Abstract:
Modified ultrafiltration (MUF) is a novel application of ultrafiltration to remove excess water after cardiopulmonary bypass in pediatric patients that was first reported in 1991. It has gained widespread use as an important adjunct to fluid management in neonates, infants, and pediatric cardiac surgery patients. Now more than two decades after its original description, the exact mechanism of action and effects of this therapy are still a matter of discussion. The aim of this study was to determine the effects of MUF on plasma fentanyl levels using a two-phase in vitro and in vivo study. We designed an in vitro experimental model to simulate MUF that allowed measurement of plasma fentanyl levels while eliminating biologic variables. Plasma fentanyl levels were measured during five consecutive operations on neonates and infants undergoing repair of congenital heart defects. Increases in plasma fentanyl levels were found in vitro as well as in vivo. Fentanyl plasma levels more than doubled after MUF (increased by a factor of 2.22, from 12.4 to 27.5 ng/ml in vivo). The increase in plasma fentanyl levels needs to be taken into account when delivering anesthetic care and when analyzing the effect of MUF on outcome variables.
Keywords: infant heart surgery, bypass, ultrafiltration, fentanyl
Modified ultrafiltration (MUF) reduces total body water, increases hematocrit, increases systemic blood pressure, increases cardiac index, decreases myocardial edema, decreases heart rate, reduces pulmonary hypertension, decreases transfusion requirements, and decreases chest drainage loss in repair of congenital heart lesions (1–4). Although MUF has been found to remove some inflammatory mediators, not all studies have shown a significant advantage. No clear benefit over conventional ultrafiltration (CUF) could be established (5,6). One of the main goals in delivering anesthetic care to neonates and infants undergoing heart surgery has been to provide a “stress-free” high fentanyl technique (7), although this may not be as important anymore as it was initially thought (8). This two-stage study was designed to establish the role of fentanyl plasma levels as a possible contributing factor to the effects of MUF.
MATERIALS AND METHODS
This study was done in a two-stage set-up: in vivo and in vitro. The same manufacturer and model hemofilter was used for both the in vitro and in vivo studies.
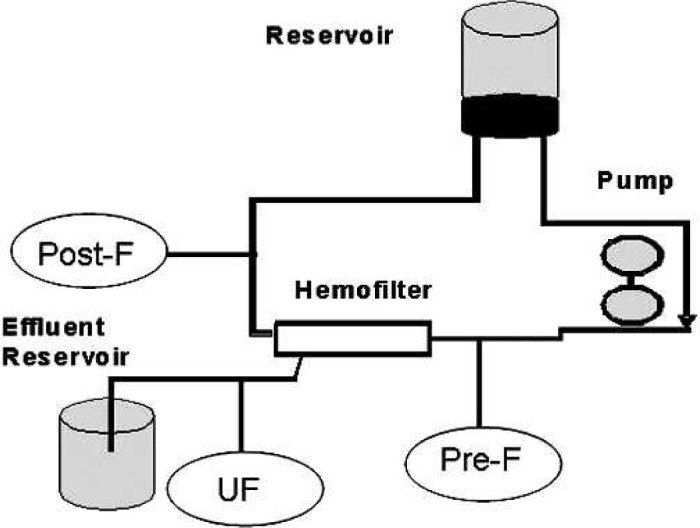
The circuit set-up consisted of a Dideco D901 oxygenator (Dideco, Modena, Italy) with integrated venous reservoir, ¼-in arterial and venous line (Terumo, Tokyo, Japan), AF02 32-μm arterial filter (Terumo), and a custom BCD VAN P 4:1 blood cardioplegia set (Sorin, Sallugia, Italy). Arterio-venous MUF is routinely used for pediatric cases in our center using a HC05S Capiox hemoconcentrator (Terumo). The Capiox hemoconcentrator is of the polysulfon type and has a surface area of 0.5 m2. The albumin (molecular weight = 66,000 d) sieving coefficient is 0.6%, meaning that 99.4% remains in circulation. MUF was accomplished through the cardioplegia delivery system that includes the heat exchanger and bubble trap components of that system (9).
