Abstract:
Oxygen pressure field theory (OPFT) was originally described in the early 1900s by Danish physiologist, Dr. August Krogh. This revolutionary theory described microcirculation of blood gases at the capillary level using a theoretical cylindrical tissue model commonly referred to as the Krogh cylinder. In recent years, the principles and benefits of OPFT in long-term extracorporeal circulatory support (ECMO) have been realized. Cardiac clinicians have successfully mastered OPFT fundamentals and incorporated them into their clinical practice. These clinicians have experienced significantly improved survival rates as a result of OPFT strategies. The objective of this study was to determine if a hyperoxic strategy can lead to equally beneficial outcomes for short-term support as measured by total ventilator time and total length of stay in intensive care unit (ICU) in the cardiopulmonary bypass (CPB) patient at a private institution. Patients receiving traditional blood gas management while on CPB (group B, n = 17) were retrospectively compared with hyperoxic patients (group A, n = 19). Hyperoxic/OPFT management was defined as paO2 values of 300–350 mmHg and average VSAT > 75%. Traditional blood gas management was defined as paO2 values of 150–250 mmHg and average VSAT < 75%. No significant differences between treatment groups were found for patient weight, CPB/AXC times, BSA, pre/post Hgb, pre/post-platelet (PLT) counts, pre/post-creatinine levels, pre/post-BUN, UF volumes, or CPB urine output. Additionally, no significant statistical differences were found between treatment groups for total time in ICU (T-ICU) or total time on ventilator (TOV). Hyperoxic management strategies provided no conclusive evidence of outcome improvement for patients receiving CPB for routine cardiac surgical repair. Additional studies into the impact of hyperoxia in short-term extracorporeal circulatory support are needed.
Oxygen pressure field theory (OPFT) was initially described by Danish physiologist August Krogh early in the 20th century as part of his work on the structure and function of capillaries, for which he won an unshared Nobel Prize in 1920. Unfortunately, the clinical significance of this has not been recognized by the medical community until relatively recently.
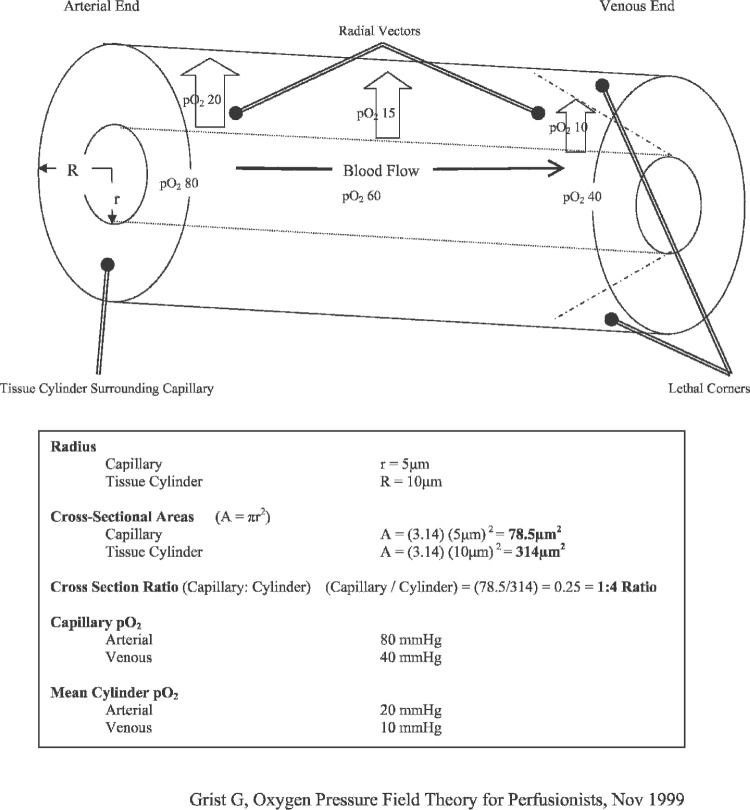
The theoretical cylindrical tissue model described in OPFT, now referred to as the Krogh cylinder (Figure 1) provides speculative microcirculatory mechanisms for blood gases at the capillary level. Krogh suggested that tissue pO2 levels are greatest and most closely resemble arterial pO2 values nearest the lumen wall on the arterial end of the capillary. Through his cylindrical model, Krogh further showed how tissue pO2 is lowest at the outside perimeter of the venous end of the capillary. Consequently, tissue pO2 levels progressively decrease while [H+] ion levels progressively increase with capillary length and diameter. As a result, venous ends of thickened, elongated capillaries are at increased risk of critical levels hypoxia and acidosis (1).
