Abstract:
Assessment of anticoagulation status during cardiac surgery can be valuable for novel therapeutics, including direct thrombin inhibitors. The ecarin clotting time (ECT) has been reported to be sensitive for monitoring of bivalirudin in cardiac surgery but is not commercially available. The activated clotting time (ACT), commonly used for heparin monitoring, may display a lack of sensitivity to alternative anticoagulants when used in on-pump cardiac surgery. Both the ACT and ECT have been successfully used for monitoring bivalirudin anticoagulation in off-pump cardiac surgery. A new ACT, the ACTT, was developed to increase the linearity of the clotting time response to bivalirudin at higher concentrations. After Ethics Committee approval, a pilot study was performed to evaluate the feasibility of using bivalirudin for on-pump cardiac surgery and to evaluate dosing of bivalirudin in terms of the pharmacokinetic and safety profile in patients undergoing coronary artery bypass graft (CABG) surgery. Secondary objectives included an assessment of the anticoagulation profile and correlation of the response seen with various ACTs and the ECT with the plasma bivalirudin concentration in the patients’ blood. After informed consent, 10 sequential patients presenting for elective cardiac surgery requiring cardiopulmonary bypass received bivalirudin anticoagulation in lieu of heparin. Dosing was fixed (1.0 mg/kg bolus followed by a 2.5 mg/kg/h infusion) and not titrated on the basis of coagulation test results. At baseline and 15-minute intervals, blood samples were collected for ACT (ACTT, Celite, kaolin, ACT+), ECT, and bivalirudin plasma level measurements. Over the range of bivalirudin plasma concentrations in this study, all clot-based systems examined were prolonged according to concentration and showed good correlation with bivalirudin plasma levels. The ACTT and the ECT showed greater sensitivity to bivalirudin (∼28.5 sec/μg/ml bivalirudin) compared with the other ACTs evaluated (∼14 sec/μg/ml). This difference in sensitivity was also evident at low concentrations of bivalirudin (<10 μg/ml), with the ECT and ACTT showing slopes near 40, and the ACT slopes varying from 18 to 27 sec/μg/ml. The ACTT assay is sensitive to levels of bivalirudin and may offer a simple method for monitoring bivalirudin during cardiac surgery.
Keywords: activated clotting time, ecarin clotting time, anticoagulation monitoring, bivalirudin, cardiopulmonary bypass
In recent years, there has been an increasing emphasis on the clinical examination of alternative anticoagulants to replace heparin, the standard anticoagulant used during both interventional cardiology and cardiac surgery procedures. These efforts are driven in part by the need for effective periprocedural anticoagulation for patients with heparin-induced thrombocytopenia (1) and in part by the variability of patient response to heparin anticoagulation (2).
While heparin is an indirect thrombin inhibitor that works in concert with antithrombin to inhibit a family of coagulation factors, the most common alternate anticoagulants are the class of direct thrombin inhibitors, including hirudin, bivalirudin, and argatroban. One of these, bivalirudin (Angiomax; The Medicines Company, Parsippany, NJ), is a synthetic 20 amino acid peptide with a plasma half-life of 25 minutes. It is indicated by the Food and Drug Administration for use in patients with unstable angina undergoing coronary angioplasty and has been successfully used in many patients during percutaneous coronary interventions (3–6). In addition, several recent reports describe its successful use in cardiac surgery, both on and off pump (7–13).
Bivalirudin showed linear, dose-proportional pharmacology and prolongs common clotting tests such as the activated clotting time (ACT) in a dose-dependent manner. In percutaneous coronary interventions (PCIs), and initial cardiac surgery studies, bivalirudin was administered as a bolus followed by an infusion, both weight-based. In contrast to heparin, it has been proposed that bivalirudin’s predictable dose-response does not necessitate titration except in patients with renal failure or receiving prolonged infusion (14). However, clotting tests are useful, particularly in the setting of cardiopulmonary bypass (CPB), to assure that the drug has been properly administered and that coagulation has been inhibited. Furthermore, the number of patients treated to date in the cardiac surgery setting may be insufficient to appreciate the true clinical response to bivalirudin. Whereas the ACT has been shown to accurately reflect the anticoagulation status of patients receiving heparin, the sensitivity of the ACT to other anticoagulants, and in particular to bivalirudin when used during cardiac surgery with CPB, has not been previously reported in conjunction with pharmacokinetic data.
