Abstract:
The devices and techniques used for pediatric cardiopulmonary bypass (CPB) undergo continuous change. New techniques and clinical comparisons of devices are frequently reported in the literature; however, information about the extent to which these techniques and devices are adopted into clinical practice at pediatric heart centers are not well described. We conducted a mail survey of North American pediatric cardiac surgery centers to gain perspective on the extent to which various devices and techniques were used for CPB along with program demographic data. In January 2005, surveys were mailed to 180 North American open heart centers. The survey was nearly identical in format and content to three earlier surveys completed in 1989, 1994, and 1999, with the exception that new questions were added to address new techniques and devices that have emerged over the years. Responses were received from 76 hospitals, for an overall response rate of 42%. Of the responding centers, 53 were performing pediatric open heart surgery and 23 were not. Twenty centers performed only pediatric open heart surgery, and 33 performed both pediatric and adult open heart surgery. The mean pediatric annual caseload of responding centers was 195 procedures/yr (range, 20–650 procedures/yr; median, 154 procedures/yr). A total of 9943 pediatric open heart procedures were performed at responding centers in 2004. Most of the centers surveyed reported use of an open venous reservoir system (88%), use of roller pumps (90%), and use of arterial line filtration (98%). Most centers used circuits that have surface treatments with heparin or some other surface-modifying agent (74%). There has been an increase in the use of all types of safety devices. Modified ultrafiltration is used at 75% of the centers surveyed. Centers reported an increase in the availability of all types of cardiac support devices including extracorporeal membrane oxygenation for postcardiotomy cardiac support (90%). This survey provides an overview of clinical practice in 2004. The series of surveys document the historical progression of clinical practice over the past 16 years. Practice surveys may also be useful for identifying gaps between evidence-based knowledge and clinical practice. These surveys document the diffusion of innovation related to CPB during the past 16 years and areas of variation in practice that need further study.
Keywords: perfusion, survey, pediatric, cardiopulmonary bypass, devices and techniques
Cardiopulmonary bypass (CPB) is used in more than 18,000 pediatric open heart surgical procedures each year in North America. New techniques and equipment are constantly emerging as a result of research efforts to improve patient care. These innovations are described in the medical literature; however, the extent to which they are actually adopted into clinical practice is unknown. We surveyed pediatric centers in 1989, 1994, and 1999 using a comprehensive survey documenting program demographics, equipment, and techniques (1–4). In January 2005, an updated survey was mailed to pediatric open heart surgery programs in the United States and Canada. The survey questions were identical to those posed in previous surveys with the exception of new questions that were added to address new devices and emerging techniques. The intent of this work was to gather information, provide an accurate description of clinical practice, and determine the extent to which surgical innovation and technological advancements have been incorporated into the clinical setting over the past 16 years. The following report describes current clinical practice along with results from previous surveys that document the trends in practice since 1989.
MATERIALS AND METHODS
An updated list of pediatric open heart programs was compiled from previous survey databases. A six-page questionnaire was mailed to each center. The questionnaire consisted of 77 questions pertaining to demographics, equipment, techniques, and patient management. Sixty-five questions were taken from the 1994 survey, and 12 new questions related to new devices and techniques were added. Sixty questions were multiple choice, and 17 required the respondent to fill in a value or series of values. In January 2005, 180 institutions were sent a survey, a cover letter explaining the purpose of the questionnaire, and a stamped return envelope. Respondents were promised a complete report of responses in return for their participation. Each survey form was individually coded to prevent duplication of responses and to identify nonrespondent centers to facilitate follow-up solicitation. If a particular institution was not an active pediatric open heart center, the respondent was instructed to write a zero next to the question regarding the number of pediatric procedures performed per year and return the survey. Two weeks after the first mailing, a follow-up phone call was placed to nonresponding centers. Subsequent attempts were made to these centers using both telephone and fax. The survey responses were compiled using Microsoft Excel (Microsoft Corporation, Redmond, WA). Survey questions where the respondents indicated a range of values, were reported as mean, SD, and median values. Questions related to the use of devices or techniques were expressed as a frequency relative to the number of active centers that responded.
