Abstract
Bone metastasis leads to skeletal-related events in final-stage cancer patients. The incidence of prostate and lung cancers increases yearly; these cancers readily invade the bone. Some recent studies have found that serum osteoprotegerin (OPG) levels may be altered in patients with bone metastasis, whereas other reports have produced inconsistent findings. Hence, we conducted a meta-analysis to examine the effects of lung and prostate cancer on serum OPG levels. A systematic literature search was conducted using PubMed, Medline, and CNKI to identify relevant studies. A total of 11 studies were included. The standardized mean difference (SMD) and 95% confidence interval (95% CI) of the bone metastasis (BM) group, the non-bone metastasis (BM-) group and healthy controls were calculated. In prostate cancer, serum OPG levels in the BM group were higher than in the BM- group and healthy controls. Additionally, no significant difference in serum OPG levels was found between the BM- group and healthy controls. In lung cancer, serum OPG levels in the BM and BM- groups were significantly increased compared with healthy controls. However, no significant difference in serum OPG levels was found between the BM and BM- groups. Studies with larger sample sizes are required to confirm these findings.
Bone metastases are often clinically manifested by patients with advanced malignant tumours and are most common in prostate and lung cancer patients1. In advanced lung cancer, metastatic deposits in bone are common and represent a source of pain and morbidity2. It is estimated that more than 35% of patients with advanced lung cancer manifest bone metastases, and a higher proportion was found in an autopsy series3. Similarly, as the second leading cause of cancer-related deaths in men, prostate cancer (PCa) has the ability to easily invade the bone, and more than 80% of PCa patients die from the development of bone metastasis4,5.
Bone metastasis incites bony destruction and skeletal-related events (SREs), such as ostealgia, pathological fracture and hypercalcaemia6. Among patients with bone metastasis, 45%–75% experience more pain due to secondary changes of bone metastasis7. Furthermore, bone metastasis results in a shorter survival time and worse prognosis8. Hence, the early detection of bone metastasis is critical for the clinical management and accurate staging of tumours9. However, only a fraction of bone metastasis cases are clinically diagnosed10.
Currently, the detection of bone metastasis mainly depends on pathology, imaging technologies and bone scans. However, most patients cannot tolerate undergoing a biopsy due to the invasiveness of the procedure. Each type of imaging technology has its own limitations: X-ray has hysteresis; CT has intense radiation; and MRI or PET/CT has a low incremental cost-effectiveness ratio. Although high-sensitivity bone scanning is widely used, the accuracy of its results is controversial due to low specificity. Hence, finding an effective and convenient detection method to diagnose bone metastasis is necessary.
It would be exciting to find a blood index that could accurately reflect bone metastasis. Recently, several new biomarkers of bone metabolism have been identified that can reflect bone turnover at an early stage. Some markers are already being widely applied in clinical practice11. However, the application of these biomarkers in the clinical diagnosis of bone metastases is not feasible10. Osteoprotegerin (OPG), also referred to as osteoclastogenesis inhibitory factor (OCIF), is a secreted glycoprotein that can suppress the function of osteoclasts (OCs); it was first observed in 199712. As a soluble tumour necrosis factor receptor, OPG has become a research hotspot in many fields, including bone metabolism. In the same year, RANKL (receptor activator of nuclear factor-κB ligand), a ligand of OPG, was discovered13. RANK, the exclusive receptor for RANKL, is found in OCs. In bone metabolism, as a functional spindle12, OPG/RANK/RANKL regulates cell maturation and differentiation. Many studies have shown that lung and prostate cancer bone metastases can increase serum OPG levels. However, other studies have shown contradictory results. Hence, we conducted this meta-analysis of relevant studies to assess the effects of lung and prostate cancer bone metastasis on serum OPG levels.
Methods
Literature Search Strategy
An electronic literature search was executed in PubMed, Medline and CNKI (China National Knowledge Infrastructure, a widely used search engine in China) to identify relevant studies published up to June 2015. The following keywords or phrases were used: “OPG”, “osteoprotegerin”, “osteoclastogenesis inhibitory factor” and “OCIF” in combination with “(lung cancer) or (prostate cancer) bone metastasis”. We manually screened the reference lists of all eligible articles to obtain more studies.
Inclusion Criteria
The inclusion criteria were as follows: 1. reports in English or Chinese; 2. cases of either primary malignancy or bone metastasis with definitive diagnoses and the collection of blood samples before the study subjects received any treatment measures; 3. the use of healthy or non-bone metastasis patients as controls; and 4. available data were supplied or obtainable through calculations.
