Abstract:
Over the past 20 years, the bulk of the literature and texts published about extracorporeal membrane oxygenation (ECMO) has been written by physicians and nurses. The consensus of this body of printed information would suggest, among other things, that (1) despite significant advancements in extra-corporeal technology, the standard ECMO circuit has remained fundamentally unchanged since originally described in 1982, and (2) perfusionists are nearly absent from the staffing algorithm at most centers. While these conclusions may be representative of the extracorporeal life support (ELSO) reporting centers, they may not be representative of the field as a whole. We hypothesized that the use of modern extracorporeal equipment and the involvement of perfusionists in ECMO patient care is largely underreported in previous studies. To study this hypothesis, we developed a standard survey instrument and queried perfusion teams from the hospitals listed on the American Society of Extra-Corporeal Technology Pediatric Registry. All centers were contacted by phone and were asked questions regarding their caseload, circuitry, and staffing algorithms. Data are reported as a percentage of respondents. ECMO is used as a method of mechanical support after neonatal open heart surgery in 94% of centers surveyed. For 60% of the centers, a silicone membrane oxygenator is used exclusively, whereas 40% of the centers have used a hollow fiber oxygenator (HFO), and of that group, 19% use a HFO routinely for neonatal post-cardiopulmonary bypass ECMO. Roller pumps are used exclusively at 65% of the centers, whereas centrifugal pumps are used routinely in 12%, and 23% have used both. Perfusionists are responsible for set-up/initiation (79%) and daily rounding/troubleshooting (71%), and provide around-the-clock bedside care (46%) at the surveyed centers. These data suggest that previously published ELSO-centric ECMO studies may significantly underestimate the contemporary application of modern technologies and the involvement of perfusionists.
Keywords: extracorporeal membrane oxygenation, membrane oxygenator, hollow-fiber oxygenator, pumps, staffing
Since the first successful use of extracorporeal membrane oxygenation (ECMO) in 1972, its application in neonates has been categorized into two primary patient populations: those with respiratory dysfunction and those with cardiac dysfunction (1). The cardiac application of ECMO contains the subset population of neonates who are unable to be weaned from cardiopulmonary bypass (CPB). These patients are at great risk of bleeding complications during ECMO support. While recent surveys suggest that ECMO circuitry has remained largely unchanged in recent decades (Figure 1) (2–4), there have been numerous antidotal reports from pediatric heart centers suggesting a shift from the “standard” circuit toward the use of heparin-treated tubing, hollow fiber oxygenators, and centrifugal arterial pumps (5–8). The purpose of this study was to identify the current state-of-the-art for cardiac ECMO technology in the United States.
Figure 1.
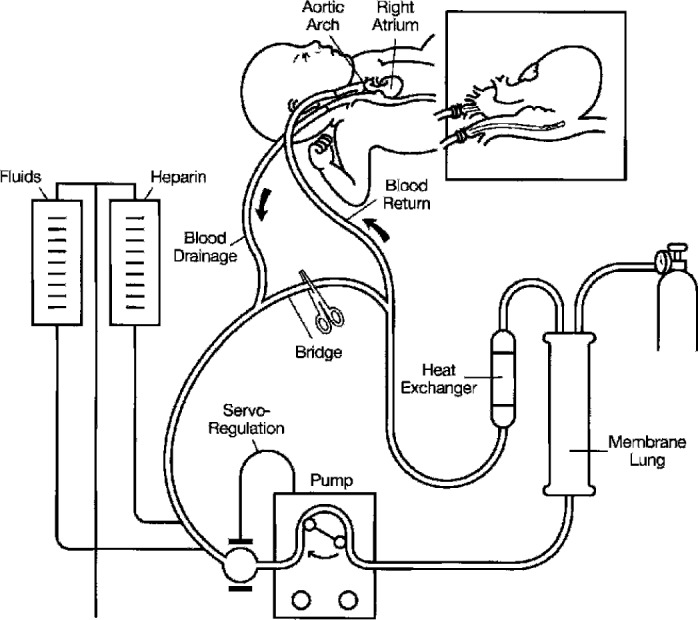
The standard ECMO circuit (reprinted by permission from the Extracorporeal Life Support Organization).
