Abstract:
The Oximetrix III Opticath (Abbott Critical Care Systems) is used for continuous measurement of venous saturation in a variety of applications, including extracorporeal membrane oxygenation (ECMO), despite clinical reports that have presented data showing poor accuracy of these devices. The CDI Blood Parameter Monitoring System 500 (Terumo) is an inline blood gas monitoring tool commonly used during cardiopulmonary bypass procedures to continuously assess oxygen saturation, blood gases, potassium, and bicarbonate. The purpose of this experiment was to compare the Opticath and the CDI 500 in trending venous blood saturation during a simulation of ECMO. An ECMO simulation circuit consisting of a silicone rubber membrane oxygenator and a stainless steel heat exchanger was constructed, and a standard venous reservoir bag was used to represent the patient. The CDI and the Opticath were incorporated side by side into a shunt that originated just before the oxygenator and returned flow to the venous line. The circuit was primed with fresh porcine blood and conditioned with the addition of CO2 to simulate typical venous blood under ECMO conditions. After an initial calibration procedure, samples were drawn and analyzed by an AVL Opti CCA (Roche/Osmetech) every 4–8 hours for a period of 7 days, with calibration of each device at sample intervals. The data were plotted, and a least squares regression line was calculated. The average error for venous saturation of the CDI and Opticath after 72 hours was 3.86 and 9.51 respectively. At 168 hours, error for the CDI was 8.37, and the Opticath had an error of 14.78. A correlation analysis of the CDI and AVL CCA analyzer yielded a correlation coefficient of r = .88 at 72 hours and r = .84 at 168 hours. Correlation between the Opticath and the AVL CCA yielded a correlation coefficient of r = .77 at 72 hours and r = .55 at 168 hours. Based on these findings, the CDI 500 is an effective tool for monitoring venous blood saturation under simulated conditions of ECMO.
Keywords: CDI 500, Opticath, extracorporeal membrane oxygenation, venous saturation
The Oximetrix III Opticath (Abbott Critical Care Systems, North Chicago, IL) is used for continuous measurement of venous saturation in a variety of applications, including extracorporeal membrane oxygenation (ECMO), despite clinical reports that have presented data showing poor accuracy of these devices (1). The Opticath uses reflectance spectrophotometry and optical fibers to calculate venous saturation. Additional information is often necessary and would be useful for patient management during critical periods of ECMO such as initiation and termination. The CDI Blood Parameter Monitoring System 500 (Terumo Cardiovascular Systems, Ann Arbor, MI) shunt sensor is an inline blood gas monitoring tool commonly used during cardiopulmonary bypass (CPB) procedures to continuously assess oxygen saturation, blood gases, potassium, and bicarbonate. The accuracy of the fluorescence technology used by the CDI has been documented in previous studies (2–4). The purpose of this study was to evaluate and compare the CDI and the Opticath in measuring venous saturation during a simulation of ECMO over the time span of 1 week.
MATERIALS AND METHODS
A diagram of the ECMO simulation circuit used for this experiment is shown in Figure 1. A standard venous reservoir bag was used to represent the patient. Blood drained by gravity from the bag through ¼-in polyvinyl-chloride (PVC) tubing to a roller pump. The blood was then pumped through a silicone rubber membrane oxygenator, which was connected to gas sources of air, oxygen, and carbon dioxide. Beyond the oxygenator, blood flowed through a stainless steel heat exchanger and finally back into the reservoir bag. A shunt was positioned just before the oxygenator. This shunt incorporated the Opticath and CDI side by side and also a stopcock manifold for sampling access and delivery of drugs. The shunt returned blood back to the venous line.
Figure 1.