Published reports of the use of bivalirudin in cardiac surgery reference several alternative monitoring methods including the ecarin clotting time (ECT), the plasma modified ACT (9), and the high-dose thrombin time (HiTT) (10) as potentially more suitable measures of bivalirudin anticoagulation than an ACT.
A new assay, the ACTT, designed to be a simple assay that is sensitive to bivalirudin at the levels required for cardiac surgery, was evaluated in the approved indication of coronary angioplasty and in vitro analyses (15). The current study expands on those data, evaluating the ACTT in cardiac surgery with direct comparison to the ECT and bivalirudin levels in the patients’ blood.
MATERIALS AND METHODS
Clinical Analyses
After Ethics Committee approval, a pilot study was performed at a single clinical center, Institute Fur Anasthesie, Deutsches Herzzentrum Berlin, Berlin, Germany, to evaluate the feasibility of using bivalirudin (Angiomax; The Medicines Company) for on-pump surgery and to evaluate the dosing of bivalirudin in this setting. Secondary objectives included the evaluation of the response seen with various ACT assays (ACTT, Celite, kaolin, and ACT+; International Technidyne Corporation, Edison, NJ) and the ECT (Pharmaceutics, Raleigh, NC) to the assayed bivalirudin concentration in the patients’ blood. After informed consent, 10 sequential patients presenting for elective cardiac surgery requiring CPB received bivalirudin anticoagulation in lieu of heparin. Demographics of the patient population and details of the bypass circuit have been previously described (16). Based on experiences gained in a previous cohort of patients (7), the dosing was fixed (intravenous bolus of 1 mg/kg followed by a continuous infusion of 2.5 mg/kg/h) and not titrated on the basis of monitoring results. CPB was initiated 10–15 minutes after the initial bolus, and the infusion was discontinued 15 minutes before the end of CPB. At baseline and 15-minute intervals beginning 5 minutes after the bolus, blood samples were collected and analyzed for ACT (ACTT, Celite, kaolin, ACT+) and ECT, and plasma samples were frozen and stored for bivalirudin level measurements. Bivalirudin plasma concentrations were assayed by Quintiles Preclinical Services (Riccarton, Edinburgh, UK) using procedures validated with The Medicines Company.
Statistical Analyses
Linear regression analyses were performed to evaluate the relationship between each point-of-care assay and bivalirudin level.
Mean clotting times and SDs were calculated for all parameters at each sampling time-point. Sensitivity of each assay was calculated by the change in clotting time per microgram of bivalirudin as determined from regression analyses.
RESULTS
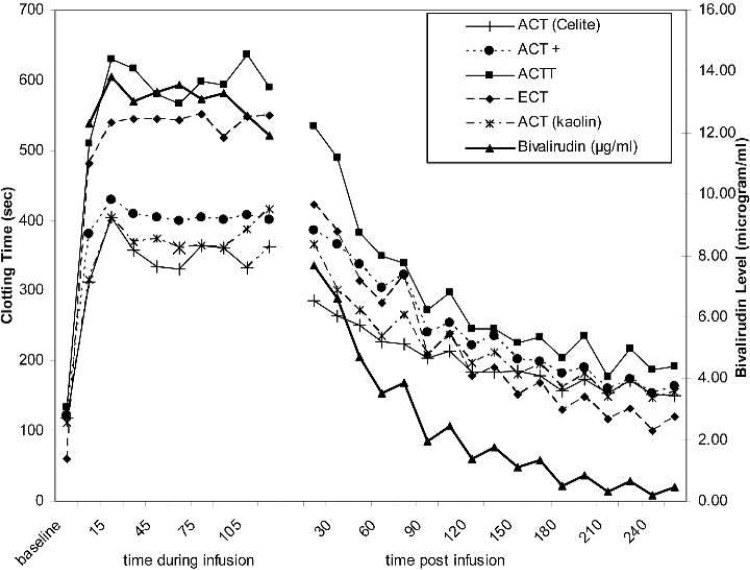
All the clotting time-based tests were prolonged significantly after the bivalirudin bolus dose and remained prolonged throughout the continuous infusion period (Figure 1). Celite, kaolin, and ACT+ did not increase to the degree seen for the ACTT and the ECT in response to increasing bivalirudin levels. After termination of the bivalirudin infusion, all clotting times decreased in a consistent manner (Figure 1). As is commonly observed with clot-based tests in an evaluation across individual patients, all tests showed wide variation at each collection time-point. The average variation, defined as SD/mean across all patients) ranged from 9.4% (ACT+) to 15% (kaolin ACT). These compare favorably with the average variation of the measured bivalirudin concentration (25.2%; data not shown). When the analysis was restricted to only those collection time-points during bivalirudin infusion (Figure 1, left of split), similar results are seen. During infusion, the variation across all patients ranged from 9.4% (ACT+) to 20.5% (kaolin ACT) and 18.8% for bivalirudin concentration.
