Abstract
Aneurysmal subarachnoid hemorrhage (SAH) is associated with high mortality. The initial hemorrhage causes death in approximately 25% of patients, with most subsequent mortality being attributable to delayed cerebral ischemia (DCI). Delayed cerebral ischemia generally occurs on post-bleed days 4 through 20, with the incidence peaking at day 8. Because of the risks of DCI, patients with SAH are usually monitored in an intensive care unit (ICU) for 14 to 21 days. Unfortunately, prolonged ICU admissions are expensive and are associated with well-documented risks to patients. We hypothesized that a subset of patients who are at low risk of DCI should be safe to transfer out of the ICU early. All patients admitted to Montefiore Medical Center from 2008 to 2013 with grade I SAH who had their aneurysms successfully protected, had an uncomplicated postoperative course, and had no clinical or ultrasonographic evidence of DCI after day 8 were retrospectively studied. The primary outcome was clinical or ultrasonographic evidence of the development of DCI after day 8. Secondary outcomes included length of ICU and hospital stay and hospital mortality. Forty patients who met the above-mentioned criteria were identified. Of these, only 1 (2.5%) developed ultrasonographic evidence of DCI after day 8 but required no intervention. The mean length of stay in the ICU was until post-bleed day 13, and the mean hospital length of stay was until post-bleed day 14. The in-hospital mortality was 0 of 40. Thus, we identified a low-risk subset of patients with grade I SAH who may be candidates for early transfer out of the ICU.
Keywords: subarachnoid hemorrhage, cerebrovascular disorders, neurocritical care, clinical specialty, vasospasm, intracranial, cerebrovascular disorders
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) strikes approximately 30 000 people per year in North America.1,2 About 25% of patients with aSAH die before reaching the hospital and another 10% die within the first hospital day. One-month mortality is estimated at 45% to 50%.3,4 Around 30% of patients who survive the initial 3 days of aSAH had delayed cerebral ischemia (DCI), a clinical syndrome of focal neurological and cognitive deficits most commonly caused by vasospasm of cerebral arteries.5,6 Mortality from DCI is nearly 30%, and permanent neurological deficits occur in 34% of affected patients.7
The risk of vasospasm typically begins at 3 to 5 days after the hemorrhage and peaks around day 8.8 After that time, the risk of new-onset vasospasm gradually decreases and becomes very uncommon after the third week. Because vasospasm requires immediate recognition and intervention to prevent clinically significant DCI during the period of high risk of vasospasm, patients with aSAH are routinely monitored in an intensive care unit (ICU) for 14 and up to 21 days after the initial bleed.9 Although patients with high-grade aSAH may require multiple interventions to maintain cardiopulmonary stability, control hydrocephalus, and prevent ongoing brain injury, patients with low-grade aSAH and uncomplicated postoperative courses often require very few interventions during their time in the ICU. The care of these patients essentially consists of frequent neurologic monitoring, prophylactic nimodipine administration, and the performance of serial transcranial doppler studies (TCDs).6,10,11
Unfortunately prolonged ICU admissions are extremely expensive. The cost of a patient care for a day in the ICU in the United States averages close to US$4000 per day, about 3 times more than on a general hospital ward.12 In addition, prolonged ICU admissions are associated with well-documented risks to patients including deconditioning, delirium, and acquisition of infection with drug-resistant organisms.13-15
It would be beneficial to recognize a subset of patients with aSAH who are at low risk of DCI and thus can be safely transferred out of the ICU early. We hypothesized that patients with Hunt and Hess Grade I aSAH who have an uncomplicated postoperative course and no clinical or ultrasonographic evidence of vasospasm by post-bleed day 8 are at low risk of subsequent development of significant DCI. This premise was based on the clinical observations of the senior investigator. We decided to examine this theory retrospectively in a cohort of patients in the neurosurgical ICU at Montefiore Medical Center.
Methods
All patients who were admitted to Montefiore Medical Center from January 1, 2008, through December 31, 2013, with the primary diagnosis of Hunt and Hess Grade I SAH who had their aneurysms successfully protected either by angiographic coiling or by surgical clipping, had an uncomplicated immediate postoperative course, and had no clinical or ultrasonographic evidence of DCI by day 8 were retrospectively studied. Exclusion criteria included any complication or comorbidity that required administration of medications for hemodynamic support within the first 8 days of hospitalization as well as hydrocephalus requiring placement of an external ventricular drain (EVD). Patients in whom an aneurysm was not identified on angiography were also excluded. The primary outcome was the incidence of clinical or ultrasonographic development of DCI from day 9 until hospital discharge. Secondary outcomes included length of ICU stay, length of hospital stay, and in-hospital mortality.
