Abstract:
Isolated limb perfusion with the administration of cytotoxic drugs has been successfully used to treat melanomas of the extremity since it was first introduced in 1958. The use of hyperthermia (40°C) combined with chemotherapy agents, primarily melphalan, has resulted in greater cytotoxicity in laboratory studies, which led to the application of hyperthermia in clinical studies during the 1960s. The effectiveness of this regional technique and the absence of any good systemic therapy made hyperthermic-isolated limb perfusion (HILP) the main treatment for patients with regionally advanced melanoma. HILP involves open surgical dissection and cannulation of the peripheral vessels and is associated with moderate morbidity rates. Blood transfusions, systemic drug leak, infection, and damage to the blood vessels and nerves are all potential hazards associated with this technique. Recently, however, there has been increased interest in an alternative technique termed isolated limb infusion (ILI), which was first reported in 1994 from the Sydney Melanoma Unit in Australia. Based on a few single institution experiences, it was found that there are fewer morbidities associated with HILP than with ILI but no compromise in patient outcomes. ILI is a less invasive procedure involving the use of angiographically placed catheters inserted percutaneously through the femoral vessels that does not require blood donor exposure or use of a heart lung machine. Preliminary data suggest that the resultant local hypoxia and acidosis induced by this procedure potentiates the cytotoxic effects of melphalan. Response rates comparing ILI to HILP seem similar, and both are markedly better than systemic chemotherapy. ILI may be a more desirable option because morbidity is greatly reduced and outcomes appear similar. There is a potential role for the perfusionist in the application of ILI, an evolving area of cancer therapy.
Keywords: isolated limb infusion, chemotherapy, melanoma, hyperthermic-isolated limb perfusion, melphalan
Isolated limb infusion (ILI) was first reported in 1994 by Thompson et al. (1) at the Sydney Melanoma Unit (SMU) in Sydney Australia. This procedure was developed as an alternative to hyperthermic isolated limb perfusion (HILP) for recurrent or advanced malignant melanoma (MM) of the extremities. ILI features the use of percutaneously placed angiographic catheters, under fluoroscopy, through the femoral artery and vein. A very low flow per-fusion system is established through the use of a syringe attached to one of two large bore stopcocks, a cardioplegia heat exchanger, and connecting small bore tubing. The technique eliminates the need for an oxygenator and heart lung machine, which is required for HILP. The delivery of a melphalan (l-phenylalanine mustard)/dactinomycin (actinomycin-D) combination, the chemotherapeutic agents of choice, are delivered through a syringe attached to a large bore three-way stopcock. Melphalan is an alkylating agent historically used to treat MM of the extremity, and dactinomycin is an antibiotic used for its antineoplastic properties in the treatment of various malignant neoplasms (2).
The objective of regionally delivered chemotherapeutic agents, with the aid of a tourniquet, is to provide a high concentration of drug to the affected limb while avoiding systemic side effects such as bone marrow suppression. The technique of ILI is technically less complex and, based on single institution studies, seems to have the same efficacy as HILP in treating patients with limb melanoma with significantly less morbidity (2). Currently, the technique of ILI is under evaluation in larger, multi-institutional studies. This article will discuss the technique used for ILI at Duke University Medical Center.
MATERIALS AND METHODS
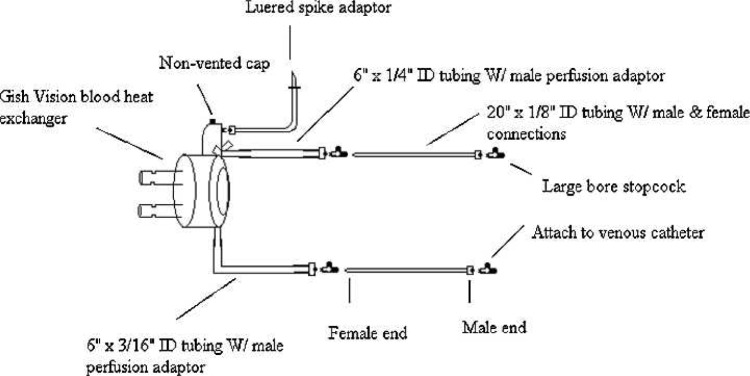
The circuit (Figure 1) consists of a standard cardioplegia heat exchanger (Gish Vision; Gish Biomedical, Rancho Santa Margarita, CA), modified by attaching a ¼-in piece of tubing to the inlet and a 3/16-in piece of tubing to the outlet. Attached to both ends of the shortened tubing are ¼-in male perfusion adapters (Medtronic, Minneapolis, MN) large bore stopcocks, 20-in luered large durometer infusion tubing (Arrow International, Reading, PA), and another set of large bore stopcocks on each end (Medex, Dublin, OH).