In Vitro
In the in vitro part of the study, we simulated an infant on cardiopulmonary bypass (CPB) by adding 300 ml of reconstituted whole blood to a typical prime volume of 450 ml for a total of 750 ml. The system was allowed to equilibrate for 30 minutes after addition of fentanyl. Controlled variables consisted of temperature, blood gas (BG), and electrolytes (including ionized calcium). At the end of the equilibration phase, the temperature was 36.8°C, hematocrit was 24%, BG = 7.38 (pH)/41 mmHg (pCO2), 163 mmHg (pO2)/27 mmHg (HCO3), and iCa = 1.13 meq/ml. The fentanyl level before initiation of MUF was 1.6 ng/ml. After completion of the filtration process, fentanyl plasma levels were determined from three locations: pre- and postfilter, as well as from the ultrafiltration port (Figure 1). Flow was initiated at 75 ml/min, and after 3 minutes was increased to 125 ml/min. Samples were drawn when the hematocrit was estimated to be 40% (T40%) and 65% (T65%).
Figure 1.
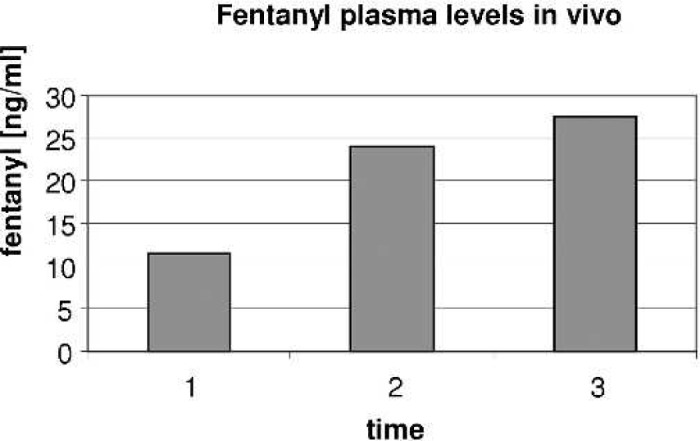
Fentanyl plasma levels in vivo (experimental set-up). T1, baseline after separation from CPB and before MUF; T2, after completion of 50% of MUF; T3, after the end of MUF.
In Vivo
In the in vivo part of the study, after approval of the institutional review board, we enrolled five infants in a 10-day period that were scheduled to undergo repair of congenital heart lesions.
A standardized anesthetic technique was used, which was provided by a single anesthesiologist (A.H.T.), CPB and MUF were performed by a single perfusionist (R.C.G.), and the operations were performed by a single surgeon (R.D.Q.). All patients received a total of 100 μg/kg fentanyl: 25 μg/kg each at time of induction, sternotomy, initiation of CPB, and during the rewarming phase. Fentanyl levels were drawn at three time-points; baseline after separation from CPB before start of MUF (T1), after completion of 50% of the MUF process (T2), and from the filtrate port (FP1), as well as at the end of MUF (T3) and from the port (FP2). MUF flow was initiated at 75 ml/min and increased to 125 ml/min as tolerated. MUF was continued until an estimated hematocrit of 40% was reached or no blood remained in the circuit.
Fentanyl levels were analyzed by high-performance liquid chromatography with tandem mass spectroscopy and spectral confirmation (Medtox, St. Paul, MN). This test is sensitive for a concentration of 0.1 ng/ml and above. Accuracy of the test is concentration dependent—approximately ±5% for higher concentrations. Statistical analysis for difference in levels was performed with a paired t test.