RESULTS
Program Demographics
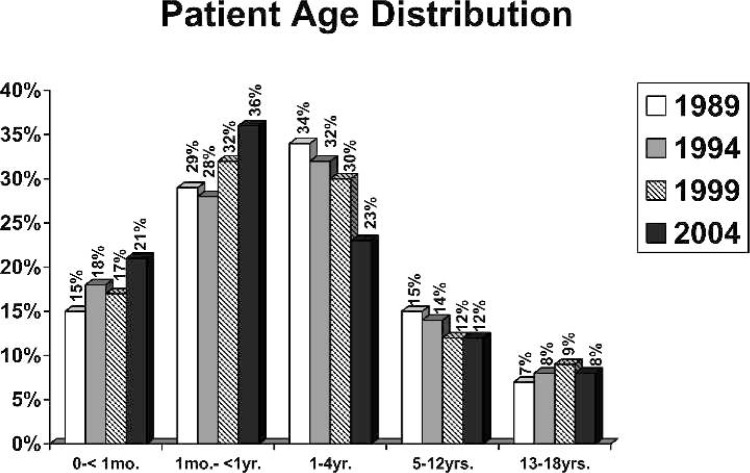
Responses were received from 76 hospitals, for an overall response rate of 42%. Respondents categorized themselves as community, university, or university-affiliated hospitals as displayed in Figure 1. Of the responding centers, 53 were performing pediatric open heart surgery, and 23 were not. Of the 53 active centers, 32 reported that they operate on both adults and children, and 21 centers reported they operate only on pediatric patients. The age distribution of patients operated on in 1989, 1993, 1999, and 2004 is shown in Figure 2. Fifty-seven percent of the patients undergoing open heart surgery in the surveyed centers were less than 1 year of age. The mean annual caseload of responding centers was 195 cases/yr/center (median, 154 cases/yr/center; range, 20–560 cases/yr/center). The average case load for each survey year is shown in Figure 3. A total of 9943 pediatric open heart procedures were performed at responding centers in 2004.
Figure 1.
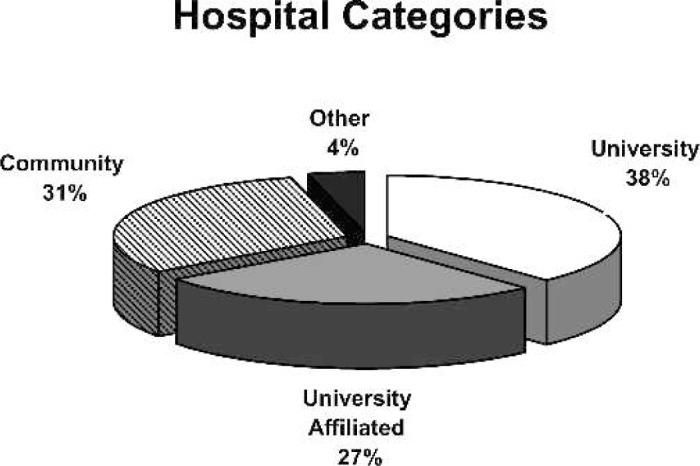
Percentage of responding centers by hospital category.
Figure 2.
Percentage of pediatric cases performed by age group.
Figure 3.
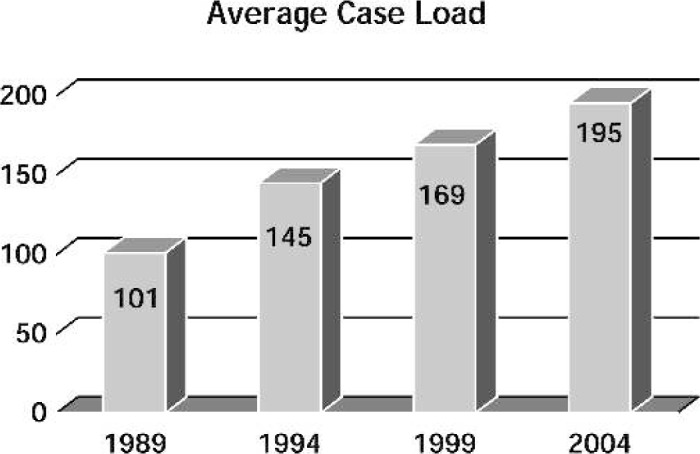
Average annual caseload per center per year.
Equipment
Table 1 shows the percent of centers that used various circuit components including safety devices by survey year. Ninety percent of the responding centers reported use of roller pumps for arterial return, whereas 2% used the centrifugal pump and 8% used some of each type. Membrane oxygenators were used exclusively. The use of open systems (hard shell) has increased to 88% of all centers, whereas closed systems (collapsible reservoirs) were used by 10% of centers, and 2% used some of each type. Surface modifications, or coatings of the CPB circuits, were reported to be used in 74% of the responding centers, an increase from 18% in 1999.