Figure 1.
Krogh cylinder.
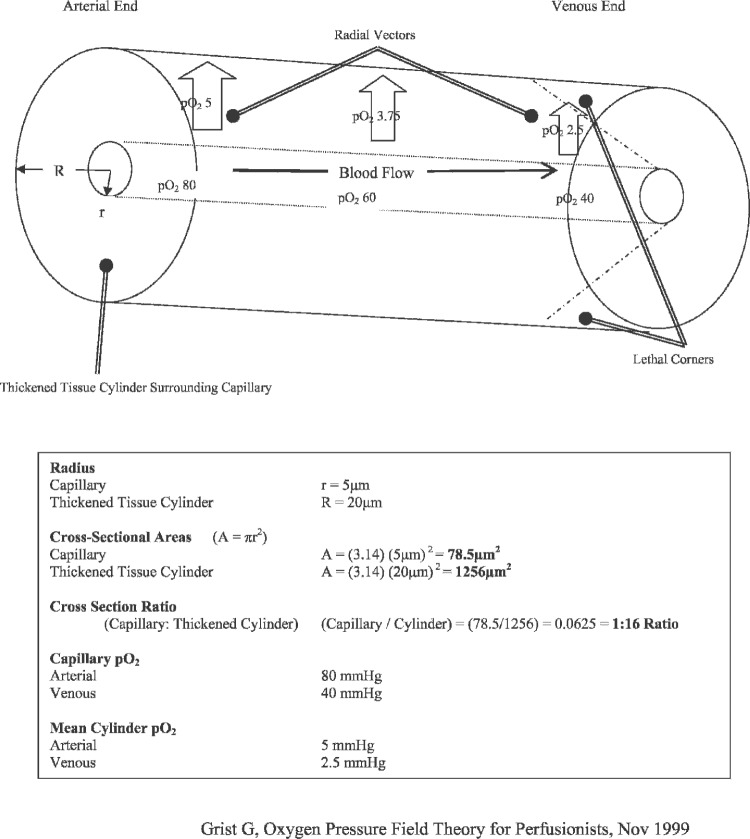
If left untreated, these hypoxic and acidotic areas can have lethal effects on cells, tissues, and entire organ systems (2). A thickened Krogh cylinder (Figure 2) shows how “lethal corners” of hypoxia, hypercapnia, acidosis, and other metabolic wastes can accumulate within the capillary, rapidly and progressively resulting in tissue death. Krogh’s suggestion for preventing or attenuating these lethal corners is primarily bifactorial: create a hyperoxic environment for tissues and minimize tissue edema.
Figure 2.
Thickened Krogh cylinder.
Arterial pO2 values are elevated beyond normal physiological levels to supersaturated/hyperoxic levels to improve O2 tissue diffusion along the capillary axis. Theoretically, this diffusion should occur in both radial and axial directions, thus increasing tissue pO2 further down the length of the capillary toward the venous end. Consequently, capillary perimeters on the venous end should experience improved oxygenation. Tissue edema is minimized to reduce capillary diameter and the resultant distance between capillary lumen and tissue perimeter. This diameter reduction shortens the distance O2 must travel to functionally perfuse the tissue. This improved tissue per-fusion results in improved oxygenation, lower [H+] ion concentration, and more efficient removal of metabolic waste products.
Mathematical and physiological studies validate Krogh’s fundamental theory and cylindrical tissue model (3–6). Other studies conclude the efficacy of OPFT and hyperoxia in clinical applications (7,8). In cases where deep hypothermic circulatory arrest (DHCA) was used, Pearl et al. (9) concluded that hyperoxia resulted in superior blood gas values regardless of specific blood gas management strategy (i.e., alpha-stat or pH stat). Wittnich et al. (10) concluded that exposure hyperoxia in the presence of ischemia provided an energy-sparing effect during the early ischemic period. However, a correlational increase in ventricular fibrillation was concluded to negate any beneficial hyperoxic effects. Of note, a significant discrepancy in clinical definitions of hyperoxia exists within the literature. This discrepancy ranges from 250 to 700 mmHg (9–13).