Figure 1.
Time-course of mean clotting times and bivalirudin levels. The mean clotting time result (left axis) is shown for baseline, postbivalirudin bolus, and 15-minute intervals throughout and after bivalirudin infusion for Celite ACT (—+—), kaolin ACT(—*—), ACT+ (- - •- -), ACTT (—▪—), and ECT(—♦—) assays. The mean bivalirudin concentration (μg/ml) measured in the patients’ plasma is also indicated (right axis, —▴—). The SDs around each point are omitted for clarity. The break in the continuity of the data indicates the time at which the continuous infusion of bivalirudin was terminated.
All assays showed good correlation by linear regression to the bivalirudin concentration (r = 0.817–0.951; Table 1). Celite, kaolin, and ACT+ showed a reduced sensitivity to bivalirudin as evidenced by the slope (change of clotting time per microgram per milliliter bivalirudin) of the regression line (13.5–15.0) compared with either the ECT (slope = 28.8) or the ACTT (slope = 28.4). These observations held true for both the population as a whole (Table 1) and each individual patient (Table 2).
Table 1.
Correlation to measured bivalirudin concentration—all patients combined.
| Assay | n | Slope | Intercept | r |
|---|---|---|---|---|
| Celite ACT | 85* | 13.5 | 169.7 | 0.875 |
| Kaolin ACT | 171 | 14.8 | 179.8 | 0.817 |
| ACT+ | 172 | 15.0 | 214.0 | 0.870 |
| ACTT | 172 | 28.4 | 224.9 | 0.896 |
| ECT | 172 | 28.8 | 154.8 | 0.951 |
Samples from only 5 of the 10 patients were tested with the Celite ACT.
Table 2.
Correlation to measured bivalirudin concentration—summary of individual patients (n = 10).
| Assay | Slope [Mean ± SD; Range] | Intercept [Mean ± SD; Range] | r [Mean ± SD; Range] |
|---|---|---|---|
| Celite ACT* | 13.8 ± 3.7 | 168.3 ± 14.3 | 0.930 ± 0.22 |
| (10.7–20.0) | (154.5–185.5) | (0.909–0.966) | |
| Kaolin ACT | 14.9 ± 4.1 | 179.5 ± 24.7 | 0.896 ± 0.067 |
| (10.5–24.4) | (142.9–222.6) | (0.729–0.963) | |
| ACT+ | 15.7 ± 2.9 | 210.5 ± 14.9 | 0.897 ± 0.038 |
| (12.4–21.1) | (181.6–232.1) | (0.810–0.947) | |
| ACTT | 28.8 ± 4.2 | 222.7 ± 19.0 | 0.920 ± 0.049 |
| (23.8–37.0) | (196.0–262.6) | (0.794–0.960) | |
| ECT | 29.7 ± 3.2 | 150.4 ± 17.2 | 0.958 ± 0.017 |
| (26.4–35.3) | (121.7–171.8) | (0.919–0.978) |
Samples from only 5 of the 10 patients were tested with the Celite ACT.
The correlation curves for the currently available ACTs (Celite, kaolin, and ACT+) show that the response of these tests and their sensitivity to increasing plasma bivalirudin concentrations is decreased at levels exceeding 10 μg/ml and that the clotting time response is better fitted with a parabolic regression (Figure 2A). The linear regression curves for the ACTT and ECT showed good sensitivity across the range of plasma levels tested (Figure 2B).
Figure 2.