Table 1. Safety devices and circuit components.
| Safety Devices and Circuit Components | 1989 (%) | 1994 (%) | 1999 (%) | 2004 (%) |
|---|---|---|---|---|
| Safety devices | ||||
| Level detector | 64.6 | 66.7 | 79.2 | 90.0 |
| Bubble detector | 72.4 | 84.8 | 87.5 | 83.0 |
| One-way valve (vent/sucker lines) | 52 | 65.7 | 69.4 | 75.0 |
| One-way purge line | 72.4 | 89.5 | 91.7 | 92.0 |
| Gas supply oxygen analyzer | 27.6 | 48.5 | 47.2 | 48.0 |
| Arterial pump | ||||
| Roller | 81.3 | 72.4 | 81.9 | 90.0 |
| Centrifugal | 10.1 | 10.5 | 13.9 | 2.0 |
| Roller and centrifugal | 8.6 | 17.1 | 4.2 | 8.0 |
| Reservoir | ||||
| Open system (hard shell) | 52.0 | 56.0 | 77.0 | 88.0 |
| Closed system (collapsible) | 41.0 | 35.0 | 23.0 | 10.0 |
| Open and closed system | 7.0 | 9.0 | 0 | 2.0 |
| Filtration | ||||
| Prebypass | 51.2 | 68.5 | 68.1 | 83.0 |
| Arterial line filter | 80.6 | 96.1 | 95.8 | 98.0 |
| Cardioplegia filter | N/A | N/A | 33.0 | 29.0 |
| Ultrafiltration device | 62.7 | 92.3 | 94.3 | 98.0 |
| Leukodepletion | N/A | N/A | N/A | 17.0 |
N/A, not applicable.
Monitoring Devices
Table 2 shows the reported use of continuous in-line monitoring devices during CPB. Continuous in-line arterial blood gas monitoring was used at 78% of centers. Continuous in-line venous blood gas monitoring was used by 22% and in-line venous saturation sensors by 94% of responding centers. Use of near infrared spectrometry cerebral monitoring was reported by 48% of responding centers. A new question was added in 2005 about the use of electronic perfusion recording systems, and 16% of the centers reported use of such systems.
Table 2. Online monitoring in the CPB circuit.
| 1989 (%) | 1994 (%) | 1999 (%) | 2004 (%) | |
|---|---|---|---|---|
| Arterial blood gases | 66.9 | 85.7 | 70.8 | 76.9 |
| Arterial saturation | 8.9 | 25.7 | 26.4 | 19.2 |
| Venous blood gases | 48.4 | 52.3 | 30.6 | 21.1 |
| Venous saturation | 48.4 | 78.1 | 87.5 | 94.2 |
Ventricular Assist Devices
The availability of ventricular assist devices (VADs) has increased since 1989, when 67% of centers reported the use of some type of VAD (Figure 4). In the recent survey, 96% of the responding centers reported the availability of assist devices at their center. Of the pediatric VADs currently available, extracorporeal membrane oxygenation (ECMO) was the most common, available at 90% of responding centers. ECMO systems components were described as follows: roller pump systems, 65%; centrifugal pump systems, 23%; both types, 12%. Oxygenators used in the ECMO systems were as follows: 55% used silicone rubber membranes, 16% used only hollow fiber membranes, and 29% used both silicone sheet and hollow fiber oxygenators. The use of a diffusion membrane oxygenator was reported by two Canadian centers for use in their ECMO system circuits. The availability of pneumatic VADs was reported by 30% of responding centers. Respondents reported that mechanical support was used on 2.7% of their cases in 2004 (∼268 support procedures in 2004).
Figure 4.

Percentage of centers with VADs.
Techniques
Ultrafiltration: Ninety-eight percent of the centers reported routine use of ultrafiltration. There were several methods of ultrafiltration reported, as shown in Figure 5. The total number of centers using modified ultrafiltration in 1994 was 43%, in 1999, it was 64%, and the number increased to 75% in 2004. Arterial-venous-modified ultra-filtration was used by 64% of the centers and venovenous-modified ultrafiltration was used by 11% of the centers in 2004.
Figure 5.