Exclusion Criteria
Studies were excluded if they met the following criteria: 1. the absence of a case-control design; 2. review articles, case reports or conference articles; 3. animal or in vitro studies; 4. research involving bone primary tumours; and 5. research involving special populations (e.g., infants or pregnant women).
Study Selection
Initially, we reviewed the titles and abstracts to identify potential studies that fulfilled the inclusion criteria. In cases of uncertainty regarding the relevance of a report, a subsequent full-text assessment was conducted. Because the data used for this study were retrieved from published literature, we did not need to obtain approval from an ethics committee.
Data Extraction
Two authors independently extracted the following information: author, publication year, nationality, mean age, sample size, and serum OPG levels (mean ± SD).
Validity Assessment
Based on the primary criteria for non-randomized and observational studies of the Newcastle-Ottawa Quality Assessment scale (NOS) for meta-analyses14, two authors completed the quality assessment. Disagreements were resolved by discussion.
Statistical Analysis
The analysis was performed with Stata 12.0 and Review Manager 5.2 software. Standard mean differences (SMDs) and the corresponding 95% confidence intervals (CIs) were used to measure serum OPG levels. Homogeneity testing was performed using the I2 statistic. In the absence of heterogeneity (I2 ≤ 50%), a fixed-effects model was used to combine the SMDs. In the opposite case, a random-effects model was used. Additionally, we performed a sensitivity analysis to assess the effect of a single study on the overall estimate by rejecting each study one at a time. Furthermore, publication bias was detected using Begg’s and Egger’s tests. p-values less than 0.05 were considered statistically significant.
Results
Study screening process
We initially identified 692 potential studies from the above databases. Most were excluded because they were not case-control studies or had incomplete data. Finally, 11 studies (PCa, 6; lung cancer, 5) were included15,16,17,18,19,20,21,22,23,24,25 (Fig. 1). These 11 eligible studies involved 448 cases of BM (PCa, 213; lung cancer, 235), 409 BM- (PCa, 215; lung cancer, 194) and 349 healthy controls. The general data from the 11 studies are summarised in Table 1 and Table 2.
Figure 1. Flow chart of the studies identified, included and excluded.
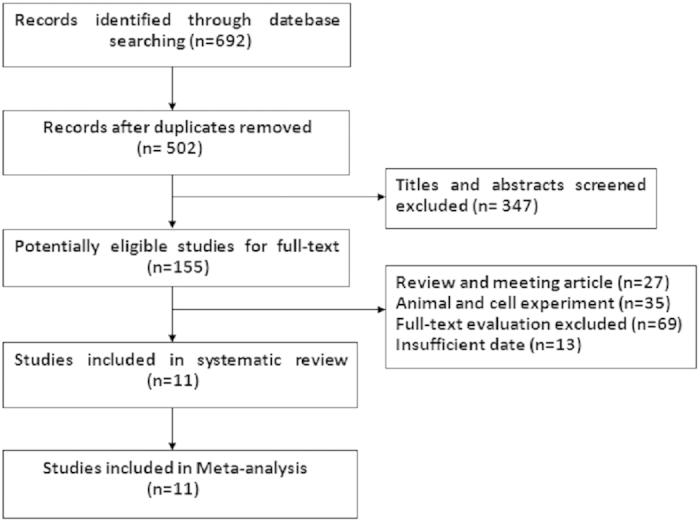
Table 1. The characteristics of included 6 prostate cancer bone metastasis studies.
| Author | Year | Country | Mean age* | Sample Size* |
|---|---|---|---|---|
| Chen et al. | 2007 | China | 68/70/48 | 30/36/30 |
| Roato et al. | 2008 | Italy | 67/64/60 | 9/37/20 |
| Zhao et al. | 2010 | China | N/N/N | 27/13/N |
| Kamiya et al. | 2011 | Japan | 69.2/66.2/62.9 | 51/101/49 |
| Bu | 2013 | China | 65.2/63.8/43.5 | 35/30/65 |
| Tan | 2014 | China | 45.5/45.8/N | 61/53/N |
Note: *: Bone metastasis group/non-bone metastasis group/healthy controls.
Table 2. The characteristics of included 5 lung cancer bone metastasis studies.
| Author | Year | Country | Mean age* | Sample Size* |
|---|---|---|---|---|
| Terpos et al. | 2009 | Greece | 66.6/69.6/65.3 | 51/28/29 |
| Yin et al. | 2009 | China | N/N/53 | 40/44/65 |
| Karapanagiotou.et al. | 2010 | Greece | 59.6/64.3/54.8 | 22/18/29 |
| Zhu et al. | 2013 | China | N/N/66 | 18/22/32 |
| Li et al. | 2013 | China | N/N/N | 104/82/30 |
Note: *: Bone metastasis group/non-bone metastasis group/healthy controls.