MATERIALS AND METHODS
Survey Instrument
In the fall of 2004, a standard survey instrument was developed that included questions in three categories: (1) clinical demographics of the responding institution, (2) circuitry questions related to ECMO components used for postcardiotomy patients, and (3) staffing questions related to the involvement of perfusionists in various stages of ECMO patient care. A contact list was generated from the nationwide databases of the American Society of Extra-Corporeal Technology Pediatric Registry and the Society of Congenital Heart Surgeons. The contact list consisted of 140 centers in the United States that performed pediatric and/or neonatal CPB.
Survey Population
Every pediatric open heart surgery team listed in the American Society of Extra-Corporeal Technology Pediatric Registry was included in the study. Perfusionists from each center were contacted by telephone. The survey questions were read to the participant, and their answers were transcribed for analysis.
Data from all participating centers were pooled and are presented as a percentage of total respondents.
RESULTS
Demographic Information
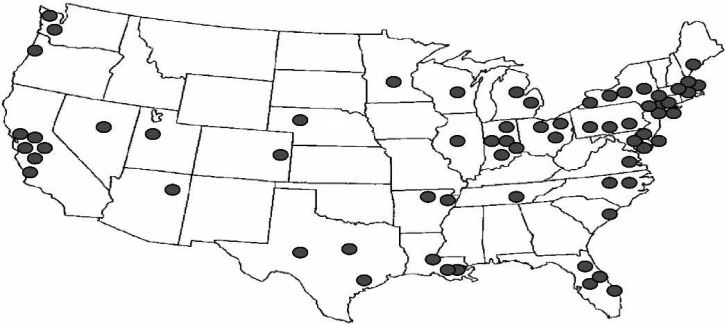
Of the 140 centers identified by the national registries, 70 centers (50%) were successfully contacted and participated in the survey. Geographically, the centers are representative of the entire continental United States (Figure 2). Only 41 (60%) of the participating institutions are members of the ELSO Registry. The clinical CPB caseload for the participating centers was either entirely pediatric (44%) or a combination of adult and pediatric cases (56%; Figure 3). The average CPB caseload and the number of perfusionists on staff for participating institutions are reported in Table 1. In the case of failure to wean from CPB for neonatal cases, 94% of the responding centers offer ECMO as a means of a ventricular assist device (Figure 4). Of these centers, 73% use the standard ECMO circuit previously described, whereas 22% routinely use a modified circuit, and 5% base their circuit configuration on patient-derived variables (Figure 5).
Figure 2.
Geographic profile of the survey.
Figure 3.
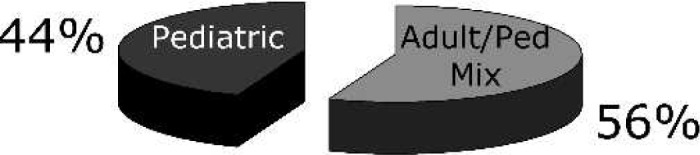
Program type.
Table 1.
Caseload and staff demographics for participating institutions.
| All Centers (n = 70) | Adult/Peds Mixed (n = 39) | Pediatric Only (n = 31) | |
|---|---|---|---|
| Annual no. pediatric | 193 ± 137 | 154 ± 112 | 240 ± 151 |
| CPB cases | (15–750) | (20–500) | (15–750) |
| No. of staff | 4 ± 2 | 5 ± 3 | 3 ± 1 |
| perfusionists | (1–11) | (1–11) | (1–6) |
Figure 4.

In the case of failure to wean from neonatal CPB, what mode of mechanical assistance is considered at your institution?
Figure 5.
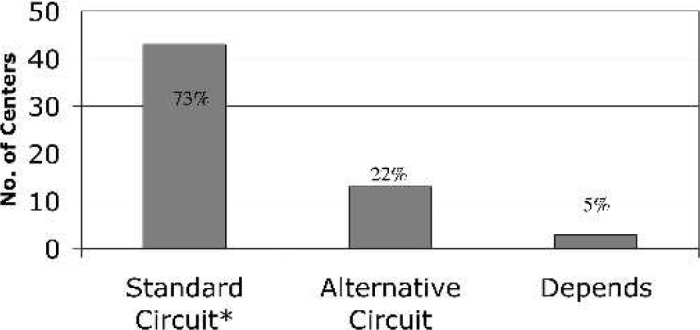
If ECMO is used for post-CPB support of the neonate, do you use a “standard” ECMO circuit?
