Abstract:
Autologous thrombin can be produced by activating the patient’s own plasma. By adding calcium chloride (CaCl2) to the anticoagulated plasma, the coagulation cascade will be initiated, and active thrombin will be produced. However, thrombin obtained by this method degrades very quickly and is not practical for use during surgery. The aim of this study was to investigate the stability of the thrombin produced using the thrombin processing device (TPD; Thermogenesis Corporation). The TPD consists of a tubular chamber containing a negatively charged surface for activation. Plasma (11 ml) and reagent (CaCl2 and ethanol, 3.75 ml) were added to the TPD, and active thrombin was harvested after a 20-minute incubation. The production of thrombin was done at 18°C (64°F), 24°C (75°F), and 27°C (81°F) (n = 4/group). The produced thrombin was stored at the production temperature, 4°C (39°F), and 35°C (95°F). The thrombin activity was assessed by time to clot formation, using a fibrinogen concentrate as substrate, after 2, 4, and 6 hours of storage. Thrombin produced at 18°C had clot times of less than 5 seconds for 2 hours (4.42 ± 1.3 seconds) when stored at 4°C, but 4 hours (4.1 ± 1.3 seconds) when stored at 35°C. In contrast, when thrombin was produced at 24°C, the clot times were 4.3 ± 0.7 and 4.6 ± 1.6 seconds at 4°C and 35°C, respectively, for up to 6 hours. Similar results were obtained for thrombin produced at 27°C. Active thrombin produced by the TPD is dependent on both the production temperature and the storage temperature. Autologous human thrombin with a stability of up to 6 hours can be obtained using the TPD when produced at 24°C or 27°C and stored at 4°C.
Keywords: human thrombin, stability, fibrinogen, clot, plasma
Hemostasis, the ability of the body to control the flow of blood after injury, is critical for the survival of the host. It is initiated when the protein tissue factor (TF) found on the surface of cells not normally in contact with the blood is exposed to the blood flow. This starts a cascade of signaling with TF-activating factor VII. This in turn activates factor X, which eventually leads to clot formation. Ultimately, thrombin is produced (1).
Thrombin has been used in surgery as a means of reducing bleeding for decades. Already in 1977, it was reported that patients treated with topical thrombin after abdominal surgery had a decreased incidence of hematomas compared with a control group not receiving treatment with thrombin (2). This finding was confirmed by other groups in a variety of surgical disciplines (3–5). The combination of thrombin with concentrated fibrinogen (i.e., cryoprecipitate) and platelets is also common clinical practice to prevent bleeding (6) and enhance wound healing (7). For example, fibrin sealants (thrombin combined with fibrinogen) are used in a variety of surgical procedures and have been shown to reduce bleeding, seroma formation, and drain use (8). In addition, thrombin used in combination with platelets is mainly used in orthopedic, oral, and maxillofacial surgery for enhancement of bone growth caused by the increased level of tissue growth factors associated with platelets (9,10).
Thrombin used in surgery has, to date, primarily been derived from bovine origin. There are major concerns with the use of bovine products. For instance, it has been shown that the use of bovine thrombin can cause antibody formation. These antibodies can cross-react with the patient’s coagulation factor V (FV) and cause a range of symptoms that may lead to adverse reactions, including severe and life-threatening bleeding (11,12). Another concern is possible exposure to bovine-derived prions causing variant Creutzfeldt-Jacob disease (vCJD) in humans (13). Thus, the ability to produce a human, preferably autologous, thrombin for use in surgery is highly desirable.
Commercially available thrombin is typically produced from a pool of citrate-anticoagulated plasma by Cohn-Oncley fractionation (14). This is typically done by a large plasma fractionation plant, pooling thousands of units of plasma from different donors. The active thrombin is produced from purified prothrombin. Several methods have been developed where purified prothrombin is used as raw material, using different coagulation proteins (15,16) or snake venom as activators (17–20). These methods are mostly suited for industrial production of thrombin from large pools of plasma and normally not for production of single donor thrombin.
The easiest way to initiate thrombin production is to add calcium ions to citrated plasma. The surplus calcium will allow the clotting cascade to initiate and thrombin to be produced. The disadvantage with this procedure is that the time of stability of the thrombin activity in the produced thrombin is short, and the activity is typically decreased to nonfunctionality within 20 minutes because of the inhibition of the coagulation cascade by protein S, protein C, and antithrombin III (1). To circumvent these limitations, a stable thrombin product can be produced when the inhibitory enzymes are inactivated.