In recent years, cardiac surgical clinicians have been implementing OPFT fundamentals and successfully incorporating them into their clinical practice. The principles and benefits of OPFT strategies in long-term extracorporeal circulatory support, (i.e., extracorporeal circulatory support [ECMO]) have been realized. As a direct result of OPFT strategies, many clinicians have experienced improved patient survival rates (1). OPFT strategies that include the tactic of hyperoxia may reduce morbidity or mortality among selected patients (14–25).
The objective of this study was to determine if a hyperoxia management strategy would result in equally beneficial outcomes for short-term support as measured by total ventilator time and total length of stay in the intensive care unit (ICU).
MATERIALS AND METHODS
Study Design
This study was designed to collect appropriate data for retrospective analysis of patients receiving one of two per-fusion management strategies while on cardiopulmonary bypass (CPB). The different management strategies can be categorized as follows—group A: those patients receiving blood gas management consistent with hyperoxic/ OPFT and management (i.e., paO2 300–350 mmHg and VSAT values >75%); group B: those patient receiving traditionally accepted blood gas management while on CPB (i.e., paO2 150–250 mmHg and average VSAT values of 70–75%).
Patient Population/Selection
A total of 36 patients were selected: 19 from group A and 17 from group B. Of the 19 group A patients, 18 received CABG and 1 received valve replacement. Among the 17 group B patients, 13 received CABG, 3 received valve replacements, and 1 received combined CABG/valve. To be included in the study, each patient had to meet both inclusion and exclusion selection criteria. The criteria for inclusion/exclusion are as follows.
Inclusion Criteria
Nonemergent cardiac surgical repair necessitating CPB and AXC
Age range: 40–80 years
BSA 1.6–2.4 m2
Preoperative HCT > 27%
Maximum CPB time 120 minutes
Ultrafiltration volume 5500–6500 mL
Exclusion Criteria
CPB time >120 minutes
Recent history, such as neurological deficits (TIA, CVA, head injury etc.)
Renal insufficiency presenting to the operating room with anuria or serum creatinine level > 1.5
Presence of pre/perioperative intra-aortic balloon pump (IABP)
Deep hypothermic circulatory arrest (DHCA)
Hypothermia < 28°C
Medical history positive for diabetes mellitus, COPD, hypothyroidism
Extracorporeal Circuit Design
Patients from both group A and group B received identical extracorporeal circuits (ECCs) run on a Sarns Modular Perfusion System 8000 (Terumo/SARNS, Ann Arbor, MI). The ECC used was a customized pack by Terumo. The pack incorporated a Terumo Capiox RX-25 hollow fiber oxygenator with open integrated hard shell venous reservoir, Capiox arterial line filter, and Capiox HC11 Hemoconcentrator. The oxygenator, venous reservoir, and arterio-venous (A-V) loop were coated with Terumo’s bio-passive X-coating. Capiox disposable centrifugal pumps were used on all cases. The Quest MPS (Quest Medical, Allen, TX) was used for cardioplegia delivery.
Other ancillary equipment consists of Sechrist air/O2 blender (Sechrist Industries, Anaheim, CA), Hemotherm heater/cooler (Cincinnati Sub-Zero, Cincinnati, OH) and a Sarns/Terumo CDI Blood Parameter Monitoring System 500 (Terumo/SARNS) for continuous in-line blood gas monitoring. All ancillary components were used on every patient in both groups.
Cardiopulmonary Bypass
All patients received 1200–1800 mL of priming solution (lactated Ringer or Normosol), 10,000 units of porcine-derived heparin, and 50 mEq NaHCO3. The use of antifibrinolytics and protease inhibitors, such as Amicar and Trasylol, are surgeon-specific and were added as indicated.
All perfusion parameters were mandated by the attending surgeon. As such, patient target temperatures, as well as other relevant physiological parameters, were performed per protocol for that particular surgeon.
