Abstract
Background:
Multiple Sclerosis (MS) is a neurological disease in which demyelination and axonal loss leads to progressive disability. Cognition impairment is among the most common complication. Studying axonal loss in the retina is a new marker for MS. The main goal of our study is to search for correlations between magnetic resonance imaging (MRI) findings and the retinal nerve fiber layer (RNFL) thickness at the macula and head of the optic nerve and Wechsler Adult Intelligence Scale-Revised (WAIS-R) Scores that assess multiple domains of intelligence, and to explore the relationship between changes in the RNFL thickness with intellectual and cognitive dysfunction.
Materials and Methods:
A prospective cross-sectional study was conducted at the University Hospital of Kashani, Isfahan, Iran, from September to December 2013. All patients were assessed with a full-scale intelligence quotient (IQ) on the WAIS-R. An optical coherence tomography study and brain MRI were performed in the same week for all the patients. Statistical analysis was conducted by using a bivariate correlation, by utilizing SPSS 20.0. A P value ≤ 0.05 was the threshold of statistical significance.
Results:
Examination of a 100 patients showed a significant correlation between the average RNFL thickness of the macula and the verbal IQ (P value = 0.01) and full IQ (P value = 0.01). There was a significant correlation between brain atrophy and verbal IQ.
Conclusion:
The RNFL loss was correlated with verbal IQ and full IQ.
Keywords: Cognition, intelligence quotient, multiple sclerosis, optic coherence tomography, retinal nerve fiber layer, Wechsler adult intelligence scale-revised
INTROUDUCTION
Multiple Sclerosis (MS) is a chronic neurological disease in which demyelination and axonal loss leads to disruption in the communication between the neurons of the central nervous system (CNS). Cognitive impairment occurs in a significant proportion of the patients with multiple sclerosis (MS).[1,2] The prevalence of cognitive impairment in persons with MS is high; The estimates range from 43 to 65%.[3] Multiple domains of cognition are impaired. According to previous studies, the most affected areas of cognition are: Performances on the cognitive tests for the intelligence quotient (IQ), executive function, processing speed, working memory, verbal memory, and attention.[3,4]
Most magnetic resonance (MR) studies have been focused on the assessment of MS plaques and their correlation with neurological disability.[5] Several studies have shown that a greater lesion burden and brain atrophy occur in MS patients with a more severe cognitive impairment.[6,7,8,9]
In recent times, measures of RNFL by optical coherence tomography (OCT) were seen to be a potentially reliable predictor outcome in MS.[10,11] Some studies have demonstrated a correlation between optical nerve atrophy measured by RNFL and the degree of brain atrophy in patients with MS.[12] Taking into account the correlation between cognitive impairment and brain atrophy and the correlation between brain atrophy and optical nerve atrophy, it is reasonable to hypothesize a correlation between cognitive decline and optical nerve atrophy, measured by OCT.
To better understand both the benefits and limitations of measuring RNFL and to define its place in the often complicated array of neurological symptoms in MS patients, further studies are needed.
Therefore, the aim of our cross-sectional study was to analyze the relationship between the thickness of the RNFL and intellectual impairment and MRI findings, including lesion load and brain atrophy.
In fact, a correlation between optical nerve atrophy measured by OCT and cognitive impairment was seen in patients with MS.[13] However, this study and some similar studies, mentioned in the article, did not evaluate the correlation of these three parameter (RNFL, MRI findings, and WAIS scores). We want to determine a new tool for assessment of patients with cognitive problems and a more reliable discrimination between individuals with and without axonal loss.
MATERIALS AND METHODS
A cross-sectional study was conducted at the University Hospital of Kashani, Isfahan, Iran, from September to December 2013. Patients were recruited from the MS Outpatient Clinic in the Kashani Hospital. The present study was approved by the Ethical Committee of the Isfahan University of Medical Sciences and informed consent was obtained from each participant. Inclusion criteria for the study were, definite MS according to the McDonald Criteria[14] for MS, irrespective of the disease subtype and disease duration and drug types. Patients have not acute relapse during the past three months.