The effect of prostate cancer bone metastasis on serum OPG levels
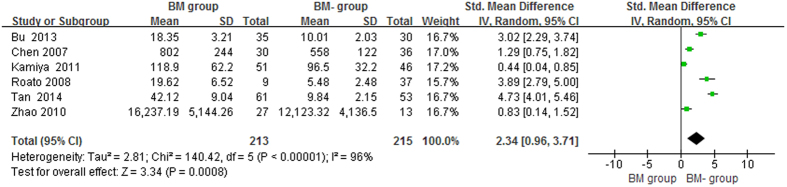
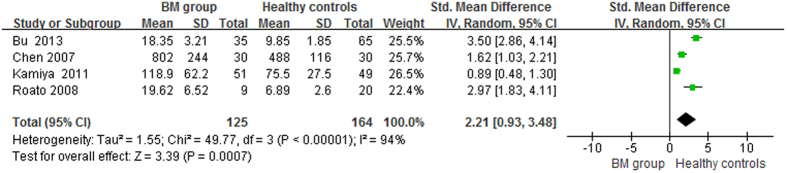
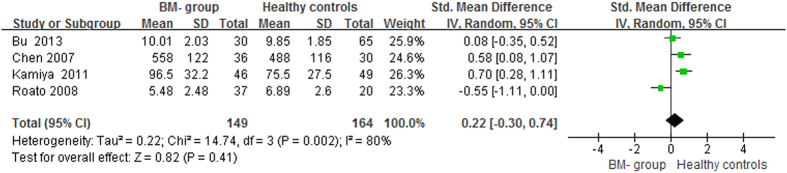
The pooled data suggested that the serum OPG levels in the BM group were significantly higher than in the BM- group and healthy controls, with pooled SMDs (95% CIs) of 2.34 (0.96, 3.71) and 2.21 (0.93, 3.48), p < 0.05, respectively (Figs 2 and 3). However, no significant difference in serum OPG levels was found between the BM- group and healthy controls (SMD = 0.22, 95% CI = −0.30 to 0.74, p = 0.41, >0.05; Fig. 4).
Figure 2. Forest plot for the comparison of serum OPG levels between BM group and BM- group in prostate cancer.
Figure 3. Forest plot for the comparison of serum OPG levels between BM group and healthy controls in prostate cancer.
Figure 4. Forest plot for the comparison of serum OPG levels between BM- group and healthy controls in prostate cancer.
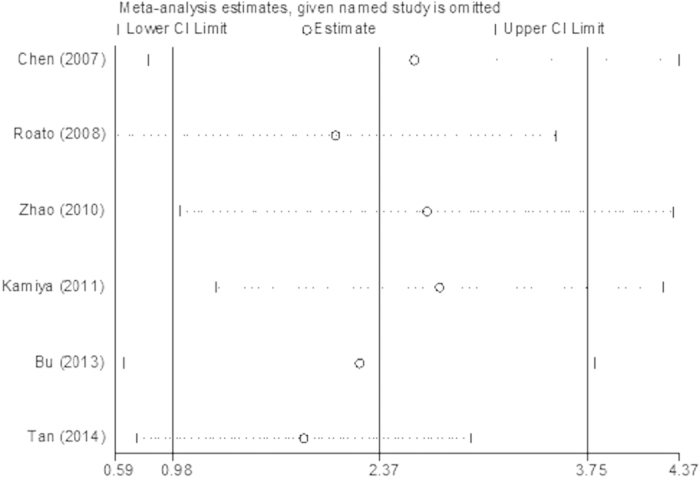
We conducted a sensitivity analysis regarding the comparison of serum OPG levels between the BM and BM- groups by sequentially eliminating one study from the relevant data; Stata 12.0 software was used to pool the SMD for the remaining studies. The results consistently suggested that no single study significantly altered the combined results (Fig. 5). Egger’s regression test indicated little evidence of publication bias (p > 0.05).
