Abstract
The diagnosis of leptomeningeal metastasis (LM) has increased in frequency, as new therapies have lengthened the survival of patients with cancer. Early diagnosis and intervention help improve quality of life and prevent further neurological deterioration in LM. The detection of LM is often established by magnetic resonance imaging examinations, cerebrospinal fluid analysis, or both. We present a series of cases where LM was identified on fluid-attenuated inversion recovery or T2-weighted image but was nonenhancing on the traditionally more sensitive postcontrast T1-weighted sequences. Nonenhancing LM is unusual and not yet fully understood but should be considered in the appropriate clinical context and may become more common with increased utilization of antiangiogenic therapies.
Keywords: brain neoplasms, nervous system neoplasms, meningeal neoplasms, imaging, techniques
Introduction
Leptomeningeal metastasis (LM) occurs when tumor cells disseminate to the cerebrospinal fluid (CSF), arachnoid mater, or pia mater. Leptomeningeal metastasis occurs in approximately 5% to 8% of patients with cancer and confers a dismal prognosis.1 While almost any cancer can cause LM, the most common are lung, breast, melanoma, acute lymphoblastic leukemia, and non-Hodgkin lymphoma.1 Better therapies have lengthened the survival of patients with cancer and have led to increased detection of LM.2 Standard treatments for LM include systemic and/or intrathecal chemotherapy and radiation to bulky or symptomatic disease. Median survival without treatment is only 4 to 6 weeks.1 Early intervention can significantly improve quality of life and increase survival to 3 months or more.1
Postcontrast T1-weighted image (T1WI) has long been recognized as the most sensitive magnetic resonance imaging (MRI) sequence for detecting LM.3,4 The typical appearance of LM is serpentine, nodular, or plaque-like enhancement within the CSF and along the surfaces of the brain, spinal cord, and nerve roots. Although tumor in the CSF may result in abnormal signal intensity on other sequences such as fluid-attenuated inversion recovery (FLAIR), it is most often characterized by contrast enhancement on T1WI. Abnormal CSF signal on T2-weighted imaging (T2WI) or FLAIR without corresponding enhancement typically indicates blood products, infection, inflammatory meningitis, or artifact such with supplemental oxygen therapy and CSF pulsations.5 This series examines imaging findings in a subset of patients with LM and positive findings on T2WI but without enhancement on postcontrast T1WI. We briefly discuss mechanisms that may account for lack of enhancement and how increased utilization of antiangiogenic agents may alter the radiologic characteristics of LM.
Materials and Methods
After a waiver of informed consent was granted by the Institutional Review Board approval, we collected clinical and imaging data from patients at our institution with known LM from January 2010 through June 2014. Two experienced neuroradiologists reviewed all pertinent images. Seven patients were found with LM evident on FLAIR and T2WI but without enhancement on post-gadolinium T1WI. Four of the 7 patients had CSF analysis or surgery within 2 weeks after their imaging that confirmed LM. Three patients without pathologic confirmation of leptomeningeal disease (LM; Patients 1, 6 and 7) initially had nonenhancing leptomeningeal abnormalities, yet later in their disease trajectory developed overt clinical LM with unequivocal imaging findings, and therefore histologic diagnosis was not pursued.
All patients had a histopathologically confirmed primary malignancy that demonstrated contrast enhancement. Patients were not included in this study if alternative explanations for their imaging findings, such as infection or subarachnoid hemorrhage, could not be reliably excluded. A chart review gathered information about patients’ primary tumors, age, sex, treatments, and clinical symptoms. The MRI sequences for the brain and spine were all performed on 1.5-T scanners (GE Medical Systems, Milwaukee, Wisconsin) with T2WI and pre- and postcontrast-enhanced T1WI.
Results
Summary of Patient Data
There were 4 male and 3 female patients with a mean age of 32.4 years (age range, 13-65 years) at the time LM was detected. All patients had imaging of the brain and spine as part of their workup. Four had nonenhancing LM in the spine and 3 had nonenhancing intracranial LM. One patient had radiographic LM in both the brain and the spine. This patient’s LM did not enhance in the brain (positive on FLAIR only) but enhanced in the spine (Figure 1). Five patients had primary brain tumors (2 medulloblastomas, 1 glioblastoma, 1 low-grade oligodendroglioma, and 1 anaplastic oligodendroglioma). The other 2 patients had lung cancer and lumbar spine chordoma. One patient was on antiangiogenic therapy (bevacizumab) at the time nonenhancing LM was detected. No patients were taking corticosteroids within 4 weeks of the diagnosis of nonenhancing LM. The clinical findings for all patients are summarized in Table 1. Representative cases are presented in Figures 1 and 2.