Patients with ophthalmic diseases that might impair or bias the OCT (e.g., diabetes, glaucoma, congenital abnormal optic disc, such as tilted nerve, myopia ≥ 4 diopters, and hypermetropia ≥ 4 diopters) and patients who could not undergo MRI, such as, those with pacemakers or claustrophobia, were excluded. We also excluded patients with a past history of serious head trauma and with major psychiatric disorders that may have an effect on the WAIS-R result. In this study, patients with a previous history of bilateral optic neuritis were excluded.
The intelligence was evaluated by a trained psychologist, using the WAIS-R, a revised form of the WAIS, which was considerably extended from the previous edition that was released in1981. It consisted of six verbal and five performance subtests.[15] In this study, a verbal IQ, performance IQ, and full scale IQ were obtained. Moreover, an association among the digit symbol subtest, vocabulary subtest, object assembly subtest, and digit symbol subtest (most affected domain in MS patients), with other parameters, were studied. In all the patients, the following variables were collected: Age, sex, other underlying disease, duration of MS, the course of MS, previous history of optic neuritis, and severity of the disease according to the Expanded Disability Status Scale Score (EDSS).
Optical coherence tomography was performed on both eyes of each patient, using a Stratus OCT 3000 (Carl Zeiss Meditec, Inc., Dublin, CA, USA), by experienced operators. Retinal nerve fiber layer thickness (RNFL) was measured using the standard protocol with three 3.4 mm circular scans. A technician, who was blind to the results of the neuropsychological studies, made the measurements. The average RNFL thickness in the macula and optic nerve was obtained in micrometers.
For all patients, brain MRIs were obtained in a single session, using the 1.5 T MRI. For every patient, the T1, T2, Fluid-attenuated inversion recovery (FLAIR), and proton density sequences in the axial and sagittal planes were obtained. The MRI scan was visually assessed by a single observer, unaware of the subject's identity. A lesion was defined as a region of prolonged T2 relaxation time with a diameter greater than 3 mm.[16] Only the supratentorial brain was considered for classification purposes. Parenchymal atrophy was defined on the basis of the appearance of cortical sulci, ventricular size, and corpus callosum thickness on the axial and sagittal Tl-weighted images. For the purposes of our investigation, the MRI images of the supratentorial brain were divided into four groups according to the following arbitrary criteria:
Group 1: Absence of typical lesions in the supratentorial brain or only a few (up to nine) very small lesions (less than 0.5 cm in diameter), without evidence of parenchymal atrophy
Group 2: Presence of disseminated (typically more than nine discrete and scattered) perivetricular lesions, without any evidence of parenchymal atrophy
Group 3: Concomitant presence of disseminated discrete and confluent lesions (especially around the lateral ventricles) and cortical and juxtacortical lesions, without parenchymal brain atrophy
Group 4: Same as Group 3, but with evidence of parenchymal atrophy.
The MRI scans were scored by an experienced neurologist, who was blind to the OCT and neuropsychological data. A maximum of three months elapsed between the MRI examinations and the neuropsychological tests.
Statistical analysis
The correlation between the RNFL thickness, WAIS-R measures, and other clinical variables was carried out using a bivariate correlation (Pearson correlation.). Statistical analysis was done by utilizing the SPSS 20.0. A P value ≤ 0.05 was considered the threshold of statistical significance. For the statistical analysis, one eye from every patient was chosen, such that, the eyes with optic neuritis were avoided.
There are reports of a reliability coefficient of 0.76 to 0.94 and a validity coefficient of 0.75 for WAIS-R, in the Iranian population. WAIS-R was widely supported for use in our patients, in the clinical context.[17]
RESULTS
One hundred multiple sclerosis patients were included in this study, 82 females and 18 males. The mean age was 33.5 ± 9.0 years (minimum 19 and maximum 60 years). The mean EDSS score of the patients with MS was 2.05 ± 1.1 (range 1-6). The mean EDSS score of patients with MS was 2.05 ± 1.1 (range 1-6). The demographical and clinical data of patients in each MRI group are shown in Table 1.