We have developed a simple method to produce a stable human thrombin from single donor plasma. To concentrate and activate thrombin, a mixture of calcium chloride and ethanol is added to citrate anticoagulated plasma in the presence of a negatively charged surface. The negative surface charge initiates the formation of the prothrombin-FV-FXa complex, whereas the mixture of calcium chloride and ethanol (the thrombin reagent) provides the chemical constituents needed to inactivate the inhibitors of thrombin and stabilize the thrombin so that it can be used hours after production.
The aim of this study was to characterize the thrombin produced using the thrombin processing device (TPD), both for activity and stability. The thrombin and these two parameters were studied at different production temperatures and different storage temperatures, because the ambient temperature in operating rooms can vary greatly. The in vitro efficacy was assessed using both a quantitative method (i.e., thrombin activity) and a qualitative method (i.e., the ability and time of the produced thrombin to form a clot).
MATERIALS AND METHODS
Preparation of Platelet-Poor Plasma
Fresh frozen plasma (FFP) was obtained from San Bernadino Blood Bank (San Bernadino, CA) and South Florida Blood Bank (St. Petersburg, FL). The plasma was obtained from citrated whole blood donated by healthy volunteers and processed according to local established procedures for the collection and processing of human blood products. Each unit was thawed in a plasma thawer (MT-202; Thermogenesis, Rancho Cordova, CA) within 1 hour of initiation of the experiment.
Thrombin Processing Device
The TPD (Thermogenesis Corporation, Rancho Cordova, CA) consists of two parts: a reaction chamber containing a negative surface charge required for initiation of the formation of thrombin and a reagent consisting of calcium chloride and ethanol (final concentration 7.2 mM and 19%, respectively; Thermogenesis; Figure 1).
Figure 1.
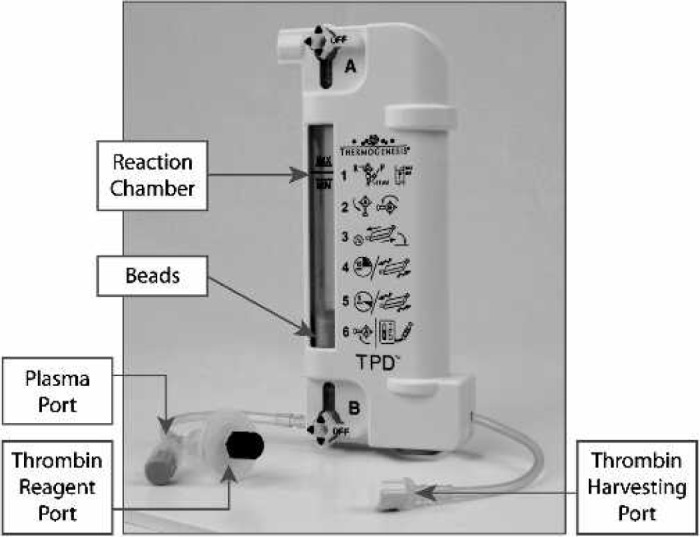
Description of TPD.
Preparation of Thrombin
Eleven milliliters of FFP and 4 ml thrombin reagent were added to the TPD reaction chamber. The contents were mixed and incubated at room temperature for 15 minutes. The TPD was agitated to break any formed fibrinogen clots and incubated for an additional 5 minutes. After the elapsed time, the TPD was shaken vigorously to dislodge the gel. The syringe was attached to the thrombin collection port, and activated serum was collected. The produced thrombin was harvested, and if not analyzed immediately, aliquoted in 3-ml samples and frozen at −80°C until analysis.
To establish the intravariability of the TPD System, the same plasma was used with 10 different TPD disposables. The intervariability was assessed by determining the thrombin activity in 25 units of thrombin produced from different plasma units using TPD disposables from the same production lot. To study the influence of the ambient temperature on the production of thrombin, TPD thrombin was prepared at 18°C (64°F), 24°C (75°F), and 27°C (81°F).