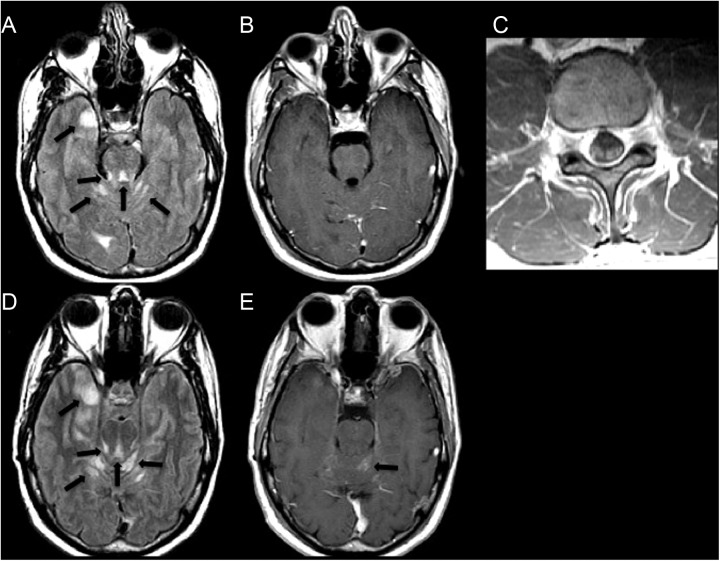
Figure 1.
A 39-year-old male with an anaplastic oligodendroglioma. A, An axial FLAIR image demonstrates abnormal hyperintense signal representing leptomeningeal metastatic disease in the cerebellar folia, around the surface of the pons and along the medial right temporal lobe (arrows). B, There is no corresponding enhancement on the axial postcontrast T1WI. C, The patient’s spine MRI acquired on the same date demonstrates abnormal enhancement of the cauda equine nerve roots on this axial postcontrast T1WI. D and E, Axial FLAIR and contrast-enhanced T1WI images acquired 15 months later demonstrate progression of LM and new enhancement of the lesion in the left vermis (arrows). FLAIR indicates fluid-attenuated inversion recovery; T1W1, T1-weighted image; MRI, magnetic resonance imaging; LM, leptomeningeal disease.
Table 1.
Demographics, Clinical, and Imaging data for the Patient Cohort.
| Patient No. | Age, Years | Sex | Clinical Presentation | Primary Tumor | Location of Nonenhancing LM | Pathologic Confirmation |
|---|---|---|---|---|---|---|
| 1 | 39 | M | Headaches and vomiting (hydrocephalus) | Anaplastic oligodendroglioma | Supratentorial and posterior fossa | No |
| 2 | 32 | M | Headaches and neck pain | Lumbar chordoma | Cervical and thoracic spine | Yes-Surgery |
| 3 | 13 | F | Asymptomatic | Medulloblastoma | Subependymal left frontal horn | Yes-Surgery |
| 4 | 65 | F | Headaches and imbalance (hydrocephalus) | Nonsmall cell lung cancer | Posterior fossa | Yes-CSF |
| 5 | 17 | M | Asymptomatic | Medulloblastoma | Posterior fossa | Yes-CSF |
| 6 | 20 | M | Right shoulder pain | Glioblastoma | Thoracic spine | No |
| 7 | 41 | F | Leg numbness | Low-grade oligodendroglioma | Cervical and thoracolumbar | No |
Abbreviations: CSF, cerebrospinal fluid; F, female; LM, leptomeningeal disease; M, male.
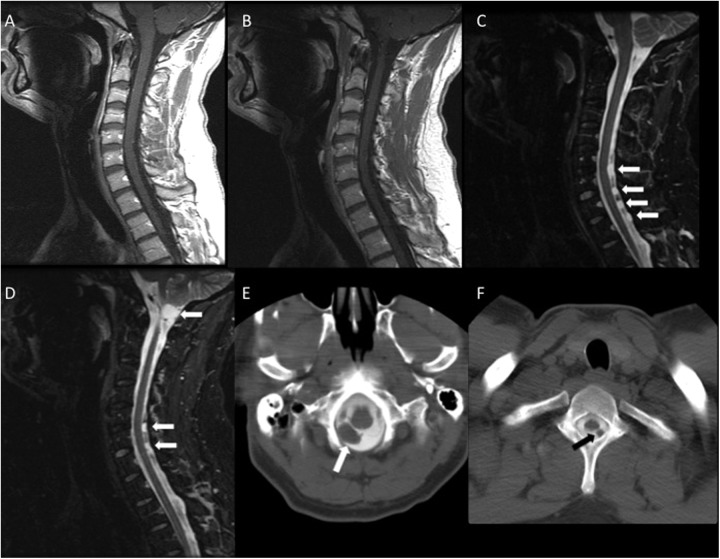
Figure 2.
