always, sometimes, never
14.17 Heparin Concentration always, nearly always, sometimes, never
14.18 TEG always, nearly always, sometimes, never
14.19 SVC/Jugular pressure always, nearly always, sometimes, never
14.20 LAP/PAP always, nearly always, sometimes, never
14.21 EEG always, nearly always, sometimes, never
15, Where are patient temperatures measured, Site, Frequency,
15.01 Naso-pharynx always, nearly always, sometimes, never
15.02 Rectal always, nearly always, sometimes, never
15.03 Bladder always, nearly always, sometimes, never
15.04 Esophageal always, nearly always, sometimes, never
15.05 Tympanic always, nearly always, sometimes, never
15.06 Jugular Bulb always, nearly always, sometimes, never
15.07 Skin always, nearly always, sometimes, never
16, What circuit temperatures are routinely monitored,
16.01 Arterial blood always, nearly always, sometimes, never
16.02 Venous Blood always, nearly always, sometimes, never
16.03 Cardioplegia always, nearly always, sometimes, never
16.04 Heat exchanger water inlet always, nearly always, sometimes, never
PRIME
17, Is the circuit flushed with CO2 always, nearly always, sometimes, never
18a, What is the routine prime solutions for adults (exclude drugs), text
18b, What is the total prime volume for routine adult CPB ml
19, What is the routine prime solutions for pediatrics (exclude drugs), text
19b, Total prime volumes for pediatric circuits (ml): circuit a ml, circuit b ml, circuit c ml, circuit d ml, circuit e ml.
20.01, What is the criteria for using blood in the prime in adults? text
20.01, What is the criteria for using blood in the prime in pediatrics? text
21, What drugs are routinely added to the prime before CPB,
a) Adults, text
b) Pediatric text
c) Neonate < 1 month text
CPB
22, Is a pump set up 24/7? always, nearly always, sometimes, never
23, How long is a dry set up pump kept for (hours) text
24, How long is a wet set up pump kept for (hours) text
25a, For OPCAB is a pump;
25.01 available in the OR, always, nearly always, sometimes, never
25.02 Available but not in the OR, always, nearly always, sometimes, never
25.03 Not available, always, nearly always, sometimes, never
25b, If a pump is available for OPCAB?
25.04 Is it set up (with perfusion circuit), always, nearly always, sometimes, never
25.05 If it is set up is it primed, always, nearly always, sometimes, never
25.06 If set up is it unprimed, always, nearly always, sometimes, never
26.01, Is a minimum ACT required for cannulation yes/no
26.02, If 26.01 = YES, what is the minimum required ACT for cannulation text, enter a range if appropriate text
27.01, Is a minimum ACT required before starting CPB? yes/no,
27.02, If 27.01 = YES, what is the minimum ACT before starting CPB, (enter a range if appropriate), text
28, What volatile agents are used when on CPB, text
29.01, What main vasoactive drugs are used when on CPB, text
29.02, What is the target range of glucose concentration during CPB, text
29.03, What is the observed range of glucose concentration during CPB, text
30, What is the minimum temperature aimed for in the following situations
30.01 Routine adult CPB (e.g., CABG x 4) text
30.02 Complex / prolonged Adult CPB (Valve redo CABG) text
30.03 Adult CPB requiring DHCA text
30.04 Acynotic Neonate (VSD) text
30.05 Neonate requiring DHCA text
31, What is the maximum temperature used during rewarming,
Arterial blood text
Naso/eosph text
Water in text
32, What maximum temperature gradients are used during rewarming (degree gradient), Blood: patient text Blood: water text
33, What is the target rate of rewarming (site, degree/min) naso or equivalent text arterial blood text
33.02, What guides your rewarming strategy most, max water temp, max blood temp, gradient, rates of rewarming
34, What is the target PaO2 range for CPB (mmHg) text
35, Acid base strategy for Adult CPB
35.01 alpha stat, always, nearly always, sometimes, never
35.02 pH stat, always, nearly always, sometimes, never always, nearly always, sometimes, never
35.03, If 35.01/02 = sometimes in what circumstances do you use pH Stat, text
36, Acid-base strategy for pediatric CPB
36.01 alpha stat cooling, always, nearly always, sometimes, never
36.02 pH stat cooling, always, nearly always, sometimes, never
36.03 alpha stat rewarming, always, nearly always, sometimes, never
36.04 pH stat rewarming, always, nearly always, sometimes, never
36.05, If 36.01/02 = sometimes in what circumstances do you use pH Stat,
37, Cardioplegia type (frequency, ratio [e.g., 4:1, variable])
37.01 Blood cardioplegia Frequency always, nearly always, sometimes, never