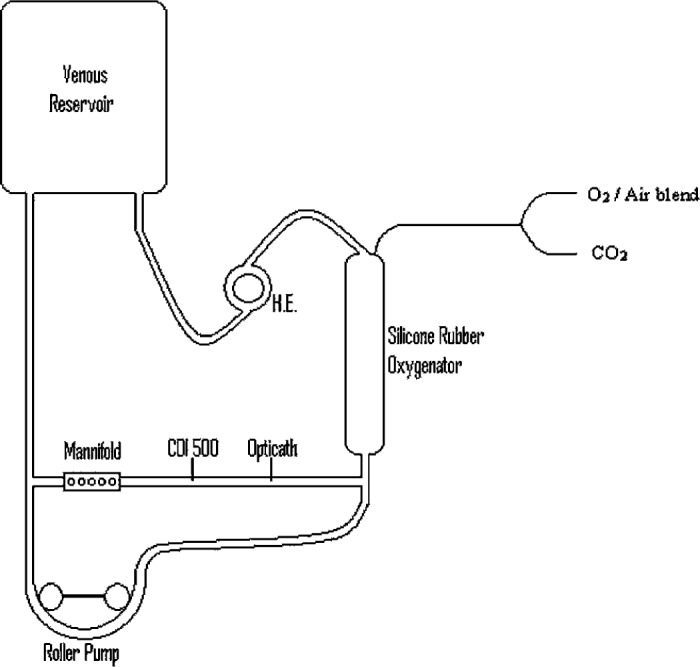
ECMO simulation test circuit. H.E., heat exchanger.
The circuit was initially primed with Plasmalyte-A, to which 500 ml of fresh porcine whole blood, anticoagulated with citrate-phosphate-dextrose-adenine (CPDA) was added. Supplemental heparin was administered (1.0 U/ml) to achieve an activated clotting time (ACT) of greater than 300 seconds. Additional dextrose (10 mg/ml) was added to the prime to ensure erythrocyte nutritional substrate availability, with a target glucose range of 65–120 mg/dl. Sodium bicarbonate was added for acid/base balance as necessary. Further administration of additional fresh porcine whole blood was necessary to replace volume lost during frequent circuit sampling for blood saturation determinations.
A hemoconcentrator was used to achieve and maintain a study defined hematocrit of 40%. Pump flow was established at 350 ml/min and was not altered during the study period. A heater-cooler was used to maintain a blood temperature of 37°C.
To simulate typical venous blood gases, the oxygenator was ventilated with a mixture of CO2, O2, and air with the following goal ranges: pH, 7.37 ± 0.05; pCO2, 46±10 mmHg; pO2, 40 ± 10 mmHg; SO2, 75% ± 10%; hematocrit, 40% ± 5%.
An initial calibration procedure was performed on both instruments according to manufacturer specifications before study initiation. After a brief stabilization period, study blood samples were collected and analyzed by an AVL Opti CCA (Osmetech Inc., Roswell, GA) blood gas analyzer. A measured hemoglobin saturation value was determined by the AVL to assure sample accuracy.
Circuit blood samples were analyzed every 4 hours for the first 2 days, and subsequently drawn every 8 hours for the next 5 days. Time-matched output readings of the CDI and Opticath were recorded at each blood sample interval. Test devices were recalibrated at each blood sample interval. Approximately 30 minutes before sample collection, ventilating gases were titrated to mimic sample conditions. A minimum of 20 minutes was allowed to elapse for circuit stabilization of blood gases before data collection.
Test circuit erythrocyte viability studies were not performed, but in vitro collection and preparation guidelines were followed according to the Guide to the Preparation, Use, and Quality Assurance of Blood Components (5). Briefly, porcine whole blood was collected in healthy donors in a solution of CPDA. Glucose and adenine are necessary components for the maintenance of erythrocyte metabolism and viability, phosphate is used to enhance glycolysis, and citrate is added to prevent in vitro hemolysis. As previously stated, each sample period included a measurement of glucose that was maintained within normal limits with the addition of dextrose (50%). Gross hemolysis was evaluated by centrifugation of test circuit blood and inspection of the supernatant fluid for color. Sample determinations of serum potassium were assessed as further indication of erythrocyte integrity. Circuit tubing occlusion was closely monitored throughout the test period to reduce circuit hemolysis. Conventional methods of determining occlusion were used.