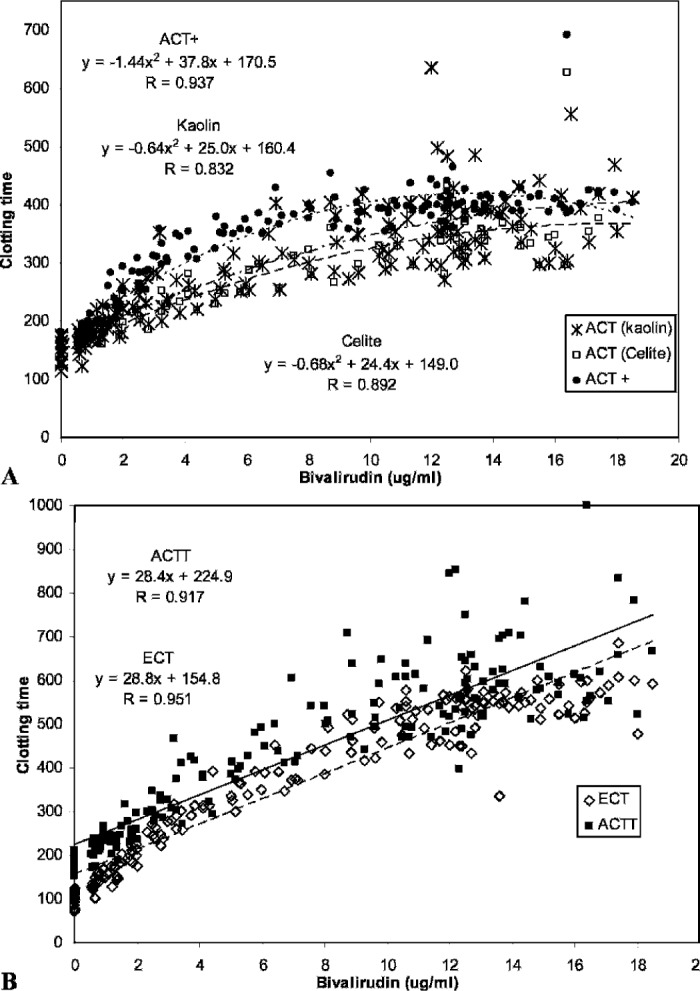
Correlation of clotting time tests to bivalirudin concentration. (A) Standard ACT assays. The clotting time data for the ACT+ (--•--), kaolin ACT (—*—), and Celite ACT (—□—) assays are better approximated by polynomial equations than by the linear regressions presented in Table 1. (B) ACTT and ECT assays. The clotting time data for the ACTT (—▪—) and ECT(—⋄—) assays are approximated equally well by the linear and polynomial regressions. Linear regressions as listed in Table 1 are shown.
The correlation analyses were repeated using only those samples with bivalirudin levels less than 10 μg/ml (Table 3). Whereas eliminating the scatter at the higher levels improved the correlation coefficient for all assays (r = 0.878–0.969), Celite, kaolin, and ACT+ sensitivities to bivalirudin (slopes 18.2–27.3) remained lower than that observed for the ACTT (slope = 41.0) and the ECT (slope = 40.4) assays. The correlation between the ACTT and ECT assays was very good (Figure 3), with a slope near 1.0 and a correlation coefficient of 0.930.
Table 3.
Correlation to measured bivalirudin <10 μg/ml.
| Assay | n | Slope | Intercept | r |
|---|---|---|---|---|
| Celite ACT | 52* | 18.2 | 156.3 | 0.923 |
| Kaolin ACT | 101 | 20.7 | 164.3 | 0.878 |
| ACT+ | 102 | 27.3 | 181.0 | 0.922 |
| ACTT | 102 | 41.0 | 190.1 | 0.938 |
| ECT | 102 | 40.4 | 121.2 | 0.969 |
Samples from only 5 of the 10 patients were tested with the Celite ACT.
Figure 3.
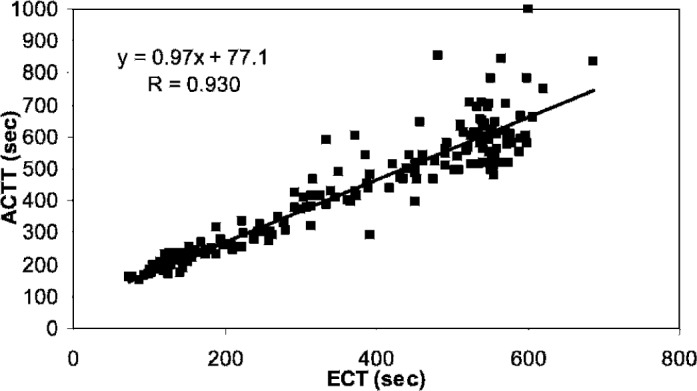
Regression analysis of ACTT to ECT. The ACTT and ECT clotting times across all bivalirudin concentrations and all patients showed a high degree of correlation.