RESULTS
In Vitro
Baseline fentanyl level was 1.6 ng/ml. Three hundred sixty-five milliliters of volume was removed, and the filtration process lasted 15 minutes. At T40% (actual hematocrit = 38%), the level drawn from proximal to the filter was 22.5 ng/ml and from the port distal to it was 23.0 ng/ml. The level from the filtrate port was 6.5 ng/ml. At T65% (actual hematocrit = 64%), the prefilter level was 31.0 ng/ml, the postfilter level was 32.0 ng/ml, and the filtrate port level was 9.1 ng/ml. For technical reasons (no port), the filtrate sample was not drawn form the ultrafiltrate reservoir. It represents a snapshot of the concentration in the line between the filter and the reservoir at the end of MUF and not the concentration in the reservoir (Table 1; Figures 1 and 2).
Table 1.
Fentanyl plasma levels in vitro.
| Baseline | T40% Prefilter | T40% Postfilter | T65% Prefilter | T65% Postfilter |
|---|---|---|---|---|
| 1.6 | 22.5 | 23.0 | 31.0 | 32.0 |
T40%, sample drawn when hematocrit was 40%; T65%, sample drawn when hematocrit was 65%.
Figure 2.
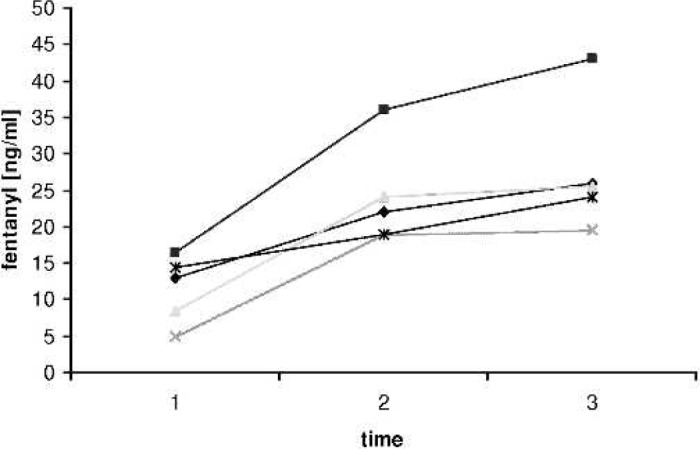
Fentanyl plasma levels in vivo. T1, baseline after separation from CPB and before MUF; T2, after completion of 50% of MUF; T3, after the end of MUF.
In Vivo
Fentanyl plasma levels increased significantly (p = 0.001) over baseline during MUF. Patients weighed between 2.7 and 5.5 kg, and were between 1 day and 3 months of age. On average, plasma levels of fentanyl increased by a factor of 2.2 (12.4 → 27.5 ng/ml). The average hematocrit before MUF was 28.8% and was 43.0% after. The average volume removed by the MUF process was 344 ml or 86.87 ml/kg. Duration of MUF was between 13 and 19 minutes. In all patients, levels increased significantly (p = .001) when 50% of the ultrafiltration process was completed (Table 2; Figure 3). At the end of MUF, BGs were in normal range for all patients.
Table 2.
Fetanyl plasma levels in vitro.
| Weight (kg) | Operation | T1 | T2 | T3 | FP1 | FP2 |
|---|---|---|---|---|---|---|
| 2.7 | Norwood | 13.0 | 22.0 | 26.0 | 2.2 | 2.4 |
| 5.5 | TOF | 16.5 | 36.0 | 43.0 | 3.0 | 3.0 |
| 4.4 | AVC | 8.5 | 24.0 | 25.5 | 1.4 | 1.8 |
| 4.0 | TGA | 5.0 | 19.0 | 19.5 | 1.2 | 2.0 |
| 3.2 | RVOT patch | 14.5 | 19.0 | 24.0 | 2.5 | 3.0 |
T1, baseline plasma level; T2, after completion of 50% of MUF; T3, after end of MUF; FP1, MUF reservoir after 50% of MUF; FP2, MUF reservoir after end of MUF; Norwood, stage I repair for hypoplastic left heart syndrome; TOF, repair of Tetralogy of Fallot; AVC, repair of atrioventricular canal; TGA, repair of transposition of great arteries; RVOT, patch of right ventricular outflow tract obstruction.
Figure 3.