A 32-year-old male with a lumbar chordoma and neck pain. A and B, Sagittal postcontrast T1-weighted images of the cervical spine demonstrate no evidence of metastases. C and D, Sagittal short tau inversion recovery (STIR) images demonstrate hyperintense nodules in the CSF of the cervical spine and upper thoracic spine (arrows). A leptomeningeal metastasis is also noted at the craniocervical junction. D and E, CT myelography confirms the presence of space-occupying lesions in the CSF (arrows). CSF indicates cerebrospinal fluid; CT, computed tomography.
Selected Case History: Patient 2
A 32-year-old man underwent lumbar discectomy for relief of pain. Preoperative MRI demonstrated an erosive T2-hyperintense mass with slight enhancement in the L4 and L5 vertebral bodies. This lesion was identified intraoperatively, and biopsy was performed for further evaluation. Histopathology was consistent with chordoma in association with a giant notochordal rest. The tumor was deemed surgically unresectable, and he was treated with imatinib when he developed severe neck pain in the cervical/suboccipital region. Cervical spine MRI did not demonstrate degenerative abnormalities or evidence of metastases to account for the patient’s symptoms. The patient responded only minimally to a variety of analgesic medications and Botox injections for presumed muscle spasm. The pain intensified over the coming months, and repeat cervical spine MRI demonstrated multiple markedly T2-hyperintense, nonenhancing, intradural extramedullary nodules in the cervical and upper thoracic spine (Figure 2). A dominant lesion noted at the craniocervical junction measured up to 15 mm. The leading diagnostic considerations were arachnoid cysts and nonenhancing LM, with cystic schwannomas and CSF pulsation artifacts considered less likely. Computed tomography myelography was performed, which confirmed the presence of space-occupying lesions that did not fill in with contrast on the delayed images (Figure 2). At this time LM was favored, despite the lack of enhancement. The patient underwent cervical laminectomies for resection of several nodules, and the pathologic diagnosis confirmed LM from the lumbar chordoma.
Discussion
Early detection of LM may optimize disease control, preserve neurologic function, and improve life expectancy.1,2 The diagnostic gold standard for LM is cytologic examination of the CSF, although this has limited sensitivity, and currently MRI is frequently the initial study for diagnosing LM.1,2,6 Leptomeningeal metastasis on MRI classically manifests as abnormal enhancement in the subarachnoid space or along the pia mater. This series demonstrates, however, that LM can manifest as abnormalities on FLAIR or T2WI without enhancement on postcontrast T1WI. Furthermore, our patient population was heterogeneous in age and primary tumor type, suggesting that nonenhancing LM can occur in a wide variety of clinical settings. Interestingly, this series of patients with nonenhancing LM is represented by a disproportionate number of primary central nervous system (CNS) tumors. The reason why nonenhancing LM would be observed in primary CNS tumors is not clear, although perhaps the radiographic evolution of primary CNS LM recapitulates that of intraparenchymal primary CNS masses, which themselves often are nonenhancing initially. From a clinical viewpoint, leptomeningeal dissemination of primary brain tumors is managed similar to LM from systemic cancers. Radiotherapy is used to palliate bulky or symptomatic disease, and chemotherapy is attempted otherwise, often with unfavorable results.
The contrast enhancement associated with CNS tumors represents a combination of intravascular and interstitial (extravascular) enhancement.7-9 Intravascular enhancement may occur secondary to neovascularity or hyperemia and is often proportional to increases in blood volume or blood flow.7,8 Interstitial enhancement is related to alterations in the blood–brain barrier (BBB) and the formation of new blood vessels that are immature and leaky.9 Extravascular enhancement can also occur with CNS metastases originating from primary tumors in other organ systems because their intrinsic vasculature does not possess a BBB.7,8
One reason that CNS metastases may not enhance is that permeability of the BBB to gadolinium takes a variable amount of time to develop and may not be present at the time of imaging. A recent MRI study tracked the development of BBB permeability over time in a mouse model of breast cancer metastases to the brain, demonstrating that metastases were initially impermeable to contrast and nonenhancing during the early stages of disease.10 One of the patients in our study with nonenhancing intracranial LM eventually developed enhancement of some lesions after 15 months (Figure 1). This patient also had enhancing LM in the spine on the same date nonenhancing LM was initially detected in the brain, raising the possibility that these had developed at different times.