Admission history and physical and daily progress notes were analyzed to establish the Hunt and Hess grade of the patient at the time of presentation and to evaluate the postintervention clinical course until the discharge from the hospital. The notes were reviewed for any change in neurologic examination thought to represent symptomatic vasospasm as well as the daily TCD reports. Evidence of vasospasm on TCD was defined as any velocity measured above 120 cm/second.16 All patients were monitored in the neurosurgical ICU with a 2:1 nursing ratio and 24:7 attending intensivist coverage. This investigation was approved by the Institutional Review Board of Montefiore Medical Center with an exemption from informed consent.
Results
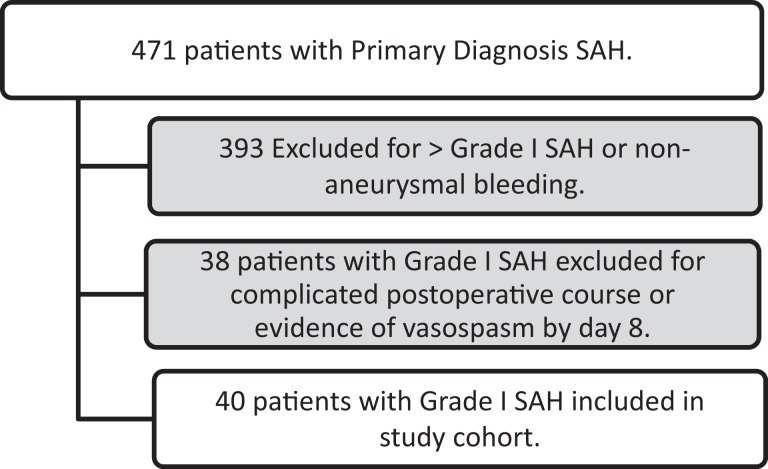
Overall, of the 471 patients admitted to the neurosurgical ICU with a primary diagnosis of aSAH over the 5-year period, 40 patients met the defined criteria for inclusion in our cohort. The reasons for exclusion of the 431 patients who were not included in the cohort are presented in Figure 1. The mean age of the study patients was 50 years (standard deviation 12 years), with 26 (65%) being female gender, 16 (40%) being African American, 14 (35%) being Hispanic, 8 (20%) being white, and 2 (5%) being Asian or other race. Of the 40 patients, 13 had surgical clipping to secure the aneurysm, while the rest had endovascular coil embolization performed. By initial radiographic imaging of the head, 2 (5%) had a Modified Fisher Scale score (MFS) of 0, 28 (70%) had an MFS of 1, 2 (5%) had an MFS of 2, and 8 (20%) had an MFS of 3.
Figure 1.
Derivation of study cohort from all patients admitted with a primary diagnosis of subarachnoid hemorrhage (SAH) between 2008 and 2013.
Of these patients, none developed clinical evidence of vasospasm after day 8, and only 1 (2.5%) of 40 developed ultrasonographic evidence of vasospam. This patient had a single mildly elevated TCD velocity of 125 cm/second in the right middle cerebral artery on day 9. This patient showed no new neurologic findings, and no new intervention was required.
The mean length of stay in the ICU was until post-bleed day 13, and the mean hospital length of stay was until post-bleed day 14. The in-hospital mortality was 0 of 40. None of the patients required medications for hemodynamic support or a repeat angiography after day 8 postprocedure. All patients received prophylactic nimodipine while in the ICU.
Discussion
The ICU is a precious resource, which provides continuous monitoring, enhanced nursing care, and the ability to provide multisystem organ support to the most vulnerable and complex patients in the hospital. However, the negative impact of prolonged ICU admission is well documented. In addition, prolonged lengths of stay may make ICU beds unavailable to patients at the initial course of their critical illness. Increased wait times for ICU beds in the emergency department and on hospital floors have been associated with worsened outcomes.17,18 Many of our ICU triage practices are not evidence based, and the methods to safely reduce the length of stay in the ICU should be explored.
The practice of observing all patients with aSAH for 14 to 21 days post-bleed is clearly one area that is not evidence based. Patients with high-grade aSAH (who often require mechanical ventilation and other organ support), those who have complex early postoperative courses, those who develop hydrocephalus requiring EVD placement, and those at high risk of DCI clearly mandate prolonged ICU care. We have identified a population who, based on their initial presentation, uncomplicated postoperative course, and lack of evidence of vasospasm in the first week should be able to be safely triaged out of the ICU after day 8. It should be noted that after the discharge from the ICU to a general neurosurgical floor, the mean duration of inpatient stay on that unit was only 1 day, after which the patients were discharged from the hospital.