DISCUSSION
In recent years, several reports have been published describing the use of various clotting tests including various ACTs, the plasma-modified ACT, the HiTT, and the ECT for monitoring levels used in cardiac surgery of the direct thrombin inhibitors hirudin and bivalirudin (7,8,11,13,17–20). However, there is little data on the actual correlation of these tests to pharmacokinetic determinations.
One whole blood ECT (Pharmanetics) was commercially available briefly and cleared for use in the United States on a compassionate basis to monitor r-hirudin anticoagulation during cardiac surgery for patients with heparin-induced thrombocytopenia. This assay was reported as a good monitoring method for hirudin anticoagulation, an alternative method being the plasma-modified ACT (13,19,20). The use of these tests has also been reported with bivalirudin anticoagulation in cardiac surgery (7,10). Both the plasma-modified ACT and the ECT assays require a preanalytical procedure in which the operator must mix the blood sample with normal human plasma before conducting the test.
The current study expanded earlier evaluations of a novel ACT, the ACTT (15), to cardiac surgery patients receiving bivalirudin anticoagulation. It also presents novel data comparing clotting time assays with the measured bivalirudin levels in this patient population. The observed variability in patient response as measured by clot-based tests (Table 2) suggests that monitoring of bivalirudin may be useful in the application. The data show that, across bivalirudin concentrations of 0–20 μg/ml, all of the tests correlated well with bivalirudin plasma concentrations. Both the ACTT and the ECT showed greater sensitivity (change in clotting time per microgram per milliliter bivalirudin) than the other ACT assays at bivalirudin levels expected to yield optimal anticoagulation during CPB (10–15 μg/ml, with clotting times in the 400- to 600-second range compared with 300–400 seconds for Celite, kaolin, and ACT+) (7).
The ACTT differs from both the ECT and the plasma-modified ACT in that no independent operator manipulation of the patient sample is required before analysis. The assay is performed in a manner identical to the ACT+, for which a sample is applied to the test cuvette and the instrument START button is depressed. This, coupled with the sensitivity of the assay to those levels of bivalirudin used in cardiac surgery, makes the ACTT an attractive alternative to ACT monitoring in this intended use. The use of the ACTT in wider applications (i.e., monitoring heparin anticoagulation) has not yet been evaluated in a clinical setting. This and expanding the patient population will be the subject of future trials.
While Angiomax is currently Food and Drug Administration approved only for PCIs, several clinical teams have reported the successful use of bivalirudin in cardiac surgery under investigational protocols (1,7–13). After completion of the pilot study of the use of bivalirudin in on-pump cardiac surgery, the data suggest feasibility of the currently used PCI dosing in this setting. At this time, however, use of bivalirudin in cardiac surgery is still off-label and mainly restricted to the exceptional scenario of patients with HiT needing urgent cardiac surgery. Further studies are ongoing to elucidate the safety and efficacy profile of this drug for this application.
At bivalirudin doses described in reports of use in cardiac surgery and evaluated in this pilot study, the ACTT is a convenient and sensitive monitoring test for the anticoagulation effect of bivalirudin.
ACKNOWLEDGMENT
The authors thank The Medicines Company for supplying the bivalirudin level data used in this manuscript.