Cardioplegia was delivered by the Quest MPS. Arrest and additive agents were administered in accordance with surgeon-directed protocols. Perfusion pressures were maintained at 55–75 mmHg. If sufficient urine output was not observed during CPB, diuretics were administered per surgeon/anesthesiologist orders.
Inhalation anesthetics (exclusively isoflurane) were routinely administered by the perfusionist during CPB. The range of isoflurane levels administered during CPB were 0.4–1.5 volume % to maintain a bispectral Index (BIS) of 40–60.
Patient hematocrits were maintained between 21% and 30%. Anticoagulation was achieved through the use of porcine-derived heparin. Anticoagulation levels were monitored by Celite-activated clotting times (ACT) and maintained >480 seconds at all times during CPB.
A maximum temperature gradient of 10°C was maintained during periods of patient cooling and rewarming; attempts to wean patients from CPB were not made until core (bladder) temperature of 35–36°C was achieved.
Data Collection
Institutional Review Board (IRB) approval was obtained before initiation of study and data collection. All data included in this retrospective analysis were collected in compliance with all applicable guidelines established by the Health Insurance Portability and Accountability Act of 1996 (HIPPA). Each patient was assigned a unique and untraceable identifier. This identifier and corresponding data (demographical, statistical, and otherwise) was entered into a database for appropriate statistical analysis. The patient database was accessible exclusively by the primary investigator. As required by HIPAA regulations, all charts and computer files were locked in a secure area out of view from the general public.
To minimize clinical variation, data was collected from two different clinicians: control data from one and treatment data from another. In terms of clinical technique and overall patient management, the clinicians were virtually identical. The primary variation between clinicians was the pO2 levels maintained throughout CPB.
To adequately meet the requirements of this study, patient data was collected from three different phases of a patient’s hospital stay: preoperatively, intraoperatively, and postoperatively. Specific data to be collected from each phase are as follows.
Preoperative Data
History and physical
Appropriate preoperative laboratory values (Hgb, Plt, Creat, BUN)
Intraoperative Data
Blood gas and Hgb data
Patient hemodynamic data (pressures, temperatures)
Perfusion data (blood flow, blender settings)
Urine output
Postoperative Data
Twelve-to 24-hour postoperative laboratory values at (Hgb, Plt, Creat, BUN)
Twenty-four-hour urine output
Length of time on ventilator (TOV)
Length of time in ICU (T-ICU)
Postoperative complications/morbidities
Statistical Analysis
Appropriate data points were recorded directly from extracorporeal perfusion records and entered into a computer database using Microsoft Excel. Additional data were collected from institutional electronic databases and merged with Excel data.
The Shapiro-Wilk test was used to test for normality of the data for the following continuous variables: ultrafiltration, ICU time, ventilator time, weight, CPB time, AXC time, BSA, urine output during CPB, and the repeatedly measured Hgb, PLT, Creat, BUN, ABG, VBG, and Vsat variables. Normal probability plots were also examined.
Ultrafiltration, weight, CPB time, and AXC time were approximately normally distributed, so a t test was used to test for differences in the means between the two treatment groups. A test for equality of variances was performed to determine whether to use a t test that assumed equal variances or a t test that assumed unequal variances should be used.
ICU time, ventilator time, BSA, and urine output during CPB were slightly skewed, so the nonparametric Wilcoxon test was used to test for differences in the medians between the two treatment groups.
The repeatedly measured variables of Hgb, PLT, Creat, BUN, ABG, VBG, and Vsat were each approximately normally distributed. A repeated-measures mixed general linear model with fixed effects for treatment, time-point, and treatment by time-point interaction was used to analyze these data. Pairwise comparisons were made between treatments at each time-point, and the p values were adjusted using the Bonferroni adjustment for multiple comparisons.
Estimates of treatment means, SDs, and SEs are provided for each continuous variable.
Statistical significance was defined as p : .05. Unless otherwise indicated, all data are expressed as mean ± SD. PC SAS version 9.1 was used for all analyses.