Thrombin Activity and Quantitative Assessment of Thrombin
Thrombin activity was analyzed using the modified Clauss method (21). Briefly, 200 μl of a 2.0 mg/ml pre-warmed (37°C) solution of fibrinogen (catalog F-4883; Sigma, St. Louis, MO) solution was added to 100 μl of thrombin sample. The time required for clot formation was recorded using a fibrometer (FibroSystem; Becton-Dickinson, Franklin Lakes, NJ). The thrombin activity in the samples was determined by correlating the time to clot formation to a standard curve generated with titrations of thrombin (catalog 50,502; Biopool US, Inc., Ventura, CA). Because each time-point on the standard curve corresponds to the thrombin activity needed to clot the standard concentration of fibrinogen, the thrombin activity in an unknown sample can be extrapolated from the time to clot formation.
Clot Time and Qualitative Assessment of Thrombin
Equal volumes (150 μl) of TPD-produced thrombin and a cryoprecipitate (fibrogen concentration range, 25–40 mg/ml) were warmed in a 35°C waterbath for 1 minute. The thrombin and the cryoprecipitate were mixed, and the time to clot formation was assessed. To establish the relationship between clot time and thrombin activity, both analyses were performed on 380 samples, and a correlation was established.
Assessment of Stability
The stability of the TPD-produced thrombin was assessed by storing 3 ml of thrombin either at (1) the production temperature (i.e., 18°C, 24°C, or 27°C), (2) 4°C, or (3) 35°C. Thrombin activity and clot times were assessed at time 0 (after preparation) and after 2, 4, and 6 hours of storage.
Statistical Analysis
All results are presented as mean and SD. Coefficient of variation (CV) was calculated for the intra- and intervariability. Significance between groups was assessed using the Student t test or the Wilcoxon signed rank test, where appropriate.
RESULTS
Intra- and Interreproducibility
Using plasma from the same unit and 10 different TPD disposables (intrareproducibility), the thrombin activity was 73 ± 5 IU/ml, with a CV of 7%. The interreproducibility was established from 25 different plasma units, using TPD disposables from the same lot. The thrombin activity was found to be 93 ± 21 IU/ml (range, 55–147 IU/ml), with a CV of 23%.
Establishment of the Correlation Between Thrombin Activity and Clot Time
The thrombin activity and clot times were analyzed in 380 samples (Figure 2). There was no significant difference between the clot times in units with a thrombin activity of 25–35 IU/ml compared with units with a thrombin activity of 75–147 IU/ml (3.4 ± 1.0 seconds, n = 64 and 3.3 ± 0.6 seconds, n = 30, respectively; no significant difference). Units with a thrombin activity of 0–25 IU/ml had significantly longer clot times compared with units with a thrombin activity of 25–35 IU/ml (7.9 ± 5.8 seconds, n = 29 and 3.4 ± 1.0 seconds, n = 64, respectively; p < .0001). The total population (n = 351) with a thrombin activity of >25 IU/ml had 2.9 ± 0.6 seconds in manual clot time.
Figure 2.
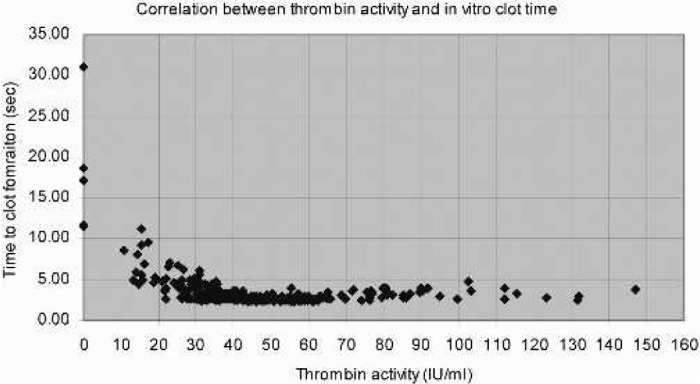
Correlation between manual clot times and thrombin activity in human thrombin produced by the TPD system (n = 380).
If a manual clot time of 5 seconds is used as the cut-off, it will predict a thrombin activity of >25 IU/ml in 97% of the cases (sensitivity, i.e., the probability that the sample really has a thrombin activity of >25 IU/ml). A clot time of >5 seconds will predict a thrombin activity of <25 IU/ml in 81% of the cases (specificity, i.e., the probability that the sample really has a thrombin activity of <25 IU/ml; Table 1).