ECMO Circuitry
For 60% of the centers, the Kolobow silicone membrane oxygenator (Medtronics Cardiopulmonary, Brooklyn Park, MN) is used exclusively, whereas 40% of the centers have used a hollow fiber oxygenator (HFO), and of that group, 19% use HFOs routinely for neonatal post-CPB ECMO (Figure 6). For the 26 centers reporting HFO use, the most common brand of HFO used for ECMO was the Medtronic Minimax (81%) (Medtronics Cardiopulmonary), although the Terumo SX05 (Terumo, Ann Arbor, MI) and Sorin Liliput (COBE Laboratories, Arvada, CO) were also used (Figure 7). Roller pumps are used exclusively at 65% of the centers, whereas centrifugal pumps are used routinely in 12%, and 23% have used both (Figure 8).
Figure 6.
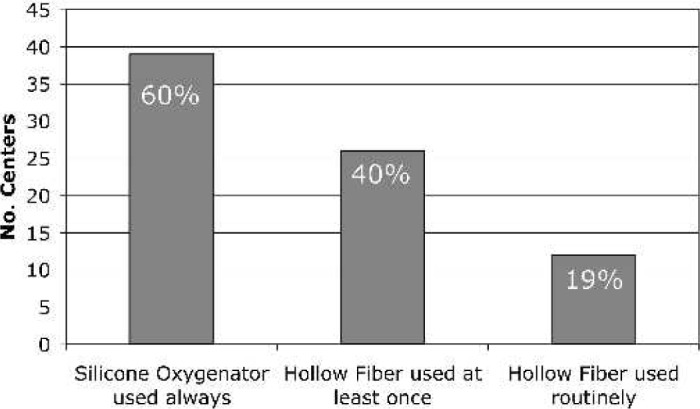
Oxygenator type used in neonates for post-CPB ECMO.
Figure 7.
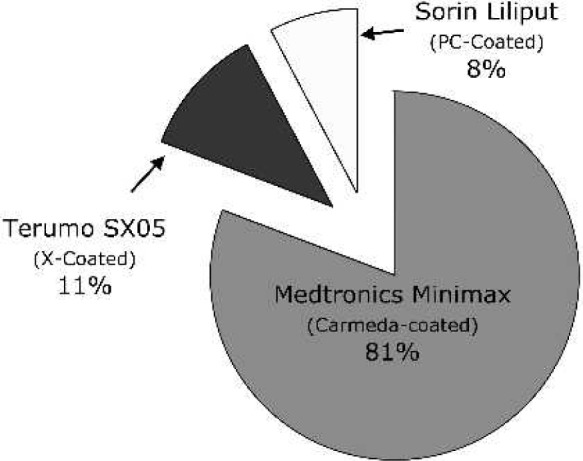
HFO brand used (n = 26 centers).
Figure 8.
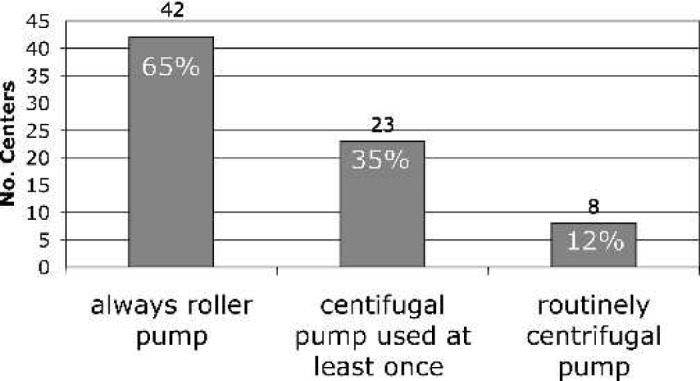
Pump type used in neonatal post-CPB ECMO.
ECMO Staffing
Perfusionists are responsible for set-up/initiation (79%), daily rounding/troubleshooting (71%), and around-the-clock bedside patient care (46%) at the centers surveyed (Table 2).
Table 2.
Staffing roles in post-CPB ECMO.
| Set-up/priming and initiation of ECMO | 79% Perfusion |
| 21% RN/RT ECMO team | |
| Sit ECMO shifts 24/7 | 46% Perfusion |
| 54% RN/RT ECMO team | |
| Daily rounding and trouble shooting | 71% Perfusion |
| 29% RN/RT ECMO team |
DISCUSSION
The ECMO circuitry and perfusion staffing results reported in this survey are in stark contrast to previously published ECMO surveys (2–4). Lawson et al. (4) surveyed ELSO centers in 2002 and reported that 97% of centers used the silicone membrane oxygenator. As many as 40% of the respondents in the current survey have used an HFO, and 19% use HFOs routinely. Lawson et al. (4) also reported that centrifugal pumps were used by only 5% of ELSO centers, whereas this survey reports that 35% have used them, and 12% use them routinely. Furthermore, the current survey reports a very high involvement of perfusionists for neonatal post-CBP ECMO (79%) compared with only a 22% involvement (set-up, priming, and initiation) reported in ELSO Centers (4).