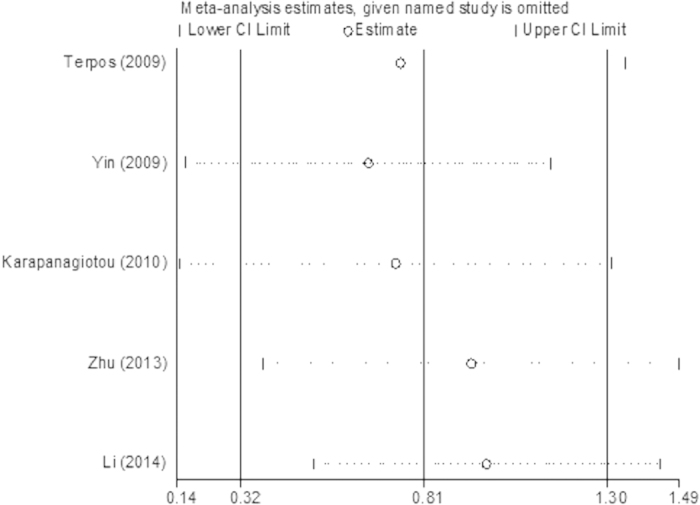
Figure 5. The plot of sensitivity analysis for the comparison of serum OPG levels between BM group and BM- group in prostate cancer.
The effect of lung cancer bone metastasis on serum OPG levels
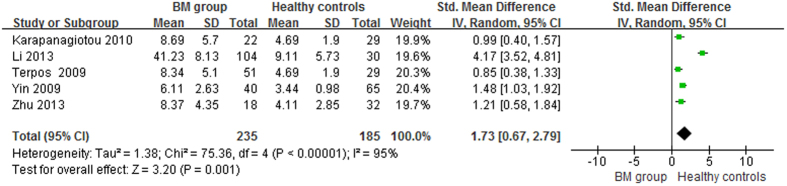
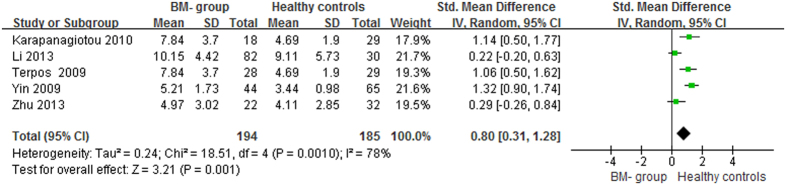
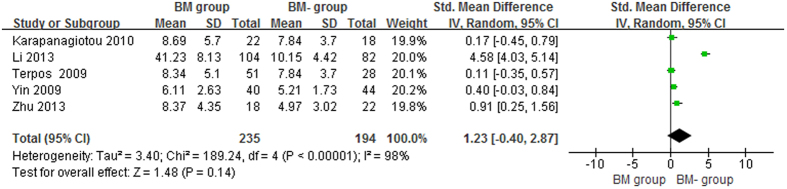
The lung cancer results were different from the prostate cancer results: compared with healthy controls, the serum OPG levels in the BM and BM- groups were significantly higher (p < 0.05), with pooled SMDs (95% CIs) of 1.73 (0.67, 2.79) and 0.80 (0.31, 1.28), respectively (Figs 6 and 7). However, no significant difference was found in serum OPG levels between the BM and BM- groups (SMD = 1.23; 95% CI = −0.40 to 2.87; p = 0.14, >0.05; Fig. 8).
Figure 6. Forest plot for the comparison of serum OPG levels between BM group and healthy controls in lung cancer.
Figure 7. Forest plot for the comparison of serum OPG levels between BM- group and healthy controls in lung cancer.
Figure 8. Forest plot for the comparison of serum OPG levels between BM group and BM- group in lung cancer.
We used Stata 12.0 software to perform the sensitivity analysis. No single study changed the combined results significantly, which indicated that the results were statistically stable and reliable (Figs 9,10 and 11). Egger’s regression test indicated little evidence of publication bias (p > 0.05).
Figure 9. The plot of sensitivity analysis for the comparison of serum OPG levels between BM group and BM- group in lung cancer.
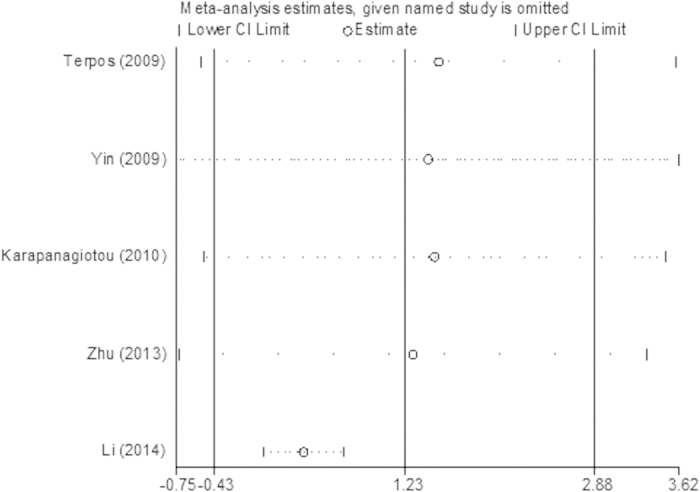
Figure 10. The plot of sensitivity analysis for the comparison of serum OPG levels between BM group and healthy controls in lung cancer.