Ratio text 37.02 Crystalloid cardioplegia
Frequency always, nearly always, sometimes, never
Ratio text
37.03 Intermittent clamping Frequency always, nearly always, sometimes, never Ratio text
37.04 Combination of above
Frequency always, nearly always, sometimes, never
Ratio text
38, At what temperature is Cardioplegia delivered? (temp range, frequency)
38.01 Warm,
Temperature text
Frequency always, nearly always, sometimes, never
38.02 Tepid
Temperature text
Frequency always, nearly always, sometimes, never
38.03 Cold
Temperature text
Frequency always, nearly always, sometimes, never
38.04 Cold + hotshot
Temperature text
Frequency always, nearly always, sometimes, never
38.05 combination of above
Temperature text Frequency always, nearly always, sometimes, never
39, Does your cardioplegia contain (component, frequency)
39.01 Potassium? always, nearly always, sometimes, never
39.02 Magnesium? always, nearly always, sometimes, never
39.03 Glutamate? always, nearly always, sometimes, never
39.04 Aspartate? always, nearly always, sometimes, never
39.05 Adenosine? always, nearly always, sometimes, never
39.06 1-arginine? always, nearly always, sometimes, never
39.07 Insulin? always, nearly always, sometimes, never
39.08 Lactobionate? always, nearly always, sometimes, never
39.09 Glucose? always, nearly always, sometimes, never
40.01, Is leukocyte filtration of blood cardioplegia used? always, nearly always, sometimes, never
40.02, Are any ancillary methods used to facilitate/ maintain, cooling of the heart other than cardioplegia? text
41, What range of mean perfusion pressure is used during routine adult CPB, (MAP range)
41.01 Normothermia, text
41.02 Hypothermia, text
42, What range of mean perfusion pressure is used during non-DHCA pediatric CPB (MAP range)
42.01 Normothermia, text
42.02 Hypothermia, text
43, Is a different target MAP range used for specific patient cohorts / conditions -comment, text
44, Rank 1–3 what methods do use to achieve the required pressure on CPB,
44.01 text
44.02 text
44.03 text
45, What range flow index is routinely used during routine Adult CPB (l/min/m2)
45.01 Normothermia Adults text
45.02 Hypothermia Adults text
45.03 Normothermia Pediatrics text
45.04 Hypothermia Pediatrics text
46, Are different flow indices used for specific patient groups or co-morbidity–comment text
47, Is pulsatile flow used, always, nearly always, sometimes, never If never go to question 50,
48, In what patient group is pulsatile flow used. Comment text
49, If using pulsatile flow are the following used
49.01 A dispersion or non std arterial cannula always, nearly always, sometimes, never
49.02 Formal assessment of the aorta (EAS/TOE) always, nearly always, sometimes, never
50, Most common anitfibrinolytic used during CPB, text
51, What are your indications for use of aprotonin in cases where CPB will be utilised? text
52 Cardiotomy blood
52.01, Is cardiotomy blood discarded? always, nearly always, sometimes, never
52.02, Is cardiotomy blood processed? always, nearly always, sometimes, never
52.03, Is unprocessed cardiotomy blood returned to the circuit? always, nearly always, sometimes, never
53, Are patients for CPB risk stratified? always, nearly always, sometimes, never
54, What Risk Score is used? text
55, Are different proscribed CPB protocols used for?
55.01 Pregnant patients? always, nearly always, sometimes, never
55.02 High neuro risk patient? always, nearly always, sometimes, never
55.03 Renal failure patient? always, nearly always, sometimes, never
55.04 Elderly Patient (>75 years)? always, nearly always, sometimes, never
56, List what your unit does differently for CPB for the high neurological risk patient? text
57, List what your unit does differently for CPB y for the high renal risk patient? text
58, Which of these are used in cases using DHCA
58.01 Profound hypothermia? always, nearly always, sometimes, never
58.02 Mild hypothermia? always, nearly always, sometimes, never
58.03 EEG/CFM? always, nearly always, sometimes, never
58.04 Somatosensory Evoked Potentials? always, nearly always, sometimes, never
58.05 CSF Drainage? always, nearly always, sometimes, never
58.06 Steroids? always, nearly always, sometimes, never
58.07 Barbiturates? always, nearly always, sometimes, never
58.08 Mannitol? always, nearly always, sometimes, never
58.09 Ca Channel Blockers? always, nearly always, sometimes, never
58.10 BIS monitor? always, nearly always, sometimes, never
58.11 cerebral oximetry? always, nearly always, sometimes, never
59, What arterial cannulation site is used where the ascending aorta is unsuitable (e.g., aneurysmal)?