Figure 1.
Diagram of isolated limb infusion circuit. ID, inner diameter.
A luered spike adapter, for washout, is attached to the top of the bubble trap of the cardioplegia heat exchanger in the place of the luered, one-way, pressure relief valve. Additionally, a nonvented cap replaces a vented cap on top of the bubble trap to seal the unit. A portable heater/cooler (BioCal; Medtronic) is used to warm the circuit and limb throughout the procedure. A temperature monitoring box (SIMS RSP, Irvine, CA) and two temperature probes (SIMS RSP) are used to monitor medial and lateral extremity temperatures.
The circuit is assembled using aseptic technique and gravity primed with approximately 80 ml of warmed Normosol-R (NR; Abbot Laboratories, Chicago, IL) using a Y-type blood administration set with bulb pump (Abbot Laboratories, Chicago, IL). The cardioplegia set is placed in its bracket and mounted to an intravenous pole. The heater is attached to the heat exchanger and set to 42°C.
The patient may be placed under light, general anesthesia, and a 6-Fr arterial and a 6- or 8-Fr venous catheter placed percutaneously through the femoral vessels and advanced to the affected limb. If a lower extremity is being treated, the catheter should be placed contralaterally to the affected lower limb to prevent interference from the proximally placed tourniquet. Once the catheters are in the proper position and confirmed by fluoroscopy in vascular radiology, the infusion procedure may begin. The surgeon inserts temperature probes to the medial and lateral side of the extremity, and the probes are connected to a temperature monitor. To assist with warming, the extremity is wrapped in a water-based warming blanket (Cincinnati Sub-Zero; Blanketrol II, Cincinnati, OH). A bolus of 5000 units of heparin should be given on catheter insertion, and the patient is systemically heparinized with 200 units/kg of porcine heparin just before starting the infusion technique (Baxter Healthcare Corp., Deerfield, IL). Before commencement of the infusion, an activated clotting time (Actalyke MAX-ACT; Helena Laboratories, Beaumont, TX) greater than 300 seconds should be achieved.
Once the patient is properly positioned, a pneumatic tourniquet is placed proximally on the affected limb but is not yet inflated. The catheters are joined to the ILI circuit, taking care to ensure air-free and sterile connections. The surgeon begins recirculating the blood through the limb using a 20-ml syringe by continuously aspirating from the venous catheter, manipulating the stopcock, and diverting the blood through the heat exchanger into the arterial catheter. This also confirms that the catheters are patent and the circuit is leak-free. Once the limb temperature reaches approximately 36°C, the chemotherapeutic agents are ordered, and the pneumatic tourniquet is inflated. The process of inflating the tourniquet is timed so that the limb temperature is at 37°C or higher when the chemotherapeutic agents are ready. This timing of tourniquet inflation also minimizes limb ischemic time. If the disease does not extend to the hand or foot, they may be isolated from the limb with Esmarch or a second pneumatic tourniquet to prevent unnecessary toxicity (2,3).