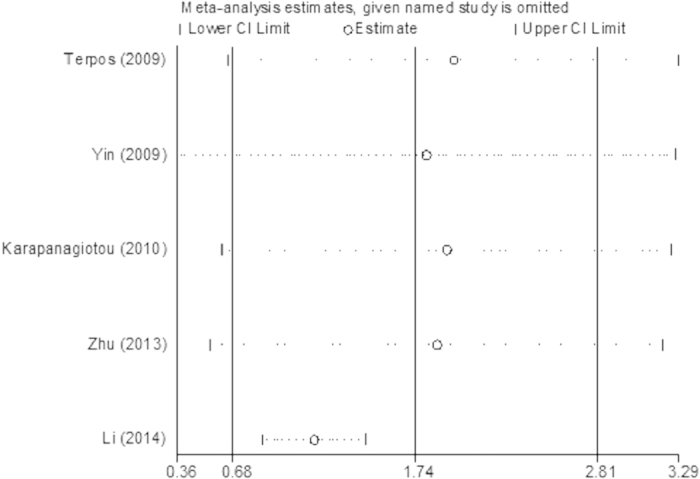
Figure 11. The plot of sensitivity analysis for the comparison of serum OPG levels between BM- group and healthy controls in lung cancer.
Discussion
Metastasis represents a major cause of mortality in cancer patients, and bone invasion is often described. Generally, lung and prostate cancers exhibit high levels of bone tropism2,26. To explain the cancer metastasis phenomenon, Stephen Paget proposed the “seed and soil” hypothesis in 1889, which suggests that the interplay between the properties of cancer cells and the particular organ microenvironment determines the selective growth advantage of cells27. Recently, most emerging evidence emphasises the crucial role of feedback interactions between tumour cells and the bone microenvironment, which lead to the establishment of a vicious cycle that acts by upregulating the physiological mechanisms that normally favour bone resorption.
Although the osteoclasia caused by bone metastases is divided into osteolytic and osteoblastic types, mixed lesions are often described. Much evidence has shown that both resorption and formation are activated in most bone metastases; therefore, both osteolytic and osteoblastic characteristics can be observed2. Osteolytic and osteoblastic metastases are only the two extremes, however. The characteristics of a lesion are mainly based on the balance between resorption and formation in the bone environment. Activation and dysregulation exist in most osseous lesions, resulting in an unbalanced bone remodelling process and reflected in the complex phenotypic outcome28,29,30.
OCs and osteoblasts play an important role in maintaining the balance of bone remodelling. Osteolytic metastases are thought to be caused by factors secreted by tumour cells, which activate OCs30. Osteoblastic metastases are believed to be caused by osteoblasts producing factors that stimulate osteoblast proliferation, differentiation and, therefore, bone formation.
Many researchers have reported that tumour cells mainly express RANKL when they adhere to the bone microenvironment31,32. In general, RANKL can bind to RANK to trigger signal transduction and thus promote the differentiation and maturity of OC precursors. OPG acts in this case as a “decoy” receptor of RANKL and could be considered a “protector” of bone33. As a paracrine regulator of OC formation, OPG was found to have an essential physiological role: OPG is produced by osteoblasts and binds to RANKL, and its constitutive production is necessary to limit the OC formation resulting from RANKL stimulation34.
The RANK/RANKL/OPG system has recently been recognised as the ultimate mediator of osteoclastogenesis30,35, and the dysregulation of the OPG–RANKL system is thought to be crucial to the bone disease connected with cancers such as prostate cancer36. Some studies have reported that the ratio of RANKL/OPG was increased in neoplastic disease patients with severe osteolysis, which has also been observed in multiple myeloma patients37,38. Recently, some studies showed that a therapy that disrupts the vicious cycle in the bone microenvironment by binding to RANKL and inhibiting its function could achieve satisfactory results. Denosumab is a fully humanised monoclonal antibody against RANKL for the prevention of SREs in patients with tumours that metastasize to bone39. Unlike denosumab, zoledronic acid (ZA) can prevent the prenylation of the small GTPase proteins that are essential for OC function and survival40,41. In 2013, Sun found that denosumab was superior to ZA in preventing complications in patients with bone metastases42. One year later, Henry reported that denosumab was more effective than ZA at either delaying SREs in solid bone tumours or preventing pain progression43. These results suggest that the RANK/RANKL/OPG system plays a key role in the process of bone metastasis from the perspective of treatment; thus, serum OPG levels could be altered in bone metastasis patients.