Table 1.
Demographic and clinical data of MS patients (mean±SD) according to the MRI groups
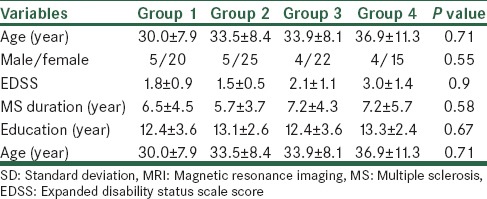
The frequencies of MS patients in the MRI groups were 25 in Group 1, 30 patients in Group 2, 26 in Group 3, and 19 patients in Group 4. Therefore, Group 2 was the most common in our patient sample (30) and Group 4 the least (19), the remaining being nearly equal in size.
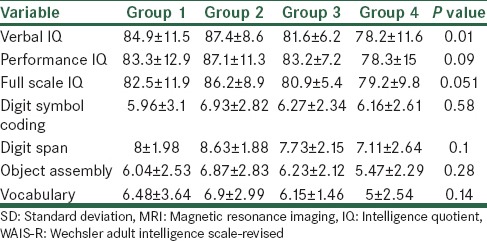
Characteristics of WAIS-R finding of MS patients according to MRI findings are shown in Table 2. According to the One-Way Analysis of Variance (ANOVA) test, there was no statistical difference between the four groups of patients in any of the variables, except the verbal IQ (P = 0.01). Also, based on the Scheffe post hoc test, Group one patients had a positive relation with patients with brain atrophy (P = 0.01).
Table 2.
Characteristics of WAIS-R finding of MS patients according to MRI findings based on mean±SD
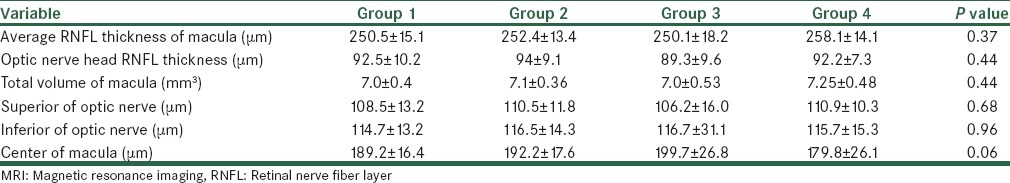
The thickness of the RNFL was measured in each eye using OCT. The average RNFL thickness in the macula and optic nerve were obtained in micrometers. In Table 3, the RNFL thicknesses of the optic nerve and macula in patients with multiple sclerosis in the four groups are shown. According to this Table, the average RNFL thickness of the macula (μm), the RNFL thickness of the optic nerve head (μm), and the total volume of macula (mm3) had no significant correlation with the MRI findings.
Table 3.
Mean of RNFL thickness and volume according to MRI findings
We examined the correlation of the RNFL thickness of the macula and the head of optic nerve, assessed with OCT and WAIS-R scores. Table 4 shows these correlations.
Table 4.
Correlation between WAIS-R scores and EDSS with RNFL thickness in MS patients*
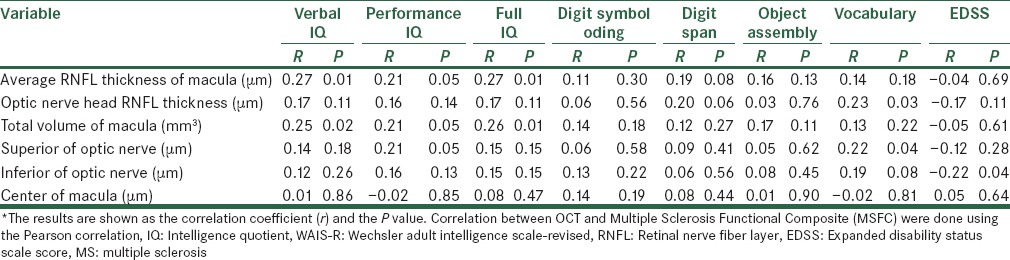
According to this Table, there was a significant correlation between the average RNFL thickness of the macula and verbal IQ (P value = 0.01) and full IQ (P value = 0.01).