Circuit setup.
DISCUSSION
MUF is used commonly in pediatric heart centers, but the techniques and endpoints are far from standardized (10). Despite its widespread use, it remains unclear if its effects are caused by hemoconcentration alone or by a decrease in the inflammatory stress response (11). Numerous studies have described a decrease in inflammatory mediators (5,12–14), but others have not (15,16). Different filters seem to have different effects on the elimination of inflammatory mediators (17). Conclusive evidence for the link between a transitory or long-lasting reduction in inflammatory proteins and alteration of significant outcome variables has not been established (18), although an association of interleukin-8 and early morbidity has been reported (19). Furthermore, there have been suggestions that a marked immune response, at least in sepsis, is good rather than bad (20).
Fentanyl plasma levels after MUF were initially studied to answer criticism that some of the changes in outcome variables (e.g., increase in systolic blood pressure) were secondary to removal of fentanyl. No change in plasma levels were found (21) by this group. The patients were older (average age, 3.8 years) and larger (average weight, 15.7 kg) than in our group. The equipment used was different as well.
Free fentanyl with a molecular weight of 528 d should be removed from circulation by a filter with a pore size of approximately 65, 000 d (as in our set-up). At a pH of 7.4, fentanyl is 80% plasma-bound. Hemoconcentration can be expected to lead to an increase in albumin and fentanyl-binding proteins, resulting in a relative increase in free and total fentanyl plasma concentrations after equilibration. This effect might depend on filter type (similar to the elimination of inflammatory proteins) and on the amount of filtrate removed.
Fentanyl plasma levels are influenced by a variety of factors, including hemodilution, hypothermia, and sequestration in lungs and other tissues. In combining our in vivo data with the in vitro model, we showed that MUF contributes to an increase in fentanyl levels and that the increase is not just a time-related coincidence of fentanyl re-entering circulation. Additionally, fentanyl levels have been shown not to change much in infants after CPB (22).
An increase in fentanyl plasma levels secondary to MUF could be a contributing factor in the reduction of the stress response associated with MUF. Some of the effects of MUF might be caused by higher fentanyl plasma levels and should be controlled for when studying inflammatory proteins and the stress response and their association with MUF in infant pediatric surgery. In the clinical setting, the fentanyl increase should be taken into account in the administration of fentanyl after MUF, especially when extubation is planned.
REFERENCES
- 1.Naik SK, Knight A, Elliott MJ.. A successful modification of ultrafiltration for cardiopulmonary bypass in children. Perfusion. 1991;6:41–50. [DOI] [PubMed] [Google Scholar]
- 2.Bando K, Turrentine MW, Palaniswamy V, et al. Effect of modified ultrafiltration in high-risk patients undergoing operations for congenital heart surgery. Ann Thorac Surg. 1998;66:821–8. [DOI] [PubMed] [Google Scholar]
- 3.Draaisma AM, Hazekamp MG, Frank M, et al. Modified ultrafiltration after cardiopulmonary bypass in pediatric cardiac surgery. Ann Thorac Surg. 1997;64:521–5. [DOI] [PubMed] [Google Scholar]
- 4.Naik SK, Knight A, Elliott M.. A prospective randomized study of a modified technique of ultrafiltration during pediatric open-heart surgery. Circulation. 1991;84(Suppl III):422–31. [PubMed] [Google Scholar]