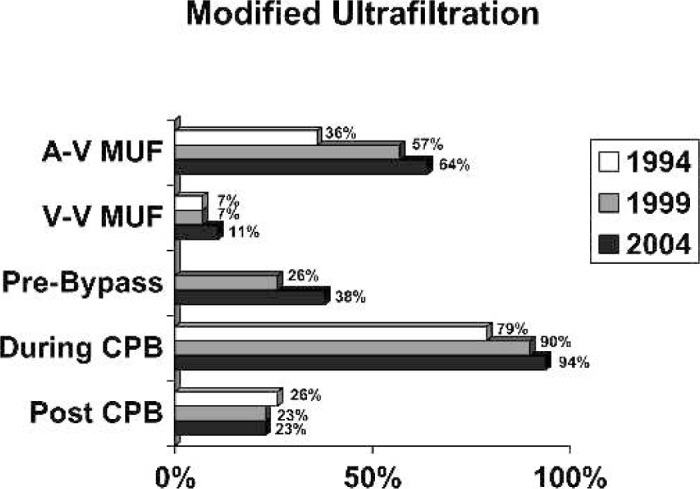
Uses of ultrafiltration.
Circuit Prime and Blood Products: Typical CPB prime solutions and drugs added to the perfusate are shown in Table 3. The majority of centers reported use of albumin in the prime (86%). Twenty-four percent of the centers reported use of whole blood for priming the neonatal CPB circuit. The number of centers that wash packed red blood cells just before adding them to the prime with a cell saver has increased (30% in 1994; 35% in 1999; and 60% in 2004).
Table 3. Prime and drug additives.
| Prime/Additives | 1989 (%) | 1994 (%) | 1999 (%) | 2004 (%) |
|---|---|---|---|---|
| Crystalloid | ||||
| Plasma-Lyte A | 40.9 | 44.8 | 58.3 | 59.6 |
| Normosol-R | 22.8 | 24.5 | 27.8 | 28.8 |
| Lactated Ringer’s | 21.3 | 14.2 | 11.1 | 5.8 |
| 5% Dextrose/lactated Ringer’s | 1.6 | 0 | 1.4 | 0 |
| Other | 13.4 | 16.9 | 1.4 | 6.0 |
| Colloid | ||||
| 5% albumin | 35.7 | 17.7 | 9.7 | 7.7 |
| 25% albumin | 34.1 | 65.4 | 84.7 | 80.8 |
| Hetastarch | 7.8 | 5.4 | 2.8 | 0 |
| 5% plasma protein | 1.5 | 0.9 | 0 | 1.9 |
| None | 32.6 | 10.2 | 2.7 | 11.5 |
| Drug | ||||
| Heparin | 92.1 | 89.7 | 95.8 | 98.0 |
| Sodium bicarbonate | 88.2 | 96.3 | 83.3 | 92.3 |
| Mannitol | 57.4 | 65.4 | 72.2 | 69.2 |
| Calcium chloride | 40.1 | 40.2 | 51.4 | 55.8 |
| Methylprednisone | 22.8 | 30.8 | 34.7 | 51.9 |
| Antibiotics | 19.8 | 20.6 | 25 | 38.5 |
| Furosemide | 10.2 | 14.1 | 11.1 | 15.4 |
| Dexamethasone | 3.1 | 2.8 | 1.4 | 8.0 |
| THAM | 1.6 | 2.8 | 2.8 | 3.8 |
| Lidocaine | 0.8 | 3.7 | 1.4 | 0 |
Hypothermia Technique: In the previous surveys, deep hypothermia with circulatory arrest (DHCA) was the preferred technique used by most centers for complex procedures for neonate patients. In 1989, DHCA was the preferred technique used at 72% of centers, and in 1999, it was the preferred technique at 63% of surveyed centers. Currently, 44% of responding centers reported use of DHCA as the preferred technique for complex procedures for neonates. The target core temperature for DHCA was reported as 15.7 ± 3.19°C (range, 13°C–20°C; median, 16°C). The use of hypothermia and low flow has become the predominant technique for neonates requiring repair of complex procedures. In pediatric patients older than 1 month, hypothermia with low flow was the predominant technique used at 56% of the centers. The target core temperature for hypothermia and low flow was reported as 18.6 ± 3.19°C (range, 16.5°C–30°C; median, 18°C). The target flow rate for hypothermia and low flow CPB was reported as 40.7 ± 19.1 mL/kg (range, 10–100 mL/kg; median, 50 mL/kg). The use of retrograde cerebral perfusion during DHCA was reported by 3.9% of centers as “routine” and 21.5% as “sometimes,” and 72.5% of surveyed centers reported they “never” use retrograde cerebral perfusion. Selective cerebral perfusion for continuous perfusion of the cerebral circulation during arch reconstruction was reported to be used “always” by 30%, “sometimes” by 48%, and “never” by 20% of the reporting centers for all such cases. During deep hypothermia, the average minimal acceptable hematocrit reported was 23.6 ± 0.04% (median, 24%; range, 15%–30%). The placement of ice packs around the head to assist with cerebral protection when cooling was reported by 91% of respondents, essentially unchanged from previous years. Surface cooling used to facilitate patient cooling before and during CPB was reported to be used at 52% of the surveyed centers. Eighty percent of the responding centers reported that they maintain a temperature gradient between the heat exchanger water bath and the blood inlet temperature while cooling. The reported mean temperature gradients while cooling was 9.1 ± 2.5°C (range, 5°C–12.5°C; median, 10°C). The reported mean temperature gradient during rewarming the patient was 8.5 ± 1.4°C (range, 6°C–12.5°C; median, 10°C). All centers reported following a maximum blood temperature protocol during rewarming (maximum blood temperature, 37.7 ± 0.29°C; range, 37°C–38.5°C; median, 38°C).