RESULTS
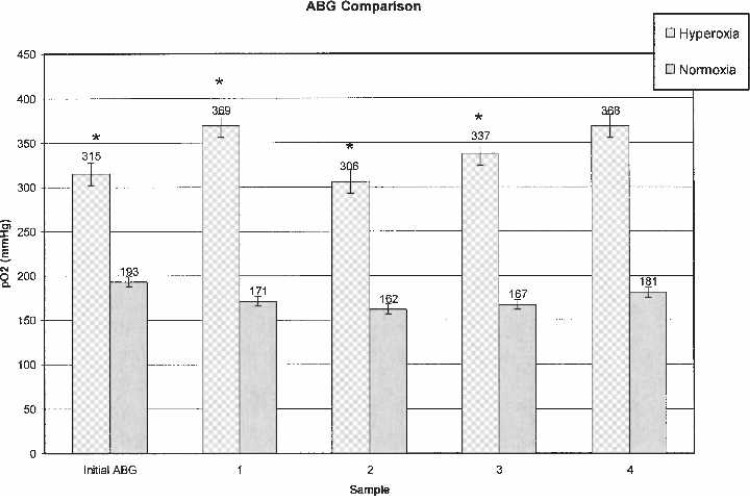
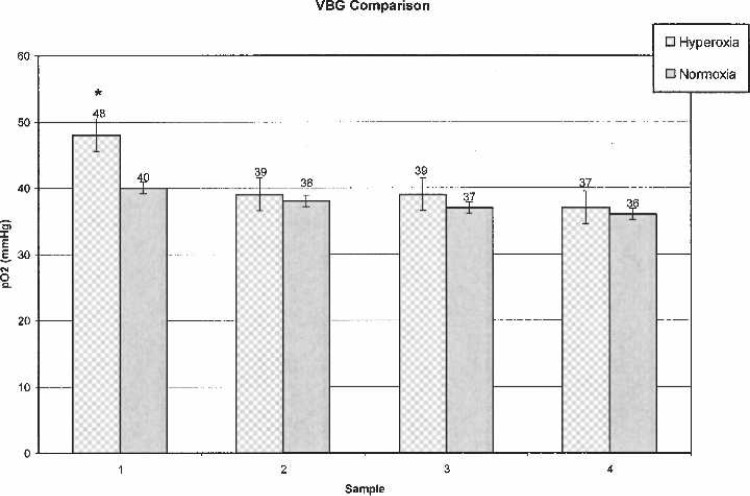
Table 1 shows patient demographic data. Laboratory and other data are outlined in Table 2. In both groups, initial ABG samples were taken 2–3 minutes after initiation of CPB to verify adequate oxygenation before placement of AXC. Subsequent ABG, VBG, and Vsat values were analyzed every 15–25 minutes throughout CPB. For clarification, subsequent samples were labeled as ABG-1, VBG-1, and Vsat-1. A comparison of sequential ABG and VBG are shown in Figures 3 and 4.
Table 1.
Patient demographics.
| Hyperoxic (n = 19) | Normoxic (n = 17) | p Value | |
|---|---|---|---|
| Patient weight (kg) | 86.2 ± 13.9 | 87 ± 16.0 | .8754* |
| Body surface area (m2) | 2.02 ± 0.20 | 1.93 ± 0.51 | .9874† |
| CPB (min) | 94.7 ± 13.3 | 86.3 ± 19.9 | .1401* |
| AXC (min) | 72.8 ± 9.74 | 66.1 ± 16.1 | .1474* |
t-test.
Nonparametric Wilcoxon (Kruskall-Wallis) test.
Table 2.
Laboratory and other patient data.