- 5.Wang M-J, Chiu I-S, Hsu C-M, et al. Efficacy of ultrafiltration in removing inflammatory mediators during pediatric cardiac operations. Ann Thorac Surg. 1996;61:651–6. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LD, McElhinney DB, Findlay P.. A prospective randomized study comparing volume-standardized modified and conventional ultrafiltration I pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2001;122:220–8. [DOI] [PubMed] [Google Scholar]
- 7.Anand KJ, Hickey PR.. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia for cardiac surgery in infants. Anesth Analg. 1984;63:117–24.6229197 [Google Scholar]
- 8.Gruber EM, Laussen PC, Casta A, et al. Stress response in infants undergoing cardiac surgery: A randomized study of fentanyl bolus, fentanyl infusion, and fentanyl-midazolam infusion. Anesth Analg. 2001;92:882–90. [DOI] [PubMed] [Google Scholar]
- 9.Groom RC, Akl BA, Albus RA, Hill AG, Munoz R, Lefreak EA.. Alternative method of ultrafiltration after cardiopulmonary bypass. Ann Thorac Surg. 1994;58:573–4. [DOI] [PubMed] [Google Scholar]
- 10.Darling E, Nanry K, Shearer I, Kaemmer D, Lawson S.. Techniques of paediatric modified ultrafiltration: 1996 survey results. Perfusion. 1998;13:93–103. [DOI] [PubMed] [Google Scholar]
- 11.Ramamoorthy C, Lynn AM.. Con: The use of modified ultrafiltration during pediatric cardiovascular surgery is not a benefit. J Thorac Cardiovasc Anesth. 1998;12:483–5. [DOI] [PubMed] [Google Scholar]
- 12.Journois D, Poupard P, Greely WJ, Mauriat P, Vouhe P, Safran D.. Hemofiltration during cardiopulmonary bypass in pediatric cardiac surgery. Anesthesiology. 1994;81:1181–9. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Huang HM, Zhu DM, Chen H, Ding WX.. Modified ultrafiltration in paediatric cardiopulmonary bypass. Perfusion. 1998;13:304–10. [DOI] [PubMed] [Google Scholar]
- 14.Henein HA, Kiziltepe U, Barst S, et al. Venovenous modified ultrafiltration after cardiopulmonary bypass in children: A prospective randomized study. J Thorac Cardiovasc Surg. 1999;117:496–503. [PubMed] [Google Scholar]
- 15.Pearl JM, Manning PB, McNamara JL, Saucier MM, Thomas DW.. Effect of modified ultrafiltration on plasma thromboxane B2, leukotriene B4, endothelin-1 in infants undergoing cardiopulmonary bypass. Ann Thorac Surg. 1999;68:1369–75. [DOI] [PubMed] [Google Scholar]
- 16.Chew MS, Brix-Christensen B, Ravn HB, et al. Effect of modified ultrafiltration on the inflammatory response in paediatric open-heart surgery:a prospective, randomized study. Perfusion. 2002;17:327–33. [DOI] [PubMed] [Google Scholar]
- 17.Berdat PA, Eichenberger E, Ebell J, et al. Elimination of proinflammatory cytokines in pediatric cardiac surgery: Analysis of ultrafiltration method and filter type. J Thorac Cardiovasc Surg. 2004;127:1688–96. [DOI] [PubMed] [Google Scholar]
- 18.Gaynor JW.. The Effect of Modified Ultrafiltration on the Postoperative Course in Patients With Congenital Heart Disease. Seminars in Thoracic and Cardiovascular Surgery. Philadelphia, PA: W.B. Saunders; 2003. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Abraham R, Weinbroum AA, Lotan D, et al. Interleukin-8 secretion following cardiopulmonary bypass in children as a marker of early postoperative morbidity. Paediatr Anaesth. 2002;12:156–61. [DOI] [PubMed] [Google Scholar]
- 20.Doecke W-D, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nature Med. 1997;3:678–9. [DOI] [PubMed] [Google Scholar]
- 21.Hodges UM, Berg S, Naik SK, Bower S, Lloyd-Thomas A, Elliot M.. Filtration of fentanyl is not the cause of elevation of arterial blood pressure associated with post-bypass ultrafiltration in children. J Cardiothorac Vasc Anesth. 1994;8:653–7. [DOI] [PubMed] [Google Scholar]
- 22.Newland MC, Leuschen P, Sarafian LB, et al. Fentanyl intermittent bolus technique for anesthesia in infants and children undergoing cardiac surgery. J Cardiothorac Anesth. 1989;3:407–10. [DOI] [PubMed] [Google Scholar]