In our study, the pooled data on PCa bone metastasis revealed that, compared with the BM- group and healthy controls, the BM group had higher serum OPG levels. However, no difference was found between the BM- group and healthy controls. The results suggest that bone metastasis increases serum OPG levels in prostate cancer patients. The specific mechanisms underlying the increase in serum OPG levels might be due to a variety of factors. The OC is a unique cell capable of dissolving bone tissue, which plays an important role in bone remodelling. It has been shown that OPG can be secreted to inhibit osteoclastogenesis and OC survival, thus preventing the establishment of tumour lesions in bone44,45,46. In vitro studies have shown that some tumour cells did not express RANKL mRNA when cultured alone. However, the co-culture of these cancer cells with osteoblastic cells or bone marrow stromal cells could induce RANKL expression. This interaction between stromal cells and tumour cells is critical for metastasis29,47. OPG can be released to upset the interaction between OCs and stromal cells48, which inhibits RANKL expression by tumour cells. Despite the potential of OPG in inhibiting OC activation, it also binds to TNF-related apoptosis-inducing ligand (TRAIL), making tumour cells resistant to apoptosis. It was shown that prostate cancer and bone marrow stromal cells could express OPG to enhance tumour cell survival by inhibiting TRAIL36,49,50. OPG can prevent the association between TRAIL and its death-inducing receptor, thereby increasing the survival of tumour cells that have metastasised to the bone microenvironment29,51,52; this may be one reason that OPG levels increase when tumours metastasise to bone.
However, we found that bone metastasis embodied a different phenomenon in lung cancer. Compared with healthy controls, the BM and BM- groups had higher serum OPG levels. No difference was found between the BM group and the BM- group. Serum OPG levels are increased in lung cancer regardless of whether it is complicated by bone metastases. The literature on the role of OPG and its involvement in metastatic bone disease is somewhat contradictory. This may suggest that OPG is not associated with skeletal metastasis at all, but rather with the tumour load per se17. In addition, preclinical data suggest that OPG plays a role in promoting angiogenesis53, which may be a less important reason for OPG expression in lung cancer bone metastasis.
The meta-analysis revealed that serum OPG levels could reflect prostate cancer bone metastasis. This has a certain clinical utility in that abnormally elevated serum OPG levels in prostate cancer patients may be associated with bone metastases. Serum OPG measurements can be used to supplement various existing diagnostic methods to increase the precision and convenience of bone metastasis diagnosis. However, we found that OPG was not associated with lung cancer bone metastasis, but rather with the presence of the tumour. This finding perhaps hints that not all bone metastases can be detected by measuring serum OPG. Recently, a study of bone metastasis treatment showed that clinical efficacy was consistent with the relative reduction observed in bone turnover markers43. This may suggest that OPG could be used as an indicator to evaluate the clinical efficacy of treatment.
We should note some limitations of our study: 1. the included studies were based on a case-control design, in which selection bias was inevitable; 2. the sample size of the included studies was relatively small, and several studies were excluded due to insufficient data; 3. we could not avoid the possibility that other unmeasured or inadequately measured factors confounded the results; and 4. our inclusion criteria might have introduced selection bias, although little statistical evidence of publication bias was observed.
Conclusion
The present meta-analysis suggests that serum OPG levels can reflect prostate cancer bone metastasis to some extent, which indicates that serum OPG measurements can be used to supplement existing diagnostic methods for bone metastasis. However, serum OPG levels may more accurately reflect the existence of lung cancer itself. Further studies on this topic with larger sample sizes are needed.
Additional Information
How to cite this article: Zang, L. et al. The effects of lung and prostate cancer bone metastasis on serum osteoprotegerin levels: a meta-analysis. Sci. Rep. 5, 18324; doi: 10.1038/srep18324 (2015).
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC, Grant no. 81570800).
Footnotes
Author Contributions Z.L.Y., M.M., H.J.X. and Q.H. wrote and revised the main manuscript text; and H.B. and C.T.W. prepared all the figures. All the authors reviewed the manuscript.