Myocardial Protection: Single-pass systems were the predominately used delivery system for cardioplegia in 2004 (62%). Recirculating systems for cardioplegia delivery are used in 32% of reporting centers in 2004. A syringe method was used in 6% of centers. The device used most to assist in delivery of cardioplegia was a roller pump, which was used at 92% of centers. Blood cardioplegia was used by 72.5% of surveyed centers in 2004, 67% in 1999, and 49% in 1989. The use of sanguineous cardioplegia has been steadily decreasing since 1989 from 48% to 27.4% in 2004. Two centers reported use of warm continuous blood cardioplegia in 1999. None of the responding centers reported the use of this method in the current survey. In 1999, 15% of surveyed centers reported routine use of a terminal dose of warm substrate enhanced blood cardioplegia, which was unchanged from 1994. Currently, 20% of centers use this technique. Cardioplegia filters were used by 30% of the centers.
Vacuum-Assisted Venous Drainage: The use of vacuum-assisted venous drainage is a technique that was first captured in the 1999 survey. Its use has increased from 24% in 1999% to 46% in 2004. The maximum acceptable negative pressure was 43.75 ± 20.2 mmHg (range, −20 to −100 mmHg; median negative, 40 mmHg).
Patient Management
Blood Gas Management: Table 4 summaries the oxygenation strategy stratified by hypothermia technique. Blood gas strategies for deep hypothermia were defined as α-stat, pH-stat, or mixed management on the same patient. During deep hypothermia, 16% of centers reported the use of α-stat, 34% used pH-stat, and 50% used a mixed strategy on the same patient.
Table 4. PaO2 management.
| PaO2 | Normothermia (%) | Hypothermia Before DHCA (%) | Hypothermia With Low Flow CPB (%) |
|---|---|---|---|
| 100 to 200 mmHg | 37.3 | 23.5 | 33.3 |
| 200 to 300 mmHg | 56.9 | 39.2 | 49.1 |
| 300 to 400 mmHg | 0 | 15.6 | 3.9 |
| >400 mmHg | 5.9 | 21.6 | 13.7 |
Blood Pressure Management: Hypotension was pharmacologically treated in 82% of programs that responded to the survey in 2004 compared with 74% in 1989, 76% in 1994, and 92% in 1999. The threshold for administering a vasoconstrictor is summarized in Table 5.
Table 5. Threshold for administering a vasoconstrictor.
| Threshold for Administering a Vasoconstrictor (mmHg) | 1989 (%) | 1994 (%) | 1999 (%) | 2004 (%) |
|---|---|---|---|---|
| >50 | 6.2 | 6.7 | 5.8 | 8.9 |
| 40 to 49 | 9.3 | 7.6 | 4.3 | 8.9 |
| 30 to 39 | 24.8 | 20.1 | 34.8 | 24.4 |
| 20 to 29 | 34.1 | 41.9 | 43.5 | 40.0 |
| Do not treat | 25.6 | 23.8 | 8.7 | 17.8 |
Inhalational Anesthetic: The use of inhalation anesthetics during CPB among centers is nearly universal (98%). The inhalation of choice was isoflurane, used by 88% of the responding centers.
Hemodiltution: The average minimal acceptable hematocrit during mild hypothermia was 27.3 ± 0.03% (range, 24%–30%; median, 28%). The average acceptable hematocrit for patients with cyanotic defects just before termination of bypass was reported as 34 ± 0.06% (range, 28%–45%; median, 35%) for cyanotic patients and 28.8 ± 0.05% (range, 21%–35%; median, 30%) for patients with noncyanotic defect.