59.01 Femoral/Iliac artery? always, nearly always, sometimes, never
59.02 distal arch? always, nearly always, sometimes, never
59.03 Axillary artery? always, nearly always, sometimes, never
59.04 innominate artery? always, nearly always, sometimes, never
60, During DHCA in adults is cerebral perfusion used? (frequency, target flow, target pressure)
60.01 Retrograde? always, nearly always, sometimes, never, Target Flow text Target Pressure text
61.02 Selective antegrade? always, nearly always, sometimes, never, Target Flow text Target Pressure text
61, Is computerised perfusion data retrieval during CPB used? always, nearly always, sometimes, never
62, Perfusion record? Handwritten only, computerised only, both, none
63, Are CPB patients preoperatively assessed by the perfusionist (notes reviewed and strategies put in place)? always, nearly always, sometimes, never
64, Does the perfusionist discuss CPB with the patient preoperatively (i.e., principles of CPB, strategies for case etc)? always, nearly always, sometimes, never
65, Do your perfusionists perform or participate in the following,
65.01 Formal pre bypass checklist, true/false
65.02 Research, true/false
65.03 Formal perfusion incident reporting, true/false
65.04 Quality assurance, true/false
65.05 Audits, true/false
65.06 Formal team meetings, weekly or more, true/false
65.07 Regular interdisciplinary meetings, true/false
65.07 M&M meetings, true/false
66, Is your hospital group involved in the following, yes/ no, number per annum
66.01 ECMO, yes/no, number per annum,
66.02 Ventricular assist (Short term e.g. centrifugal pump), yes/no, number per annum,
66.03 Ventricular assist (medium term e.g. Abiomed), yes/ no, number per annum
66.04 Ventricular assist (long term e.g. Thoratec), yes/no, number per annum
66.05 Transplants Heart, yes/no, number per annum
66.06 Transplants Lung, yes/no, number per annum
66.07 Transplants Liver, yes/no, number per annum
66.08 Isolated Limb / regional, yes/no, number per annum
REFERENCES
- 1.Wajon PR, Walsh RG, Symons NLP.. A survey of cardiopulmonary bypass perfusion practices in Australia in 1992. Anaesth Intens Care. 1993;21:814–21. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins OF, Morris M, Simpson JM.. Australasian perfusion incident survey. Perfusion. 1997;12:279–88. [DOI] [PubMed] [Google Scholar]
- 3.Silvay G, Ammar T, Reich DL, Vela-Cantos F, Joffe D, Ergin AM.. Cardiopulmonary bypass for adult patients: A survey of equipment and techniques. J Cardiothorac Vasc Anesth. 1995;9:420–4. [DOI] [PubMed] [Google Scholar]
- 4.Stammers AH, Mejak BL, Rauch ED, Vang SN, Viessman TW.. Factors affecting perfusionists’ decisions on equipment utilization: Results of a United States survey. J Extra Corpor Technol. 2000;32:4–10. [PubMed] [Google Scholar]
- 5.Lilley A.. The selection of priming fluids for cardiopulmonary bypass in the UK and Ireland. Perfusion. 2002;17:315–20. [DOI] [PubMed] [Google Scholar]
- 6.Cecere G, Groom R, Forest R, Quinn R, Morton J.. A 10-year review of pediatric perfusion practice in North America. Perfusion. 2002;17:83–9. [DOI] [PubMed] [Google Scholar]
- 7.Charriere JM, Durand C, Mandon N, et al. French national survey of the use of monitoring devices during cardiopulmonary bypass in 2001. Ann Fr Anesth Reanim. 2003;22:414–20. [DOI] [PubMed] [Google Scholar]
- 8.Groom RC, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 Survey. J Extra Corpor Technol. 2005;37:343–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Australasian Society of Cardiovascular Perfusionists Inc. Standards of practice in clinical perfusion within Australasia. Available at www.perfusion.com.au Accessed March 23, 2006.
- 10.Cockroft SJ.. Use of monitoring devices during anesthesia for cardiac surgery: A survey of practices at public hospitals within the United Kingdom and Ireland. J Cardiothorac Vasc Anesth. 1994;8:382–5. [DOI] [PubMed] [Google Scholar]
- 11.Feneck RO.. Standards of monitoring. J Cardiothorac Vasc Anesth. 1994;8:379–91. [DOI] [PubMed] [Google Scholar]
- 12.Recommendations for Standards of Monitoring and Alarms during Cardiopulmonary Bypass. Available at www.sopgbi.org, www.acta.org.uk, and www.scts.org Accessed March 23, 2006.
- 13.Grocott HP, Newman MF, Croughwell ND, White WD, Lowry E, Reves JG.. Continuous jugular venous versus nasopharyngeal temperature monitoring during hypothermic cardiopulmonary bypass for cardiac surgery. J Clin Anesth. 1997;9:312–6. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RI, Fox MA, Grayson A, Jackson M, Fabri BM.. Should we rely on nasopharyngeal temperature during cardiopulmonary bypass? Perfusion. 2002;17:145–51. [DOI] [PubMed] [Google Scholar]
- 15.Marino M, Cheng W, Romagnoli A, Walding D, Nussmeier N.. Temperature bias: Jugular bulb venous temperature versus conventional sites. Ann Thorac Surg. 2000;70:1792. [Google Scholar]
- 16.Shann KG, Likosky DS, Murkin JM, Baker RA, Baribeau YR, De-Foe GR, Dickinson TA, Gardner TJ, Grocott HP, O’Connor GT, Rosinski DJ, Selke FW, Willcox TW.. An evidence-based approach to improving the practice of cardiopulmonary bypass in adults. A focus on neurological injury, glycemic control, hemodilution and the inflammatory response. J Thorac Cardiovasc Surg (in press). [DOI] [PubMed]