RESULTS
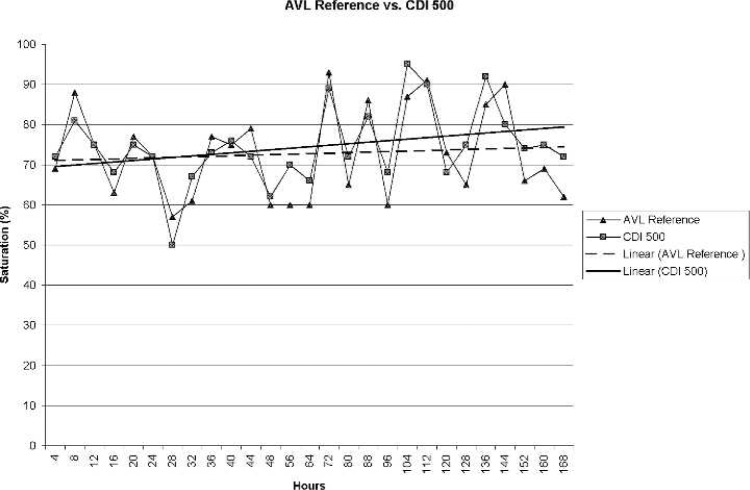
Figure 2 displays blood gas reference saturation values (AVL) and corresponding CDI values. CDI values did not differ statistically (α = .05) from reference values (p = .16). A Pearson correlation coefficient for the CDI device was r = .88 for the first 72 hours and r = .84 at 168 hours.
Figure 2.
AVL reference saturation vs. CDI 500 values (r = .84).
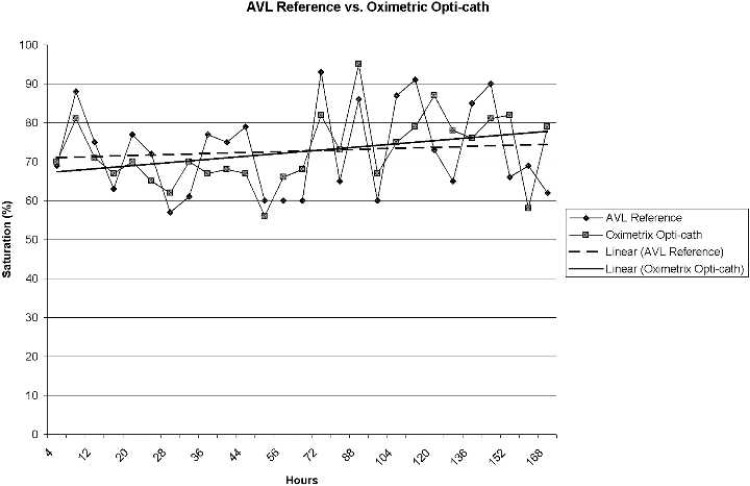
Figure 3 displays blood gas reference saturation values (AVL) and corresponding Opticath values. Opticath values did not differ statistically (α = .05) from reference values (p = .92). Correlation values for the Opticath were r = .77 for the first 72 hours and r = .55 at 168 hours.
Figure 3.
AVL reference saturation vs. Oximetrix Opticath (r = .55).
A least squares regression determination revealed an average error, compared with blood gas reference, of the CDI and Opticath devices to be 3.86 and 9.51, respectively, at 72 hours. At 168 hours, the error was determined to be 8.37 for the CDI device and 14.78 for the Opticath.
DISCUSSION
Accurate assessment and precise monitoring of blood gas values is an essential component in the management of critically ill individuals undergoing extracorporeal circulation. Advancements in patient monitoring capabilities have successfully provided near “real-time” indications of patient status under these conditions.
ECMO is a dynamic extracorporeal therapy that requires prompt response and intervention under quickly changing clinical conditions. Optimum patient monitoring during ECMO, therefore, would include the ability to continuously assess blood gas values without the need for excessive laboratory sampling.
The CDI 500 is a commonly used device used during CPB procedures to continually monitor blood gases, potassium, acid/base status, hemoglobin, and hematocrit. This technology has been proven to be effective tool in the management of intraoperative blood gases during CPB (2–4); however, its use during ECMO has not been widely documented. In this study, we attempted to evaluate the performance of the CDI 500 in a simulated ECMO circuit to better understand its ability to be successfully used as a monitoring tool.