Table 1.
Relation between clot times and thrombin activity in thrombin produced by the TPD system using a cut-off clot time of 5 seconds and a cut-off thrombin activity of 25 IU/ml.
| Thrombin Activity | Clot Time |
Total Number of Units | |
|---|---|---|---|
| <5 seconds | >5 seconds | ||
| >25 IU/ml | 347 | 4 | 351 |
| <25 IU/ml | 12 | 17 | 29 |
| Total number of units | 359 | 21 | 380 |
Quality of TPD-Produced Thrombin at Different Temperatures
Because the ambient temperature in the operating room, where the TPD thrombin would be produced, can vary, we assessed the quality of the thrombin at 18°C, 24°C, and 27°C (corresponding to 64°F, 75°F, and 81°F, respectively). Within 10 minutes of production, there was no significant difference between the thrombin activity in the thrombin produced at 24°C and 27°C (75 ± 16 and 80 ± 17 IU/ml, respectively). However, thrombin produced at 18°C had significantly lower thrombin activity (61±9IU/ml, p < .03) compared with thrombin produced at either 24°C or 27°C. In no case was the thrombin activity <25 IU/ml or the clot time >5 seconds (Table 2).
Table 2.
Thrombin activity and clot times, expressed as mean ± SD, for thrombin produced at 18°, 24°, and 27°C (n = 9/group).
| Test | Thrombin Produced at |
||
|---|---|---|---|
| 18°C | 24°C | 27°C | |
| Thrombin activity (IU/ml) | 61 ± 8 | 75 ± 16 | 80 ± 17 |
| Clot time (sec) | 3 ± 0.4 | 3 ± 0.3 | 3 ± 0.4 |
Stability of TPD-Produced Thrombin
The stability of the TPD-produced thrombin was assessed by thrombin activity and clot time at 2, 4, and 6 hours after production. The stability was determined at the production temperature (18°C, 24°C, and 27°C), 4°C, and 35°C.
Stability of TPD-Produced Thrombin at 18°C
In contrast, thrombin produced at a lower ambient temperature (18°C) did not retain its thrombin activity or its ability to clot fibrinogen as well as thrombin produced at higher ambient temperatures. After 2-hour storage at 18°C, six of nine units had a clot time of >5 seconds, and the thrombin activity decreased to 24 ± 15 IU/ml. After 4 hours of storage at 4°C and 35°C, we found two and zero of nine units with a clot time of >5 seconds, respectively (Figure 3A).
Figure 3.
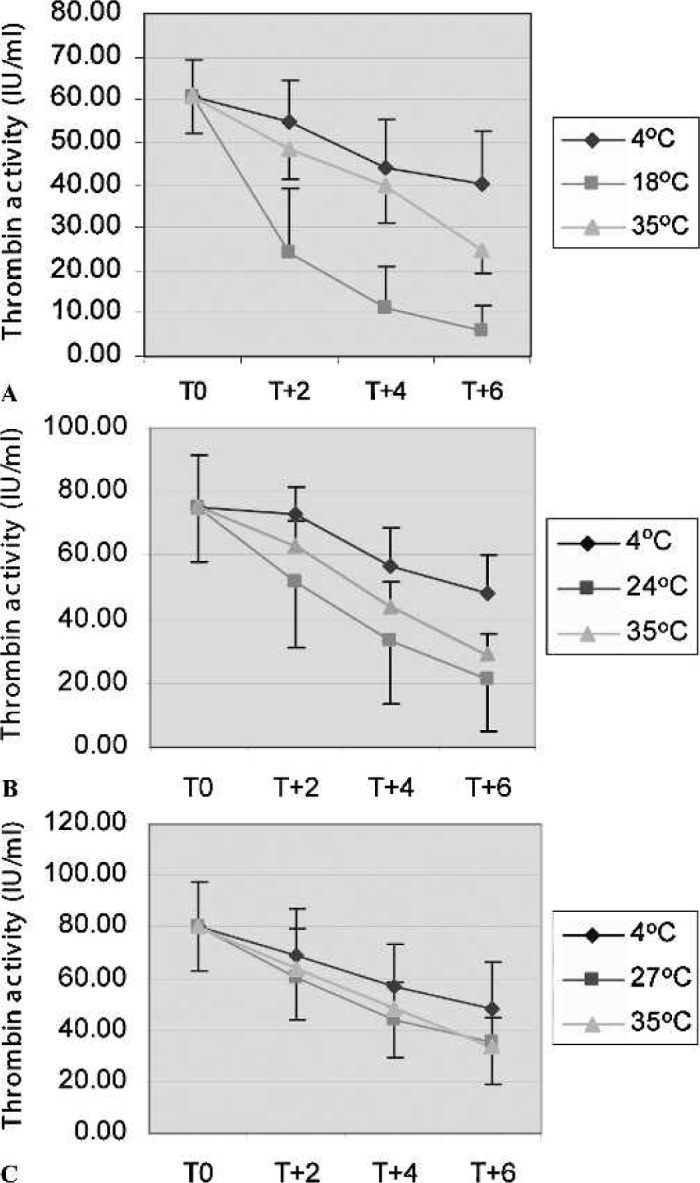
The stability of thrombin activity in thrombin produced by the TPD system. Thrombin was produced at 18°C (A), 24°C (B), or 27°C (C) and stored for 2, 4, and 6 hours at different temperatures. ♦, storage at 4°C; ▪, storage at the production temperature (i.e., 18°C, 24°C, or 27°C); ▾, storage at 35°C (n = 9/group).
Stability of TPD-Produced Thrombin at 24°C