References
- Riccio A. I., Wodajo F. M. & Malawer M. Metastatic carcinoma of the long bones. Am. Fam. Physician 76, 1489–1494 (2007). [PubMed] [Google Scholar]
- Mundy G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002). [DOI] [PubMed] [Google Scholar]
- Yin J. J., Pollock C. B. & Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 15, 57–62 (2005). [DOI] [PubMed] [Google Scholar]
- Keller E. T. et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 20, 333–349 (2001). [DOI] [PubMed] [Google Scholar]
- Bubendorf L. et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 31, 578–583 (2000). [DOI] [PubMed] [Google Scholar]
- Coleman R. E. Skeletal complications of malignancy. Cancer 80, 1558–1594 (1997). [DOI] [PubMed] [Google Scholar]
- Furukawa M., Ohta T. & Xiong Y. Activation of UBC5 ubiquitin-conjugating enzyme by the RING finger of ROC1 and assembly of active ubiquitin li-gases by all cullins. J. Biol. Chem. 277, 15758–15765 (2002). [DOI] [PubMed] [Google Scholar]
- Destombe C. et al. Investigation for bone metastasis from an unknown primary. Joint Bone Spine 74, 85–89 (2007). [DOI] [PubMed] [Google Scholar]
- Voorzanger-Rousselot N. et al. Association of 12 serum biochemical markers of angiogenesis, tumour invasion and bone turnover with bone metastases from breast cancer: a crossectional and longitudinal evaluation. Br. J. Cancer 95, 506–514 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. et al. Value of C-telopeptide-cross- linked Type I collagen, osteocalcin, bone-specific alkaline phosphatase and procollagen Type I N-terminal propeptide in the diagnosis and prognosis of bone metastasis in patients with malignant tumors. Med. Sci. Monit. 17, CR626–CR633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A. & Delmas P. D. Markers of bone turnover in bone metastases. Cancer 88, 2952–2960 (2000). [DOI] [PubMed] [Google Scholar]
- Penno H. et al. Osteoprotegerin secretion from prostate cancer isstimulated by cytokines, in vitro. Biochem. Biophys. Res. Commun. 293, 451–455 (2002). [DOI] [PubMed] [Google Scholar]
- Simonet W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997). [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of non-randomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- Chen H. X. et al. Serum osteoprotegerin as a novel marker of bone metastasis in prostate cancer. Chin. J. Surg. 45, 412–414 (2007). [PubMed] [Google Scholar]
- Roato I. et al. Osteoclasts are active in bone forming metastases of prostate cancer patients. Plos One 3, e3627 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E. et al. The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications. Anticancer Res. 29, 1651–1658 (2009). [PubMed] [Google Scholar]
- Yin J. L. & Chen X. Y. The clinical value of serum osteoprotegerin level in the diagnosis of lung cancer. Exp. Lab. Med. 27, 7–9 (2009). [Google Scholar]
- Zhao X. Z. et al. Clinical value of measuring serum osteoprotegerin in patients with prostate cancer. Clinical Medicine of China 26, 1242–1243 (2010). [Google Scholar]
- Karapanagiotou E. M. et al. Serum bone turnover markers may be involved in the metastatic potential of lung cancer patients. Med. Oncol. 27, 332–338 (2010). [DOI] [PubMed] [Google Scholar]
- Kamiya N. et al. Significance of serum osteoprotegerin and receptor activator of nuclear factor κB ligand in Japanese prostate cancer patients with bone metastasis. Int. J. Clin. Oncol. 16, 366–372 (2011). [DOI] [PubMed] [Google Scholar]
- Zhu B., Shao K. J., Yuan H. & Xu Y. F. The clinical assessment on lesions shown 99mTc-MDP whole body bone scan and determination of serum levels of OPG, BSP and TRACP-5b in lung cancerpatients with bone metastasis. J. Radioimmunology. 26, 545–547 (2013). [Google Scholar]
- Bu J. S. Changes and clinical significance of serum OPG, BMP-7 and PSA in patients with prostatic cancer. Chinese Journal of Health Laboratory Technology 23, 168–170 (2013). [Google Scholar]
- Li L., Tan B. Y., Liu Z. W., Chen M. & Zhang Y. The diagnostic value of serum osteoclast differentiation factor and inhibitory factor in bone metastasis of lung cancer. Int. J. Lab. Med. 34, 1930–1934 (2013). [Google Scholar]
- Tan Y. C. Significance of ODF and OCIF in diagnosis of prostate cancer bone metastases. China Journal of Modern Medicine 24, 37–40 (2014). [Google Scholar]
- Schaller B. et al. Prostate-specific antigen in the cerebrospinal fluid leads to diagnosis of solitary cauda equina metastasis: a unique case report and review of the literature. Br. J. Cancer 77, 2386–2389 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 8, 98–101 (1989). [PubMed] [Google Scholar]