Studies have also demonstrated that not all BBB disruptions are equivalent in size.11,12 Some disruptions may have many small fenestrations that would not allow passage of typical gadolinium chelate with a hydrodynamic diameter of 1 nm, while other tumors can result in fewer but larger BBB fenestrations that do allow extravasation.11,12 Additional variables can influence the presence or absence of enhancement including local blood flow, capillary surface area, and the activity of energy-dependent pumps that can transport molecules (such as contrast material) from the interstitial space back across the BBB faster than they can accumulate.12,13 Ultimately, the reasons why some CNS disease processes enhance and others do not enhance are not fully understood.
Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor (VEGF) and has been approved for treatment of several cancers, including glioblastoma. Studies suggest that anti-VEGF medications result in regression of microvessels, normalization of mature vessels, and inhibition of neovascularization. Reports have demonstrated the effect bevacizumab had on the MRI appearance of parenchymal and leptomeningeal brain metastases from lung cancer and focused on how challenging it was to make a definitive diagnosis based on the absence of enhancement.14,15 One of the seven patients in our study was receiving bevacizumab as part of his treatment regimen (patient 6 with glioblastoma). In the future, increased use of antiangiogenic drugs will decrease the likelihood of LM diagnosis exclusively on postcontrast T1WI, and closer examination of T2WI may be necessary to identify LM in these patients.
Conclusions
The development of enhancement identifiable by imaging is a dynamic process that arises from the complex interplay of multiple biological events. Abnormal enhancement of the leptomeninges on postcontrast T1WI is typically relied upon to confidently make the diagnosis of LM. We presented a small series of patients with nonenhancing LM who were reliably detected on FLAIR and T2WI. The introduction of new drugs that affect tumor angiogenesis and the BBB will make identification of CNS metastases based exclusively on contrast enhancement more difficult. Noncontrast sequences should be carefully examined when evaluating for LM in patients with cancer in the correct clinical context.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Groves MD. Leptomeningeal disease. Neurosurg Clin N Am. 2011;22:67–78. [DOI] [PubMed] [Google Scholar]
- 2. Clarke JL, Perez HR, Jacks LM, Pangeas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74:1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh SK, Agris JM, Leeds NE, Ginsberg LE. Intracranial leptomeningeal metastases: Comparison of depiction at FLAIR and contrast-enhanced MR imaging. Radiology. 2000;217:50–3. [DOI] [PubMed] [Google Scholar]
- 4. Singh K, Leeds NE, Ginsberg LE. MR imaging of leptomeningeal metastases: Comparison of three sequences. AJNR Am J Neuroradiol. 2002;23:817–21. [PMC free article] [PubMed] [Google Scholar]
- 5. Stuckey SL, Goh TD, Heffernan T, Rowan D. Hyperintensity in the subarachnoid space on FLAIR MRI. AJR Am J Roentgenol. 2007;189:913–21. [DOI] [PubMed] [Google Scholar]
- 6. Olson ME, Chornik NL, Posner JB. Infiltration of the leptomeninges by systemic cancer. A clinical and pathologic study. Arch Neurol. 1974;30:122–37. [DOI] [PubMed] [Google Scholar]
- 7. Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525–51. [DOI] [PubMed] [Google Scholar]
- 8. Sage MR, Wilson AJ, Scroop R. Contrast media and the brain: the basis of CT and MR imaging enhancement. Neuroimag Clin N Am. 1998;8:695–707. [PubMed] [Google Scholar]
- 9. Jain R, Griffith B, Narang J, et al. Blood-brain-barrier imaging in brain tumors: concepts and methods. Neurographics. 2012;2:48–59. [Google Scholar]
- 10. Percy DB, Ribot EJ, Chen Y, et al. In vivo characterization of changing blood-tumor-barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiolol. 2011;46:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamasson B, Serduc R, Maisin C, et al. Monitoring blood-brain barrier status in a rat model of glioma receiving therapy: dual injection of low-molecular-weight and macromolecular MR contrast media. Radiology. 2010;257:342–52 [DOI] [PubMed] [Google Scholar]
- 12. Sorensen AG. Science to practice: blood-brain leakage—one size does not fit all. Radiology. 2010;257:303–4. [DOI] [PubMed] [Google Scholar]
- 13. Ueno M. Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr Med Chem. 2007;14:1199–206. [DOI] [PubMed] [Google Scholar]
- 14. Karimi S, Lis E, Gilani S, D’Ambrosio N, Holodny A. Nonenhancing brain metastases. J Neuroimaging. 2011;21:184–7. [DOI] [PubMed] [Google Scholar]
- 15. Kleinschmidt-DeMasters BK, Damek DM. The imaging and neuropathological effects of Bevacizumab (Avastin) in patients with leptomeningeal carcinomatosis. J Neurooncol. 2010;96:375–84. [DOI] [PubMed] [Google Scholar]