When thrombin was prepared at normal ambient temperature (24°C) and stored at that temperature, the thrombin activity declined to 33 ± 19 IU/ml after 4 hours, and we found four of nine units had a clot time >5 seconds. However, storing the same thrombin at 4°C, the decrease in the thrombin activity was not as pronounced as storing at 24°C (48 ± 13 and 21 ± 16 IU/ml, respectively, after 6 hours), and all units had an acceptable clot time (<5 seconds after 6 hours). After 6-hour storage at 35°C, two of nine units had a clot time >5 seconds, and the thrombin activity decreased to 29 ± 6 IU/ml. Thus, the data showed that thrombin prepared at normal ambient temperature can be stored up to 4 hours at both 24°C and 35°C and still maintain its effectiveness. In addition, it can be stored for up to 6 hours at 4°C (Figure 3A).
Stability of TPD-Produced Thrombin at 27°C
Producing thrombin at a higher ambient temperature, 27°C, preserved the thrombin activity for a longer period of time. After 6-hour storage at 27°C, the thrombin activity decreased to 35 ± 16 IU/ml, but only two of nine units had clot times >5 seconds (6 and 7 seconds). Storing the same thrombin at 4°C and 35°C, we found zero and one of nine units, respectively, with a clot time of >5 seconds after 6-hour storage (Figure 3C).
DISCUSSION
Thrombin in surgery is commonly used in a variety of situations (3–10). The majority of the thrombin used today is of bovine origin, causing concern about adverse reactions (e.g., antibody formation against human FV leading to bleeding episodes (11,12), and transmission of bovine prions possibly causing vCJD (13)). To generate thrombin from a human source, preferably autologous (i.e., from the patient’s own blood), is thus highly advantageous. In addition, maintaining the stability of the produced thrombin is critical for delivery of an efficacious product.
In this study, we evaluated the stability of thrombin produced from human plasma. The plasma source we used was blood bank–produced FFP (plasma frozen within 8 hours of collection). In surgery, the plasma will be taken directly from the patient; thus, the thrombin will be produced from plasma that never was frozen. When prepared according to American Association of Blood Banks standards, FFP retains approximately 90% of all the clotting factors present in fresh blood (22). The freezing and thawing of the plasma does not seem to affect the levels significantly (22). Tests done previous to this study showed that there was no significant difference in the thrombin activity in thrombin produced by the TPD using fresh plasma compared with FFP (122.3 ± 6.0 vs. 121.3 ± 7.9 IU/ml).
The level of thrombin activity in the thrombin to be used either by itself or in combination with a cryoprecipitate or platelets is important for the speed of clot formation (23). For most preparations, bovine thrombin is diluted to 500 and 1000 IU/ml. The thrombin produced in this study ranged in activity from 55 to 147 IU/ml, with a mean of 93 IU/ml. This thrombin activity should be adequate for use in most clinical applications. In addition, we previously showed that TPD-produced thrombin can activate platelets to release α-granule content, and consequently growth factors, comparably with commercially available thrombin (24).