The melphalan dose for ILI is 7.5 mg/L of limb volume for the lower extremity (maximum dose < 55 mg) and 10 mg/L of limb volume for the upper extremity. The dactinomycin dose for ILI is 75 μg/L of limb volume for the lower extremity and 100 μg/L of limb volume for the upper extremity. Once the limb temperature is greater than 37.5°C, the premixed drug combination (mixed in 400 ml normal saline for the lower limb or 300 ml normal saline for the upper limb) is injected into the venous side stopcock through a blood administration set. The stopcock on the venous catheter is closed to the patient, which directs the drug to pass through the heat exchanger, warming it, so that the limb temperature does not drop, and ensuring that the full amount of the drug mix is delivered. When the entire dose of melphalan has been delivered, the time is noted so that it can be recirculated within the patient for 30 minutes. Using a 20-ml syringe attached to the venous side stopcock, blood is continuously aspirated from the venous limb and directed to the arterial limb of the circuit, passing through the heat exchanger. Blood gases are drawn at 25 and 30 minutes to determine the degree of hypoxia and acidosis. Adequacy of the tourniquet may be determined by assessing the pO2 of these blood gasses. If the blood is hypoxic, it is confirmed that there was no leaking across this tourniquet. After 30 minutes, NR is used to wash the chemotherapeutic drug out of the extremity. To accomplish this, the arterial side stopcock is turned off to the circuit, and the remaining NR that was used for priming is attached to the arterial side stop-cock through the fluid administration set with a manual pump. The spiked port with an empty bag attached at the top of the cardioplegia heat exchanger is opened to collect the effluent. A 20-ml syringe attached the venous stopcock is used to aspirate the toxic blood from the limb and flush it into the empty effluent bag attached to the luered spike at the top of the bubble trap. This is done until approximately1Lof Normosol-R is delivered or until the surgeon is satisfied with the washout. On completion of the washout, the tourniquets are removed from the extremity. The circuit is disposed of using standard procedures for biohazardous material and taking care to avoid blood spills.
The catheters are removed, and direct pressure is applied for 30 minutes. Protamine is given, if indicated, and the patient is placed on bedrest with the limb elevated for 24 hours. Subcutaneous heparin and aspirin, for deep vein thrombosis prophylaxis, are continued until the patient is ambulating. Aspirin therapy may be continued for several months.
RESULTS AND DISCUSSION
Isolated limb infusion, reported in 1994 by Thompson et al. (1), has only recently gained acceptance at cancer centers in the United States. Before the introduction of HILP and ILI, the only viable treatment option available for patients with MM of an extremity was amputation (4). In patients treated with HILP, complete tumor remission has been reported to be approximately 40%–50%, and partial remission in 35%–40% (2,5). However, HILP is a very demanding, elaborate, and expensive procedure using an abundance of staff and operating room time. ILI seems to have a similar efficacy, based on a few single center experiences, to HILP while incurring less morbidity (2). Another advantage of the ILI technique is that no banked blood products are required versus the routine use of blood products for HILP (2). The minimally invasive nature of this technique also limits the amount of time, equipment, and staffing needed compared to an HILP procedure.
For the perfusionist, ILI is a very simple technique to perform. ILI uses disposable equipment that is commonly stocked in cardiac operating rooms. Our primary modification of the circuit described by Thompson et al. (1) is the use of an efficient cardioplegia heat exchanger instead of a coil placed in a water bath. Our choice to use the Gish Vision heat exchanger was based on its heparin coating, our familiarity with the product, and its availability at our institution.
ILI requires light anesthesia and has been reported to be better tolerated than HILP in elderly or frail patients (1,2,6). Although general anesthesia is used during ILI, the patients receive less anesthesia for a shorter time than conventional HILP. The operative techniques, including a pneumatic tourniquet technique, used for ILI are generally less extensive and less traumatic than those used for HILP. Despite this, ILI has a very rare incidence of toxic drug leakage into the systemic circulation (2). Because of very low flows and pressures, there is no need to assess systemic leaks from the treated extremity into the systemic circulation.