| Hyperoxic (n = 19) | Normoxic (n = 17) | p Value | |
|---|---|---|---|
| Pre-CPB Hgb (g/dL) | 12.2 ± 1.27 | 13.1 ± 1.25 | .1014‡ |
| 12-to 24-hour postoperative Hgb (g/dL) | 9.3 ± 1.28 | 10.1 ± 1.39 | .1831‡ |
| Pre-CPB Plt (K/ μL) | 232 ± 53 | 226 ± 71 | 1.000‡ |
| 12-to 24-hour postoperative Plt (K/ μL) | 140 ± 30 | 139 ± 28 | 1.000‡ |
| Pre-CPB creatinine (mg/dL) | 0.96 ± 0.28 | 0.94 ± 0.18 | 1.000‡ |
| 12-to 24-hour postoperative creatinine (mg/dL) | 1.04 ± 0.45 | 1.01 ± 0.20 | 1.000‡ |
| Pre-CPB BUN (mg/dL) | 16.1 ± 6.93 | 14.7 ± 5.47 | .9986‡ |
| 12-to 24-hour postoperative BUN (mg/dL) | 15.3 ± 6.28 | 12.9 ± 4.46 | .4512‡ |
| Ultrafiltrate (mL) | 5995 ± 272 | 6041 ± 292 | .6241* |
| CPB urine (mL) | 193 ± 159 | 240 ± 130 | .0988† |
| Time in ICU (hr) | 38.6 ± 21.5 | 29.2 ± 14.51 | .4185† |
| Time on ventilator (hr) | 9.38 ± 4.75 | 6.97 ± 4.81 | .0986† |
t-test.
Nonparametric Wilcoxon (Kruskall-Wallis) test.
Bonferroni adjusted p value.
Figure 3.
Arterial blood gases.
Figure 4.
Venous blood gases.
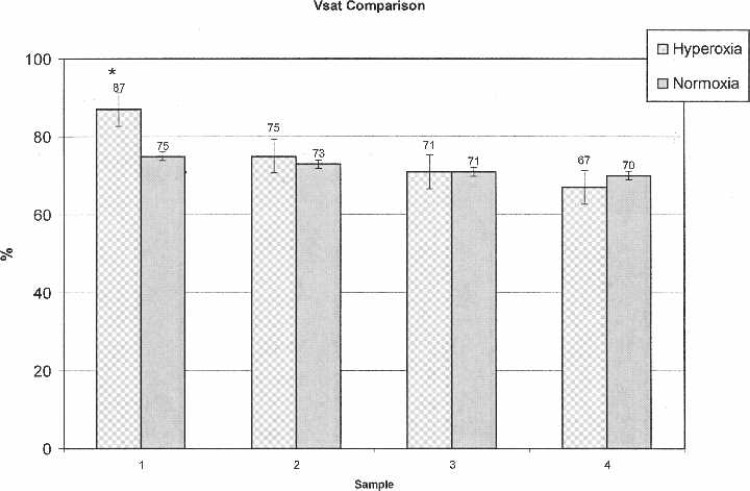
ABG values were statistically significantly higher (p < .0010) at every time interval in the OPFT group (Figure 3). VBG-1 (p = .0044) and Vsat-1 (p < .0010) values were significantly higher in the OPFT group; subsequent VBG and Vsat values revealed no statistically significant differences (Figures 4 and 5).
Figure 5.
Venous saturations.
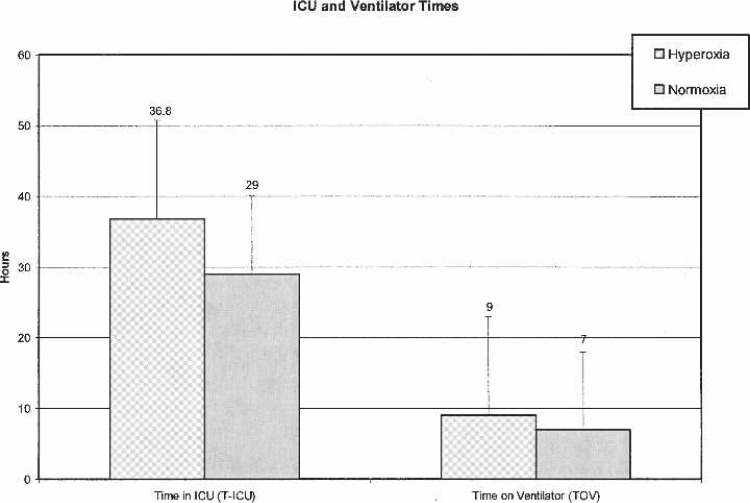
All laboratory values (Hgb, Plt, Creat, and BUN) exhibited normal distributions and showed no statistically significant differences between treatment groups. Patient weights were found to be normally distributed (means ± SD), whereas BSA values were found to be slightly skewed between groups, requiring the use of a nonparametric Wilcoxon test (p = .9874). CBP and AXC times were found to be normally distributed between groups with no statistically significant differences noted. Ultrafiltrate (UF) values were slightly higher in the non-OPFT group (6041 ± 292 vs. 5994 ± 272 in the OPFT/hyperoxic group), but revealed no statistical significance. Mean CPB urine outputs in the non-OPFT/normoxic group (240 ± 130) were slightly higher than the OPFT group (193 ± 159), but were not statistically significant. No significant differences were found in T-ICU (p = 04185) or TOV (p = .0986). T-ICU and TOV data are shown in Figure 6.