When 10 units of TPD thrombin were produced from the same unit of plasma, the CV was 7%, indicating the ability of the TPD system to produce thrombin with consistent thrombin activity. Furthermore, when plasma from different donors was used, the CV increased to 23%. Thus, there is variability between donors/plasma in the ability to produce active thrombin using the TPD system. This would be expected because availability of prothrombin and other coagulation factors needed for formation of the prothrombinase complex can vary between donor plasma. The results suggest that the TPD is able to produce a relatively consistent thrombin with acceptable activity.
Using the manual clot time as a qualitative measure of the efficacy of thrombin, we found that there was no difference between the clot time in units with a thrombin activity of 25–35 IU/ml and units with a thrombin activity of >75 IU/ml. One limit to the manual clot time is that it is not possible to accurately measure times to clot formation that are <2.5 seconds. In most cases, a clot time of <5 seconds was equal to instant clotting, because of the manual variability of the test. Thus, it would not be possible to differentiate between a thrombin activity of 25 and 500 IU/ml using this method. Because the manual clot time indicates the in vivo ability of the thrombin to form a clot, lower levels of thrombin activity than what is commonly used today would be sufficient for adequate clot formation. In fact, using a combination of thrombin and fibrinogen in a fibrin sealant, it has been shown that a thrombin activity of 50–100 IU/ml had a higher adhesiveness compared with using thrombin with a thrombin activity of 500 IU/ml (25).
Enzymes are typically sensitive to temperature, and the rate of activation and degradation is dependent on the specific optimal temperature for each enzyme. Our data showed that, using the TPD system, thrombin produced at 24°C and 27°C had a higher activity and retained its activity for a longer time compared with thrombin produced at a lower temperature (18°C). This observation is very important for the user. Because thrombin is an active enzyme, the user has to be aware of both the production temperature (the ambient temperature in the room) and the storage temperature for optimal efficacy of the product. For example, if the ambient temperature is 24–27°C, the TPD thrombin can be stored for up to 4 hours without any special precautions (i.e., it can be stored on the bench). If longer storage time is needed, the thrombin should be stored at 4°C or 35°C. However, if the ambient temperature is lower, as is required in certain surgical procedures, the shelf-life of the thrombin is shorter, and it should be used within 2 hours of production.
In conclusion, we have shown that the TPD system can produce active and effective thrombin at low, normal, and high ambient temperatures. However, the thrombin activity and clot times in the thrombin are dependent on both the production temperature and the storage temperature. Thrombin produced at 24°C and 27°C retained its efficacy for 6 hours when stored at 4°C and for 4 hours when stored at 35°C. Further studies need to be conducted to establish the clinical efficacy of TPD-produced thrombin.
REFERENCES
- 1.Butenas S, Mann KG.. Blood coagulation. Biochemistry (Moscow). 2002;67:5–15. [DOI] [PubMed] [Google Scholar]
- 2.Jasani B, Baxter-Smith DC, Donaldson LJ, Selvam A, Sokhi GS.. Topical thrombin and control of wound haematoma. Lancet. 1977;2:332–3. [DOI] [PubMed] [Google Scholar]
- 3.Hashemi K, Donaldson LJ, Freeman JW, Sokhi GS, Gyde OH, Smith HV.. The use of topical thrombin to reduce wound haematoma in patients receiving low-dose heparin. Curr Med Res Opin. 1981;7:458–62. [DOI] [PubMed] [Google Scholar]
- 4.Ofodile FA, Sadana MK.. The role of topical thrombin in skin grafting. J Natl Med Assoc. 1991;83:416–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Milne A, Murphy W, Reading S, Ruckley C.. Fibrin sealant reduces suture line bleeding during carotid endarterectomy: A randomized trial. Eur J Vasc Endovasc Surg. 1995;10:91–4. [DOI] [PubMed] [Google Scholar]