Use of Aprotinin: Aprotinin was used by 96% of centers in 2004, which is a 33% increase from 1999. The two predominant indications for use of aprotinin therapy in 1999 were reoperations (57%) and complex procedures (34%). In 2004, the indications for the use of aprotinin were as follows: reoperations (60%), complex procedures (54%), and indicated for all procedures (40%).
DISCUSSION
Survey methodology is a useful way to obtain a description of opinions and practice patterns of a population and are useful for determining trends and to make comparisons between regional and local practices. Surveys are subjected to two major sources of error: incomplete sampling and misinterpretation of the survey questions by respondents. The former results in sampling bias error and the latter produces responses that do not accurately reflect clinical practice or respondent opinions. Both types of error could result in ascribing inappropriate inferences from the responses. The response rate of the recent survey was 42%, representing 9943 cases, approximately one half of the annual caseload for North America. The group of respondents represented current practice, primarily in large and experienced centers. Our findings related to the use of ECMO and VAD support were identical to a telephone survey of pediatric centers conducted by Gunst et al. (5) earlier this year. Their survey showed use of ECMO support at 94% of centers compared with 90% reported by our respondents. Their survey showed that silcone membranes are used at 60% of centers, hollow fiber membrane oxygenators exclusively at 19%, and sometimes used at 40% of the centers, compared with 55%, 16%, and 35%, respectively, in our survey. They reported roller pump use at 65%, centrifugal pump use at 12%, and use of both at 35%. This compares to 65%, 22%, and 12%, respectively, reported in our recent survey.
To test the clarity of the survey questions, we piloted the questionnaire on five perfusionists from our center, and questions that lacked clarity were reconstructed or eliminated before mailing the survey. Furthermore, the majority of questions remained consistent since the first survey in 1989, and responses to the questions seem reasonably consistent. While we chose heart centers as the unit of analysis for the survey, we realize that sometimes there may be practice variation within centers, and this could represent another source of error. To prevent this type of error, most questions were constructed in a way that multiple responses could be made to a question by one responding center to capture intracenter variation.
Our series of surveys provides a periodic description of clinical practice in North America and should not be the criteria for selecting a new technique, although it does provide a comparison of individual practice to regional practice. Over the past 15 years, these surveys have documented how new devices and technical innovations have been applied to clinical practice. The surveys may also be useful for identifying areas where there are gaps between evidence-based knowledge and clinical practice. In their recent intensive review of the published literature related to CPB, Bartels et al. (6) suggested that lack of evidence is a barrier preventing consensus and the development of conclusive practice guidelines for CPB. Variation also occurs when technical innovations emerge. Rodgers (7) developed a theoretical framework that describes the diffusion of innovations. In his model, he described five characteristics that effect the rate at which innovations are adopted; relative advantage, compatibility, complexity, trial ability, and observe ability. Clearly all of these factors are at play in the diffusion of innovation in CPB. Rodgers further went on to describe five categories of adopters of new ideas: innovators, early adopters, the early majority, the late majority, and laggards. Each of these categories of practitioner provide value to the change. The latter add historical context, and although the rate of change is diminished by this group, they add value in that they require more complete evidence before making a change. They are less deliberate but more cautious. The rate of diffusion may be related to a learning curve, to the lack of sufficient evidence that shows the benefit of the proposed change, or to social factors related to those proposing the change. Some new techniques and devices were adopted early. For example, modified ultrafiltration was first described in 1990 and subsequently examined with a randomized prospective study (8,9). In 1994, 43% of centers performed either veno-venous— or arterial-venous—modified ultrafiltration (MUF). In 2004, 75% of centers performed either veno-venous— or arterial-venous—modified ultrafiltration, which is a 32% increase in 10 years.