- Gregory R. & Mundy M. D. Mechanisms of bone metastasis. Cancer 80, 1546- 1556 (1997). [DOI] [PubMed] [Google Scholar]
- Neville-Webbe H. L. et al. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res. Treat. 86, 269–279 (2004). [DOI] [PubMed] [Google Scholar]
- Hofbauer L. C. & Schoppet M. Clinical implications of the osteoprotegerin/ RANKL/RANK system for bone and vascular diseases. JAMA 292, 490–495 (2004). [DOI] [PubMed] [Google Scholar]
- Nagai M., Kyakumoto S. & Sato N. Cancer cells responsible for humoral hypercalcaemia express mRNA encoding a secreted form of ODF/TRANCE that induces osteoclast formation. Biochem. Biophys. Res. Commun. 269, 532–536 (2000). [DOI] [PubMed] [Google Scholar]
- Huang L., Cheng Y. Y., Chow L. T., Kumta S. M. & Zheng M. H. Tumor cells produce receptor activator of NF-kB ligand (RANKL) in skeletal metastases. J. Clin. Pathol. 55, 877–878 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. et al. Osteoprotegerin in serum as a novel marker of bone metastatic spread in prostate cancer. Clin. Chem. 47, 2061–2063 (2001). [PubMed] [Google Scholar]
- Udawela M., Hay D. L. & Sexton P. M. The receptor activity modifying protein family of G protein coupled receptor accessory proteins. Semin. Cell Dev. Biol. 15, 299–308 (2004). [DOI] [PubMed] [Google Scholar]
- Theoleyre S. et al. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodelling. Cytokine Growth Factor Rev. 15, 457–475 (2004). [DOI] [PubMed] [Google Scholar]
- Holen I. & Shipman C. M. Role of osteoprotegerin (OPG) in cancer. Clin. Sci. 110, 279–291 (2006). [DOI] [PubMed] [Google Scholar]
- Grimaud E. et al. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am. J. Pathol. 163, 2021–2031 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E. et al. Soluble receptor activator of nuclear factor kappa-B ligand- osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102, 1064–1069 (2003). [DOI] [PubMed] [Google Scholar]
- Rosen L. S. et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 21, 3150–3157 (2003). [DOI] [PubMed] [Google Scholar]
- Russell R. G., Watts N. B., Ebetino F. H. & Rogers M. J. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 19, 733–759 (2008). [DOI] [PubMed] [Google Scholar]
- Rogers M. J., Crockett J. C., Coxon F. P. & Mönkkönen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 49, 34–41 (2010). [DOI] [PubMed] [Google Scholar]
- Sun L. & Yu S. Efficacy and safety of denosumab versus zoledronic acid in patients with bone metastases: a systematic review and meta-analysis. Am. J. Clin. Oncol. 36, 399–403 (2013). [DOI] [PubMed] [Google Scholar]
- Henry D. et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer 22, 679–87 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Osteoprotegerin inhibits prostate cancer induced osteoclastogenesis and prevents tumor growth in the bone. J. Clin. Invest. 107, 1235–1244 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E. et al. RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol. Cancer Ther. 7, 2160–2169 (2008). [DOI] [PubMed] [Google Scholar]
- Hakeda Y. et al. Osteoclastogenesis inhibitory factor (OCIF) directly inhibits bone-resorbing activity of isolated mature osteoclasts. Biochem. Biophys. Res. Commun. 251, 796–801 (1998). [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Roodman D., Freeman C. & Mohla S. Mechanisms of tumor metastasis to the bone: challenges and opportunities. J. Bone Miner. Res. 18, 190–194 (2003). [DOI] [PubMed] [Google Scholar]
- Akatsu T. et al. Osteoclastogenesis inhibitory factor suppresses osteoclast survival by interfering in the interaction of stromal cells with osteoclast. Biochem. Biophys. Res. Commun. 250, 229–234 (1998). [DOI] [PubMed] [Google Scholar]
- Holen I., Crouche P. I., Hamdy F. C. & Eaton C. L. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 62, 1619–1623 (2002). [PubMed] [Google Scholar]
- Nyambo R. et al. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J. Bone Miner. Res. 191, 712–1721 (2004). [DOI] [PubMed] [Google Scholar]
- Park H. R. et al. Expression of osteoprotegerin and RANK ligand in breast cancer bone metastasis. J. Korean Med. Sci. 18, 541–546 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holen I. et al. Osteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo-a role in tumour cell survival? Breast Cancer Res. Treat. 92, 207–215 (2005). [DOI] [PubMed] [Google Scholar]
- Cross S. S. et al. Osteoprotegerin (OPG)-a potential new role inthe regulation of endothelial cell phenotype and tumour angiogenesis? Int. J. Cancer 118, 1901–1908 (2006). [DOI] [PubMed] [Google Scholar]