There are several contributing factors influencing these results.
Survey Population
The ELSO member organizations are the only institutions that submit data to the ELSO Registry of ECMO data. The membership of the ELSO has hovered for many years around 110–125 member institutions. On the other hand, many centers performing pediatric CPB do not report their ECMO experience to ELSO. This unintentional exclusion of ECMO centers is suspected to lead to the inadvertent underreporting of cardiac ECMO cases in the ELSO registry. In 2002, the registry reported that there were twice as many neonatal respiratory cases performed as all cardiac cases performed for the same year. If pediatric open heart centers reported their information to the registry, we could assume that these numbers would be much closer. Additionally, by not participating in the registry, these institutions are skewing the workforce data to the detriment of the perfusion profession. Our data suggest that there is a very large perfusion involvement in the cardiac ECMO arena that is not captured in the ELSO Registry.
Clinical Patient Demographics
The post-CPB neonatal ECMO patient represents a unique challenge for the ECMO clinician. Bleeding from large suture lines can lead to coagulopathies and intravascular volume management problems. These complications emphasize the need to maximize the patients anticoagulation management. In such cases, it is not uncommon to completely discontinue heparin administration until hemostasis can be achieved. Additionally, while the patients often have reasonable lung function, they often require total cardiac support for the first half of their care. These special clinical requirements may influence the clinicians decision to use a standard ECMO circuit or to design a circuit that better meets the needs of this patient population. The use of smaller circuits with centrifugal pumps and HFOs has been reported in the literature and anectedotally from many progressive clinicians (9). This, however, has not generally been captured in the ECMO surveys because of the sampling issue discussed above.
In ECMO, surveys designed to determine the national standards have often misrepresented the national norms for equipment and workforce. This study underscores the need for future surveys to enroll a truly representative sample population and challenges centers that offer cardiac ECMO to participate in data collection efforts. We believe that these data suggest that perfusionists are more involved in clinical ECMO than previously reported and that parallel with their involvement is the implementation of pioneering technologies that represent the future standards of ECMO care.
ACKNOWLEDGMENTS
We thank all of the centers that participated in the survey.
REFERENCES
- 1.Hill JD, De Leval MR, Fallat RJ, et al. Acute respiratory insufficiency. Treatment with prolonged extracorporeal oxygenation. J Thorac Cardiovasc Surg. 1972;64:551–62. [PubMed] [Google Scholar]
- 2.Allison PL, Kurusz M, Graves DF, Zwischenberger JB.. Devices and monitoring during ECMO: Survey results. Perfusion. 1990;5:193–201. [DOI] [PubMed] [Google Scholar]
- 3.Hultquist K.. ELSO equipment survey X 1993-94. ECMO PumpTalk. 1994;6:14–16. [Google Scholar]
- 4.Lawson DS, Walczak R, Lawson AF, et al. North American neonatal extracorporeal membrane oxygenation (ECMO) devices: 2002 survey results. J Extra Corpor Technol. 2004;36:16–21. [PubMed] [Google Scholar]
- 5.Kawahito S, Maeda T, Motomura T, et al. Feasibility of a new hollow fiber silicone membrane oxygenator for long-term ECMO applications. J Med Invest. 2002;49:156–62. [PubMed] [Google Scholar]
- 6.Lawson DS, Darling E, Searles B.. Perfusionists should take a second look at ECMO. AmSECT Today. 2004;7:13. [Google Scholar]
- 7.Walker G, Liddell M, Davis C.. Extracorporeal life support—state of the art. Paediatr Respir Rev. 2003;4:147–52. [DOI] [PubMed] [Google Scholar]
- 8.Shanley CJ, Hultquist KA, Rosenberg DM, McKenzie JM, Shah NL, Bartlett RH.. Prolonged extracorporeal circulation without heparin. Evaluation of the Medtronic Minimax oxygenator. ASAIO J. 1992;38:M311–6. [PubMed] [Google Scholar]
- 9.Horton S, Thuys C, Bennett M, et al. Experience with the Jostra Rotaflow and QuadroxD oxygenator for ECMO. Perfusion. 2004;19:17–23. [DOI] [PubMed] [Google Scholar]