The results of our study indicate that the CDI was at least as capable as the Opticath device in predicting venous saturations during simulated ECMO. Data analysis revealed an insignificant statistical difference between each device and control values (CDI vs. AVL, p = .16; Opticath vs. AVL, p = .92). Analysis of correlation between control values and test devices revealed the superiority of the CDI 500 device in predicting actual venous saturations. The correlation coefficient for the CDI device at 3 days was r = .88, whereas for the Opticath device was r = .77. The long-term effectiveness of the CDI 500 device in predicting venous saturation was apparent, with an overall correlation value of r = .84 at 1 week of testing. Testing of the Opticath device showed a relatively poor correlation of r = .55 during the same period of time. A least squares regression analysis indicated the magnitude of the error of each test device. Error in each device increased with respect to time, although the magnitude of the “drift” was much smaller in the CDI 500 device at 1 week of testing (CDI error = 8.37, Opticath error = 14.78). Possible explanations for the “drift” in each device may be related to platelet or other cellular debris collecting on the surfaces of the optical components of the devices. It is reasonable to assume that some platelet deposition occurred early in device evaluations, but it is unlikely, given the circuit design, that platelets were preserved to any extent. Platelet viability studies were not performed in this investigation. The addition of fresh whole porcine blood during the experimental phase may have additionally contributed to device error trends. Detailed examination of the sensor apparatus for platelet deposition was beyond the scope of this study, but gross examination provided no basis for a contributory conclusion. Of note, the sensor head of the CDI 500 device is treated with heparin, which may impart additional protection from platelet adhesion.
Our evaluation of the CDI 500 device exceeded the recommended operational limits suggested by the manufacturer in an effort to assess long-term accuracy of the device. As such, it is subject to “off-label” interpretation of the results. Manufacturer-suggested inaccuracies are caused by a myriad of potential causes such as inadequate flow through the sensor head, clots at the sensor, and exposure to freezing temperatures. In our experimental protocol, we controlled for these variables to the extent possible by assuring adequate blood flow through the shunt, supplementing the anticoagulation of the circuit blood, maintaining normothermic conditions, and verifying that the sensor was not exposed to an incongruent environment before use.
The advantages of using the CDI 500 device in monitoring venous saturation during simulated ECMO are apparent in this study.
The ability of this device to provide venous blood gases, in addition to potassium, bicarbonate, and saturation, offers the clinical specialist the opportunity to continuously monitor these parameters during critical periods of extra-corporeal circulation. Correlation of the CDI 500 with known standards over a period of 1 week revealed an overall value of r = .84. Evaluation of a more commonly used ECMO device, the Oximetric Opticath, revealed a much poorer overall correlation with actual saturations (r = .55). In addition, the ability to incorporate or replace the sensor at any time provides the operator the ability and flexibility to obtain a more accurate clinical patient profile. Based on our experimental data, we believe the CDI 500 Blood Parameter Monitoring System is an effective and reliable indicator of venous blood saturation in a simulated ECMO environment.
REFERENCES
- 1.Scuderi PE, MacGregor DA, Bowton DL, James RL.. A laboratory comparison of three pulmonary artery oximetry catheters. Anesthesiology. 1994;81:245–53. [DOI] [PubMed] [Google Scholar]
- 2.Fried DW, Leo JJ, Mattioni GJ, et al. CDI Blood Parameter Monitoring System 500Ua new tool for the clinical perfusionist. J Extra Corpor Technol. 2000;32:25–30. [PubMed] [Google Scholar]
- 3.Southworth R, Sutton R, Mize S, et al. Clinical evaluation of a new in-line continuous blood gas monitor. J Extra Corpor Technol. 1998;4:166–70. [PubMed] [Google Scholar]
- 4.Trowbridge CC, Vasquez M, Stammers AH, et al. The effects of continuous blood gas monitoring during cardiopulmonary bypass: A prospective, randomized study—part I. J Extra Corpor Technol. 2000;32:120–8. [PubMed] [Google Scholar]
- 5.Council of Europe. Guide to the Preparation. Use and Quality Assurance of Blood Components. 10th ed. Strasbourg, France: Council of Europe Publishing. [Google Scholar]