Our suggested optimal transfer location for these patients would be to a neurological observation or stepdown unit with a 4:1 nursing ratio. Once on the floor, we would suggest that they continue to have neurologic examinations performed at least once every 2 hours. We would also recommend continuing to perform serial TCD studies on these patients. This subset of patients should also be considered for early mobilization to prevent deconditioning and delirium. If any clinical or ultrasonographic evidence of vasospasm were to develop, they would need to be transferred back to the ICU for more intensive monitoring and management.
Weaknesses of this study include that it is from a single center and that the grading system used at this center (Hunt and Hess) is not specifically designed or demonstrated to correlate with the risk of vasospasm.19 The MFS is superior for this.20 The Hunt and Hess score does, however, correlate with overall mortality, which is a common and reasonable factor used in ICU triage decisions. It would be unwise to triage a patient with serious neurological sequelae of aSAH out of an ICU early based on a low modified Fisher score on initial imaging. Another limitation to this study is that only a minority of all patients admitted with aSAH fit our predefined criteria. Larger studies would need to be performed to see whether other subsets of patients would be good candidates for early transfer out of the ICU. Since some patients may have a clinical course that does not adhere to predictive models, careful clinical judgment will remain mandatory before such an important decision as transfer out of the ICU is made.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. [DOI] [PubMed] [Google Scholar]
- 2. King JT., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997;7(4):659–668. [PubMed] [Google Scholar]
- 3. Al-Shahi R, White PM, Davenport RJ, Lindsay KW. Subarachnoid haemorrhage. BMJ. 2006;333(7561):235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25(7):1342–1347. [DOI] [PubMed] [Google Scholar]
- 5. Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: incidence and effects. J Clin Neurosci. 1994;1(1):19–26. [DOI] [PubMed] [Google Scholar]
- 6. Feigin VL, Rinkel GJ, Algra A, Vermeulen M, van Gijn J. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology. 1998;50(4):876–883. [DOI] [PubMed] [Google Scholar]
- 7. Kassell NF, Torner JC, Haley EC, Jr, Jane JA, Adams HP, Kongable GL. the international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg. 1990;73(1):18–36. [DOI] [PubMed] [Google Scholar]
- 8. Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978;48(2):173–178. [DOI] [PubMed] [Google Scholar]
- 9. Keyrouz SG, Diringer MN. Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11(4):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM. Role of transcranial doppler monitoring in the diagnosis of cerebral vasospasm aftersubarachnoid hemorrhage. Neurosurgery. 1999;44(6):1237–1247. [PubMed] [Google Scholar]
- 11. Allen GS, Ahn HS, Preziosi TJ, et al. Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308(11):619–624. [DOI] [PubMed] [Google Scholar]
- 12. Johnson DW, Schmidt UH, Bittner EA, Christensen B, Levi R, Pino RM. Delay of transfer from the intensive care unit: a prospective observational study of incidence, causes, and financial impact. Crit Care. 2013;17(4):R128–R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varley AJ, Williams H, Fletcher S. Antibiotic resistance in the intensive care unit. Contin Educ Anaesth Critical Care Pain. 2009;9(4):114–118. [Google Scholar]
- 14. Timmers TK, Verhofstad MH, Moons KG, van Beeck EF, Leenen LP. Long-term quality of life after surgical intensive care admission. Arch Surg. 2011;146(4):412–418. [DOI] [PubMed] [Google Scholar]
- 15. Pandharipande PP, Girard TD, Jackson JC, et al. BRAIN-ICU study investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brass LM, Pavlakis SG, DeVivo D, Piomelli S, Mohr JP. Transcranial doppler measurements of the middle cerebral artery. Effect of hematocrit. Stroke. 1988;19(12):1466–1469. [DOI] [PubMed] [Google Scholar]
- 17. Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP; DELAY-ED study group. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35(6):1477–1483. [DOI] [PubMed] [Google Scholar]
- 18. Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230. [DOI] [PubMed] [Google Scholar]
- 19. Hunt WE, Hess RM. Surgical risk as related to the time of intervention in the repair of intracranial aneurysms J Neurosurg. 1968;28(1):14–20. PMID: 5635959. [DOI] [PubMed] [Google Scholar]
- 20. Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21–27. [DOI] [PubMed] [Google Scholar]