Management of pH and PCO2 represents a technique that has undergone drastic change over the past two decades. Use of α-stat pH management was popularized in adult cardiac surgery in 1984 (10). The use of this α-stat management was based mainly on two theoretic hypotheses: (a) tissue metabolism is more optimal in an alkaline milieu and reduced cerebral blood flow produced by low reduced PCO2 and (b) cerebral blood flow autoregulation is preserved preventing excess or luxury perfusion to the brain and thereby decreased the embolic burden to the brain. Many centers applied the α-stat strategy to blood gas management for pediatric patients. In 1989, 80% of the centers used α-stat, and 17.6% used pH-stat. Subsequently, it became apparent that α-stat may not be advantageous in pediatric patients with aorta to pulmonary shunts, because low arterial blood CO2 tension would result in cerebral vasoconstriction, pulmonary vasodilation, and a concomitant reduction in cerebral blood flow and insufficient cooling of the brain before DHCA, particularly in cyanotic infants. Areas that were inadequately cooled would be rendered susceptible to neurological injury during DHCA. In the late 1980s, it was noted that children subjected to low CO2 tensions and a shorter period of cooling before DHCA had a higher incidence of brain injury (11). Subsequently, several studies have confirmed pH-stat as a superior blood gas management strategy before DHCA because it provides more sufficient cooling and cerebral metabolic suppression (12–14). In the 2004 survey, only 17% of respondents reported use of α-stat for hypothermic circulatory arrest procedures. Thirty-three percent use pH-stat, and 50% use a mixed strategy where pH-stat is used during cooling and then switched to α-stat management just before DHCA or low-flow CPB.
Another example was the change in the management of PaO2 during CPB during cooling before DHCA. In 1999, Nollert et al. (15,16) conducted a series of studies on oxygenation strategies in piglets before DHCA. These studies showed a benefit to hyperoxygenation (PaO2 of > 400 mmHg) before deep hypothermic circulatory arrest. In 2000, Pearl et al. (17) showed improved acid-base balance in children when hyperoxygenation was used before DHCA. Twenty-two percent of the centers that responded to the 2004 survey practiced hyperoxygenation before DHCA.
An example of slower diffusion of innovation in practice was the use of surface-treated CPB circuits. Heparin-bonded CPB circuits have been available since the late 1980s. It has taken 20 years for the use of modified circuits to become used routinely by a majority of centers (18% used them in 1999 and 74% used them in 2004). The clinical advantages of coated circuits have been well documented in the literature for adults and pediatric patients (18–21). Stammers et al. (22) documented that cost pressures may have great influence over decisions to use various devices even when they are know to have superior safety and effectiveness. It is important to recognize that a survey should not be used as a practice guideline. Techniques used by the majority of centers may prove in time to be the wrong choice. Currently, there seems to be less diversity in CPB system components. Most centers reported use a roller pump, “hardshell” open venous reservoir system, an arterial line filter, and a system that is surface coated. However, recently, several randomized clinical trials have found superior clinical outcomes with systems that have a closed reservoir and use a centrifugal arterial pump (23,24).
Understanding the patterns of past decision-making provides instructive insight into the diffusion of change in clinical practice. Uncertainty and diversity will continue to emerge along with new techniques and devices. Further randomized trials and observational research efforts will fill some of the gaps in knowledge and lead to future certainty and consensus. This will point the way to improved care for neonate, infant, and pediatric patients that undergo cardiac surgery with CPB.
ACKNOWLEDGMENT
The authors thank Brian Mejak, MS, CCP, for assistance with the validation of the program database.
REFERENCES
- 1.Hill AG, Groom RC, Akl BF, Lefrak EA, Kurusz M.. Current paediatric perfusion practice in North America. Perfusion. 1993;8:27–38. [DOI] [PubMed] [Google Scholar]
- 2.Groom RC, Hill AG, Akl BF, Albus RA, Kurusz M, Lefrak EA.. Neonatal cardiopulmonary bypass—a review of current practice in North America. Cardiol Young. 1993;3:353–69. [Google Scholar]
- 3.Groom RC, Hill AG, Kurusz M, et al. Paediatric perfusion practice in North America: an update. Perfusion. 1995;10:393–401. [DOI] [PubMed] [Google Scholar]