Figure 6.
Time in ICU and time on ventilator.
Some clinical investigators have concluded OPFT and hyperoxic management results in an increased occurrence of myocardial reperfusion injury caused by excessive free radical and diene production (11–13). These investigators maintain that myocardial reperfusion injuries result in markedly reduced ventricular function/contractility and reduced cardiac output. No evident signs of myocardial reperfusion injury were observed in either group.
DISCUSSION
By providing tissues with a hyperoxic environment and minimizing fluid overload as theorized by Krogh and shown in his cylindrical model, cardiac clinicians have experienced improved outcomes for their patients undergoing long-term ECMO. The goal of this retrospective study was to determine if OPFT/hyperoxic management strategies had an impact on outcomes for patients receiving CPB for routine cardiac surgical repair. The primary indicators for improved outcomes were T-ICU and TOV.
Mean ABG, VBG, and Vsat values were numerically higher at every time interval in the OPFT group except for Vsat at the final interval. Statistically significant higher values of ABG were noted at every time interval in the hyperoxia group; however, only the first VBG and Vsat samples were significantly different.
No statistically significant differences were found in any other variables. Preoperative and postoperative laboratory values for Hgb, Plt, Creat, and BUN were not statistically significant. No statistical differences were found in patient weight, BSA, CPB/AXC times, UF volumes, CPB urine output, T-ICU, or TOV.
In conclusion, our study failed to provide conclusive evidence on the impact of a hyperoxic management on outcomes for patients receiving CPB for routine cardiac surgical repair. Further studies on hyperoxia in short-term extracorporeal circulatory support are warranted.
ACKNOWLEDGMENTS
The authors thank Valerie K. Shostrom for outstanding and patient statistical guidance and Angela Ask and Rick Kuntz for insightful editorial assistance and ongoing support.
REFERENCES
- 1.Grist G, Thomas D.. Blood anion gaps and venoarterial carbon dioxide gradients as risk factors in long-term extracorporeal support. J Extra Corpor Technol. 1997;29:6–10. [PubMed] [Google Scholar]
- 2.Gutierrez G, Lund N, Bryan-Brown CW.. Cellular oxygen during multiple organ failure. Crit Care Clin. 1989;5:271–87. [PubMed] [Google Scholar]
- 3.Sharan M, Gupta S, Popel AS.. Parametric analysis of the relationship between end-capillary and mean tissue pO2 as predicted by a mathematical model. J Theor Biol. 1998;195:439–49. [DOI] [PubMed] [Google Scholar]
- 4.Schumacker PT, Samsel RW.. Analysis of oxygen delivery and re-uptake relationships in the Krogh tissue model. J Appl Physiol. 1989;67:1234–44. [DOI] [PubMed] [Google Scholar]
- 5.Walfridsson H, Lund N.. Tissue oxygen pressure in normal myocardium and across the border zone during coronary artery occlusion in the pig. Effects of different arterial oxygen pressures. Basic Res Cardiol. 1990;85:467–80. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Tokunu S, Tahepold P, et al. Preconditioning Protects the Severely Atherosclerotic Mouse Heart. Ann Thorac Surg. 2001;71: 1296–304. [DOI] [PubMed] [Google Scholar]
- 7.Schumacker PT, Samsel RW.. Oxygen delivery and uptake by peripheral tissues: physiology and pathophysiology. Crit Care Clin. 1989;5:255–69. [PubMed] [Google Scholar]