Because of the complexity of the HILP procedure, it is generally performed only once. Many patients may benefit from several courses of regional chemotherapy, particularly if concentrated within predetermined intervals. These intervals can be within weeks of the previous treatment (2). This type of strategy is advantageous, and the less traumatic, minimally invasive nature of ILI allows for multiple treatments. If multiple exposures can be replicated in a relatively short amount of time, the more resistant tumor cells may be more susceptible to the chemotherapeutic agents (2).
ILI, because it isolates the limb and does not use an oxygenator, creates a progressively hypoxic and acidotic environment at the time the chemotherapeutic drugs are infused. The blood gases drawn at 25 and 30 minutes of melphalan recirculation help assess the level of hypoxia and acidosis. Data have suggested that the effects of melphalan are significantly enhanced in the setting of hypoxia and acidosis (6–8).
Originally, increased temperature (40°C) was reported to enhance the effects of melphalan (9). Lindnèr et al. (6) suggest that a subcutaneous temperature of more than 37.8°C in the limb at the conclusion of ILI is associated with an increase in survival. However, it has been suggested that the avoidance of hypothermia may be a more important factor in achieving a successful outcome than varying degrees of hyperthermia (6,10). Because of the very low flows achieved during ILI, normothermia is achieved by the use of a warming blanket wrapped around the extremity and the infusion and recirculation of melphalan through an efficient, cardioplegia heat exchanger. The temperature, hypoxia, and acidosis enhance the ability of melphalan to suppress tumors (6–8).
In conclusion, ILI is a safe, simple, economical, and effective technique for the treatment of advanced or recurrent limb melanoma. Perfusionists are the ideal professional to assist with these techniques because of their knowledge of circuitry, their attentiveness to detail, and their desire to continually improve technology and techniques that lead to better patient care. Continued efforts to refine the ILI technique need to be made to further reduce morbidity and improve its effectiveness. Multicenter clinical trials are currently underway to show the efficacy of this technique versus HILP.
REFERENCES
- 1.Thompson JF, Waugh RC, Saw RM, Kam P.. Isolated limb infusion with melphalan for recurrent limb melanoma: a simple alternative to isolated limb perfusion. Reg Cancer Treat. 1994;7:188–92. [Google Scholar]
- 2.Thompson JF, Kam PC, Waugh RC, Harman R.. Isolated limb infusion with cytotoxic agents: A simple alternative to isolated limb per-fusion. Semin Surg Oncol. 1998;14:238–47. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JF, Lai DM, Ingvar C, Kam PC.. Maximizing efficacy and minimizing toxicity in isolated limb perfusion for melanoma. Melanoma Res. 1994;4(Suppl 1):45–50. [PubMed] [Google Scholar]
- 4.Thompson JF, deWilt JF.. Isolated limb perfusion in the management of patients with recurrent limb melanoma: an important but limited role. Ann Surg Oncol. 2001;8:564–5. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JF, Hunt JA, Shannon KF, Kam PC.. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg. 1997;132:903–7. [DOI] [PubMed] [Google Scholar]
- 6.Lindnèr P, Doubrovsky A, Kam PC, Thompson JF.. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–36. [DOI] [PubMed] [Google Scholar]
- 7.Siemann DW, Chapman M, Beikirch A.. Effects of oxygenation and pH on tumor cell response to alkylating chemotherapy. Int J Radiat Oncol Biol Phys. 1991;20:287–9. [DOI] [PubMed] [Google Scholar]
- 8.Skarsgard LD, Skwarchuk MV, Vinczan A, Kristl J, Chaplin DJ.. The cytotoxicity of melphalan and its relationship to pH, hypoxia, and drug uptake. Anticancer Res. 1995;15:219–23. [PubMed] [Google Scholar]
- 9.Cavaliere R, Ciocatto RC, Giovanella BC, et al. Selective heat sensitivity of cancer cells: Biochemical and clinical studies. Cancer. 20:1351–81. [DOI] [PubMed] [Google Scholar]
- 10.Kroon BR.. Regional isolation perfusion in melanoma of the limbs: Accomplishments, unsolved problems, future. Eur J Surg Oncol. 1998;14:101–10. [PubMed] [Google Scholar]