- 4.Cecere G, Groom RC, Forest R, Quinn R, Morton J.. A 10-year review of pediatric perfusion practice in North America. Perfusion. 2002;17:83–9. [DOI] [PubMed] [Google Scholar]
- 5.Gunst G, Terry B, Melchoir R, Searles B, Darling E.. Survey of ECMO in the neonate following open-heart surgery. J Extra Corpor Technol. 2005;4:351–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels C, Gerdes A, Babin-Ebell J, et al. Working group on extra-corporeal circulation and mechanical ventricular assist devices of the German Society for Thoracic and Cardiovascular Surgery. Cardiopulmonary bypass: evidence or experience based? J Thorac Cardiovasc Surg. 2002;124:20–7. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers EM.. Diffusion of Innovations. 4th ed. New York: The Free Press; 1995:7–31. [Google Scholar]
- 8.Naik SK, Knight A, Elliott M.. A prospective randomized study of a modified technique of ultrafiltration during pediatric open-heart surgery. Circulation. 1991;84(5 Suppl):III422–31. [PubMed] [Google Scholar]
- 9.Swan H.. The importance of acid-base management for cardiac and cerebral preservation during open heart operations. Surg Gynecol Obstet. 1984;158:391–414. [PubMed] [Google Scholar]
- 10.Lawson DS, Walczak R, Lawson AF, et al. North American neonatal extracorporeal membrane oxygenation (ECMO) devices: 2002 survey results. J Extra Corpor Technol. 2004;36:16–21. [PubMed] [Google Scholar]
- 11.Jonas RA.. Hypothermia, circulatory arrest, and the pediatric brain. J Cardiothorac Vasc Anesth. 1996;10:66–74. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto T, Kurosawa H, Shin’oka T, Aoki M, Isomatsu Y.. The influence of pH strategy on cerebral and collateral circulation during hypothermic cardiopulmonary bypass in cyanotic patients with heart disease: results of a randomized trial and real-time monitoring. J Thorac Cardiovasc Surg. 2004;127:12–9. [DOI] [PubMed] [Google Scholar]
- 13.du Plessis AJ, Jonas RA, Wypij D, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1001. [DOI] [PubMed] [Google Scholar]
- 14.Skaryak LA, Chai PJ, Kern FH, Greeley WJ, Ungerleider RM.. Blood gas management and degree of cooling: effects on cerebral metabolism before and after circulatory arrest. J Thorac Cardiovasc Surg. 1995;110:1649–57. [DOI] [PubMed] [Google Scholar]
- 15.Nollert G, Nagashima M, Bucerius J, et al. Oxygenation strategy and neurologic damage after deep hypothermic circulatory arrest. II. hypoxic versus free radical injury. J Thorac Cardiovasc Surg. 1999; 117:1172–9. [DOI] [PubMed] [Google Scholar]
- 16.Nollert G, Nagashima M, Bucerius J, Shin’oka T, Jonas RA.. Oxygenation strategy and neurologic damage after deep hypothermic circulatory arrest. I. Gaseous microemboli. J Thorac Cardiovasc Surg. 1999;117:1166–71. [DOI] [PubMed] [Google Scholar]
- 17.Pearl JM, Thomas DW, Grist G, Duffy JY, Manning PB.. Hyperoxia for management of acid-base status during deep hypothermia with circulatory arrest. Ann Thorac Surg. 2000;70:751–5. [DOI] [PubMed] [Google Scholar]
- 18.Stammers AH, Christensen KA, Lynch J, Zavadil DP, Deptula JJ, Sydzyik RT.. Quantitative evaluation of heparin-coated versus nonheparin-coated bypass circuits during cardiopulmonary bypass. J Extra Corpor Technol. 1999;31:135–41. [PubMed] [Google Scholar]
- 19.Grossi EA, Kallenbach K, Chau S, et al. Impact of heparin bonding on pediatric cardiopulmonary bypass: a prospective randomized study. Ann Thorac Surg. 2000;70:191–6. [DOI] [PubMed] [Google Scholar]
- 20.Ozawa T, Yoshihara K, Koyama N, Yamazaki S, Takanashi Y.. Superior biocompatibility of heparin-bonded circuits in pediatric cardiopulmonary bypass. Jpn J Thorac Cardiovasc Surg. 1999;47:592–9. [DOI] [PubMed] [Google Scholar]
- 21.Jensen E, Andreasson S, Bengtsson A, et al. Changes in hemostasis during pediatric heart surgery: impact of a biocompatible heparin-coated perfusion system. Ann Thorac Surg. 2004;77:962–7. [DOI] [PubMed] [Google Scholar]
- 22.Stammers AH, Mejak BL, Rauch ED, Vang SN, Viessman TW.. Factors affecting perfusionists’ decisions on equipment utilization: results of a United States survey. J Extra Corpor Technol. 2000;32:4–10. [PubMed] [Google Scholar]
- 23.Jensen E, Andreasson S, Bengtsson A, et al. Influence of two different perfusion systems on inflammatory response in pediatric heart surgery. Ann Thorac Surg. 2003;75:919–25. [DOI] [PubMed] [Google Scholar]
- 24.Morgan IS, Codispoti M, Sanger K, Mankad PS.. Superiority of centrifugal pump over roller pump in paediatric cardiac surgery: prospective randomized trial. Eur J Cardiothorac Surg. 1998;13:526–32. [DOI] [PubMed] [Google Scholar]