- 6.Rousou JA, Engelman RM, Breyer RH.. Fibrin glue: An effective hemostatic agent for nonsuturable intraoperative bleeding. Ann Thorac Surg. 1984;38:409–10. [DOI] [PubMed] [Google Scholar]
- 7.Amrani DL, Diorio JP, Delmotte Y.. Wound healing. Role of commercial fibrin sealants. Ann NY Acad Sci. 2001;936:566–79. [PubMed] [Google Scholar]
- 8.Moore M, Burak W Jr, Nelson E, et al. Fibrin sealant reduces the duration and amount of fluid drainage after axillary dissection: A randomized prospective clinical trial. J Am Coll Surg. 2001;192:591–9. [DOI] [PubMed] [Google Scholar]
- 9.Fennis JP, Stoelinga PJ, Jansen JA.. Mandibular reconstruction: A clinical and radiographic animal study on the use of autogenous scaffolds and platelet-rich plasma. Int J Oral Maxillofac Surg. 2002;31:281–6. [DOI] [PubMed] [Google Scholar]
- 10.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT.. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. [DOI] [PubMed] [Google Scholar]
- 11.Kajitani M, Ozdemir A, Aguinaga M, Jazieh AR, Flick JT, Antakli T.. Severe hemorrhagic complication due to acquired factor V inhibitor after single exposure to bovine thrombin product. J Card Surg. 2000;15:378–82. [DOI] [PubMed] [Google Scholar]
- 12.Ortel TL, Mercer MC, Thames EH, Moore KD, Lawson JH.. Immunological impact and clinical outcomes after surgical exposure to bovine thrombin. Ann Surg. 2001;233:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beghi E, Gandolfo C, Ferrarese C, et al. Bovine spongiform encephalopathy and Creutzfeldt-Jacob disease: fact and uncertainties underlying the causal link between animal and human disease. Neurol Sci. 2004;25:122–9. [DOI] [PubMed] [Google Scholar]
- 14.Cohn E, Oncley J, Strong L, Hughes W Jr, Armstrong H Jr.. Chemical, clinical, and immunological studies on the products of human plasma fractionation I. The characterization of the protein fractions of human plasma. Clin Invest. 1944;23:417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton J II, Fasco M, Stackrow A.. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977;252:3587–98. [PubMed] [Google Scholar]
- 16.Seegers W.. Activation of purified prothrombin. Proc Soc Exp Biol Med. 1949;72:677–80. [DOI] [PubMed] [Google Scholar]
- 17.Denson K.. Coagulant and anticoagulant action of snake venoms. Toxicon. 1969;7:5–11. [DOI] [PubMed] [Google Scholar]
- 18.Masci P, Whitaker A, DeJersy J.. Purification and characterization of a prothrombin activator from venom of the Australian brown snake, Pseudonaja textilis. Biochem Int. 1988;17:825–35. [PubMed] [Google Scholar]
- 19.Rosing J, Tans G.. Structural and functional properties of snake venom prothrombin activators. Toxicon. 1992;30:1515–27. [DOI] [PubMed] [Google Scholar]
- 20.Stocker K, Hauer H, Muller C, Triplett DA.. Isolation and characterization of Textarin, a prothrombin activator from eastern brown snake (Pseudonaja textilis) venom. Toxicon. 1994;32:1227–36. [DOI] [PubMed] [Google Scholar]
- 21.Clauss A.. Gerinnungsphzysiologische schnellmethode zur bestimmung des fibrinogens. Acta Haematol. 1957;17:237–46. [DOI] [PubMed] [Google Scholar]
- 22.Smith J, Ness P, Moroff G, Luban N.. Retention of coagulation factors in plasma frozen after extended holding at 1–6 degrees C. Vox Sang. 2000;78:28–30. [DOI] [PubMed] [Google Scholar]
- 23.Alving B, Weinstein M, Finlayson J, Menitove J, Fratantoni J.. Fibrin sealant: Summary of a conference on characteristics and clinical uses. Transfusion. 1995;35:783–90. [DOI] [PubMed] [Google Scholar]
- 24.Semple E, Madsen T, Semple J.. Quality of thrombin produced from the patient’s own plasma using the TPD@, a new thrombin processing device. J Extra Corpor Technol. 2005;37:196–200. [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida H, Hirozane K, Kamiya A.. Adhesive strength of autologous fibrin glue. Biol Pharm Bull. 2000;23:313–7. [DOI] [PubMed] [Google Scholar]