REFERENCES
- 1.Greinacher A.. The use of direct thrombin inhibitors in cardiovascular surgery in patients with heparin-induced thrombocytopenia. Semin Thromb Hemost. 2004;30:315–27. [DOI] [PubMed] [Google Scholar]
- 2.Bull BS, Huse WM, Brauer FS, Korpman RA.. Heparin therapy during extracorporeal circulation. II. The use of a dose-response curve to individualize heparin and protamine dosage. J Thorac Cardiovasc Surg. 1975;69:685–9. [PubMed] [Google Scholar]
- 3.Bittl JA, Chaitman BR, Feit F, Kimball W, Topol EJ.. Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: Final report reanalysis of the Bivalirudin Angioplasty Study. Am Heart J. 2001;142:952–9. [DOI] [PubMed] [Google Scholar]
- 4.Lincoff AM, Bittl JA, Kleiman NS, et al. The REPLACE 1 trial: A pilot study of bivalirudin versus heparin during percutaneous coronary intervention with stenting and GP IIb/IIIa blockade. J Am Coll Cardiol. 2002;39:16A. [Google Scholar]
- 5.Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–63. [DOI] [PubMed] [Google Scholar]
- 6.Lincoff AM, Bittl JA, Kleiman NS, et al. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the randomized evaluation of PCI linking angiomax to reduced clinical events [REPLACE]-1 trial). Am J Cardiol. 2004;93:1092–6. [DOI] [PubMed] [Google Scholar]
- 7.Koster A, Chew D, Grundel M, Bauer M, Kuppe H, Spiess BD.. Bivalirudin monitored with the ecarin clotting time for anticoagulation during cardiopulmonary bypass. Anesth Analg. 2003;96:383–6. [DOI] [PubMed] [Google Scholar]
- 8.Koster A, Spiess B, Chew D, et al. Effectiveness of bivalirudin as a replacement for heparin during cardiopulmonary bypass in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2004;93:356–9. [DOI] [PubMed] [Google Scholar]
- 9.Jabr K, Johnson JH, McDonald MH, et al. Plasma-modified ACT can be used to monitor bivalirudin (Angiomax) anticoagulation for on-pump cardiopulmonary bypass surgery in a patient with heparin-induced thrombocytopenia. J Extra Corpor Technol. 2004;36:174–7. [PubMed] [Google Scholar]
- 10.Baker T, Chan R, Hill F.. Anticoagulant monitoring techniques in a heparin-induced thrombocytopenia patient undergoing cardiopulmonary bypass using bivalirudin anticoagulant. J Extra Corpor Technol. 2004;36:371–4. [PubMed] [Google Scholar]
- 11.Clayton S, Acsell J, Crumbley AJ, Shackelford A, Uber WE.. Cardiopulmonary bypass with bivalirudin in type II heparin-induced thrombocytopenia. Ann Thorac Surg. 2004;78:2167–9. [DOI] [PubMed] [Google Scholar]
- 12.Merry AF, Raudkivi PJ, Middleton NG, et al. Bivalirudin versus heparin and protamine in off-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:925–31. [DOI] [PubMed] [Google Scholar]
- 13.McDonald SB, Kattapurum BM, Saleem R, Joist HJ, Avidan M, Despotis GJ.. Monitoring hirudin anticoagulation in two patients undergoing cardiac surgery with a plasma-modified ACT method. Anesthesiology. 2002;97:509–12. [DOI] [PubMed] [Google Scholar]
- 14.Caron MF, McKendall GR.. Bivalirudin in percutaneous coronary intervention. Am J Health Syst Pharm. 2003;60:1841–9. [DOI] [PubMed] [Google Scholar]
- 15.Measday MA, Zucker MLF.. Optimizing mangement of hirudin anticoagulation. J Extra Corpor Technol. 2005;37:66–70. [PubMed] [Google Scholar]
- 16.Koster A, Yeter R, Buz S, et al. Assessment of hemostatic activation during cardiopulmonary bypass for coronary artery bypass grafting with bivalirudin: Results of a pilot study. J Thorac Cardiovasc Surg. 2005;129:1391–4. [DOI] [PubMed] [Google Scholar]
- 17.Potzsch B, Madlener K, Seelig C, Riess CF, Greinacher A, Muller-Berghaus G.. Monitoring of r-hirudin anticoagulation during cardiopulmonary bypass-assessment of the whole blood ecarin clotting time. Thromb Haemost. 1997;77:920–5. [PubMed] [Google Scholar]
- 18.Fabrizio MC.. Use of ecarin clotting time (ECT) with lepirudin therapy in heparin-induced thrombocytopenia and cardiopulmonary bypass. J Extra Corpor Technol. 2001;33:117–25. [PubMed] [Google Scholar]
- 19.Despotis GJ, Hogue CW, Saleem R, et al. The relationship between hirudin and activated clotting time: Implications for patients with heparin-induced thrombocytopenia undergoing cardiac surgery. Anesth Analg. 2001;93:28–32. [DOI] [PubMed] [Google Scholar]
- 20.Koster A, Despotis G, Gruendel M, et al. The plasma supplemented modified activated clotting time for monitoring of heparinization during cardiopulmonary bypass: a pilot investigation. Anesth Analg. 2002;95:26–30. [DOI] [PubMed] [Google Scholar]