- 8.Vallet B, Tavernier B, Lund N.. Assessment of tissue oxygenation in the critically-ill. Eur J Anaesthesiol. 2000;17:221–9. [DOI] [PubMed] [Google Scholar]
- 9.Pearl JM, Thomas DW, Grist G, et al. Hyperoxia for management of acid-base status during deep hypothermia with circulatory arrest. Ann Thorac Surg. 2000;70:751–5. [DOI] [PubMed] [Google Scholar]
- 10.Wittnich C, Torrance SM, Carlyle CE.. Effects of hyperoxia on neonatal myocardial energy status and response to global ischemia. Ann Thorac Surg. 2000;70:2125–31. [DOI] [PubMed] [Google Scholar]
- 11.Ihnken K, Buckberg GD, Winkelmann B, et al. Reduced oxygen tension during cardiopulmonary bypass limits myocardial damage in acute hypoxic immature piglet hearts. Eur J Cardiothorac Surg. 1996;10:1127–34. [DOI] [PubMed] [Google Scholar]
- 12.Kaneda T, Ku K, Inoue M, et al. Post-ischemic reperfusion injury can be attenuated by oxygen tension control. Jpn Circ J. 2001;65:213–8. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T, Ku K, Kaneda T, et al. Cardioprotective effects of lowering oxygen tension after aortic unclamping on cardiopulmonary bypass during coronary artery bypass grafting. Circ J. 2002;66:718–22. [DOI] [PubMed] [Google Scholar]
- 14.Lund N.. Skeletal muscle surface oxygen pressure fields in normal human volunteers and in critically ill patients. In: Ehrly AM, ed. Determination of Tissue Oxygen Pressure Fields in Patients. Oxford, New York: Pergamon Press;1983:53–9. [Google Scholar]
- 15.Kruezer F.. Oxygen supply to tissues: the Krogh model and its assumptions. Experientia. 1982;38:1415–26. [DOI] [PubMed] [Google Scholar]
- 16.Grist G.. Oxygen Pressure Field Theory: A Primer For Perfusionists. Part 2, Canadian Perfusion Canadienne. 1996;9:21–25. [Google Scholar]
- 17.Grist G.. Oxygen Pressure Field Theory: A Primer For Perfusionists. Part. 1. Canadian Perfusion Canadienne 1996;8:19–24. [Google Scholar]
- 18.Meier J, Kemming G, Meisner F, et al. Hyperoxic ventilation enables hemodilution beyond the critical myocardial hemoglobin concentration. Eur J Med Res. 2005;10:462–8. [PubMed] [Google Scholar]
- 19.Kemming GI, Meisner FG, Meier J, et al. Hyperoxic ventilation at the critical hematocrit: effects on myocardial perfusion and function. Acta Anaesthesiol Scand. 2004;48:951–9. [DOI] [PubMed] [Google Scholar]
- 20.Kemming GI, Meisner FG, Kleen M, et al. Hyperoxic ventilation at the critical haematocrit. Resuscitation 2003;56:289–97. [DOI] [PubMed] [Google Scholar]
- 21.Habler O, Kleen M, Kemming G, Zwissler B.. Hyperoxia in extreme hemodilution. Eur Surg Res. 2002;34:181–7. [DOI] [PubMed] [Google Scholar]
- 22.Nollert G, Nagashima M, Bucerius J, et al. Oxygenation strategy and neurologic damage after deep hypothermic circulatory arrest. II. hypoxic versus free radical injury. J Thorac Cardiovasc Surg. 1999;117:1172–9. [DOI] [PubMed] [Google Scholar]
- 23.Shnier CB, Cason BA, Horton AF, Hickey RF.. Hyperoxemic reperfusion does not increase myocardial infarct size. Am J Physiol. 1991;260:H1307–12. [DOI] [PubMed] [Google Scholar]
- 24.Zwemer CF, Whitesall SE, D’Alecy LG.. Hypoxic cardiopulmonary-cerebral resuscitation fails to improve neurological outcome following cardiac arrest in dogs. Resuscitation. 1995;29:225–36. [DOI] [PubMed] [Google Scholar]
- 25.Pearl JM, Thomas DW, Grist G, et al. Hyperoxia for management of acid-base status during deep hypothermia with circulatory arrest. Ann Thorac Surg. 2000;70:751–5. [DOI] [PubMed] [Google Scholar]