DISCUSSION
Recognition of new tools for MS evaluation is crucial to improve our ability to predict disease evolution and for monitoring of responses to therapy.[18] The goal of our study was to search for correlations between the RNFL thickness of the macula and head of the optic nerve and the MRI findings, with WAIS-R scores in patients with multiple sclerosis.
In this study, there was significant correlation between the average RNFL thickness of the macula (μm) and total volume of the macula (mm3), with verbal IQ and full IQ. There was no significant correlation between the RNFL layer in the head of the optic nerve and MRI findings (lesion load and brain atrophy) in MS patients. There was a near-significant correlation between the RNFL in the center of the macula and the MRI findings (P = 0.06) toward the lower RNFL, in patients with evidence of parenchymal atrophy (Group 4). Patients with brain atrophy (Group 4) had a lower verbal IQ than patients without brain atrophy and lower lesion load (P value = 0.01). Also there was near-significant correlation between thefull IQ and brain atrophy (Group 4) with P value = 0.051.
The relationship between brain atrophy measured with MRI and cognitive impairment is well- established in patients with MS.[19] In this study, we have also found a correlation between brain atrophy and verbal IQ and near-significant correlation between brain atrophy and full IQ. In a new study by Ryann, in 2012, it has been found that MS patients had significantly lower subtest and composite scores, with the help of WAIS, for evaluation of IQ. The patients’ mean scores were consistently in the low-average to average range, and the patterns of performance across the groups did not differ significantly, although there was a trend toward higher scores on the Verbal Comprehension Index and lower scores on the Processing Speed Index. Approximately 78% of the patients had actual Full Scale IQs that were significantly lower than the pre-illness, demographically-based IQ estimates.[4] In other studies also, it was concluded that the central and whole brain atrophy accounted for more variance in MS cognition than in the lesion burden.[20,21] Our findings demonstrated lower IQ (full and verbal) in patients with brain atrophy.
In another study, it was seen that global IQ seemed not to be impaired on baseline and over time; consequently they analyzed the patients’ performances of each WAIS subtest to better clarify the individual differences. Overall, most verbal performance WAIS subtests (comprehension, arithmetic, vocabulary, and digit span) showed mild worsening in an increased number of patients after the follow-up period. This may be indicative of impairments in information processing, activation to recall, and organizing the right response for word meaning and problem solving.[22] In our study also, patients with brain atrophy had a lower verbal IQ. However, full IQ had only a near significance correlation with brain atrophy.
The size of the corpus callosum appears to be relevant to cognition in people with MS. In fact, atrophy of the whole corpus callosum predicted test performance on measures of mental processing speed, and rapid problem-solving, whereas, atrophy of the anterior callosum strongly affected the performance on the verbal fluency task.[23]
In our study, we have found a near-significant correlation between RNFL and brain atrophy in MRI. Data regarding the relation between OCT parameters and MRI measures for neurodegeneration are not yet consistent and a consensus on the most relevant parameter has yet to be reached.[24,25] However, a similar finding on correlation MRI atrophy and RNFL thickness is shown in another study.[26]
We have also evaluated the relationship between RNFL thickness and the scores of the WAIS-R test. We found average RNFL thickness of the macula and the total volume of the macula had a significant correlation with verbal IQ and full IQ.
The OCT is a noninvasive, easy-to-perform, inexpensive procedure that has been correlated with brain atrophy detected by MRI in multiple sclerosis patients,[27,28] indicating its ability to detect brain abnormalities. Although we did not find any significant correlation between the lesion load and brain atrophy with RNFL layer thickness in our patients, there was a relationship between the WAIS-R scores and RNFL layer thickness.
Imaging of the optic pathway and RNFL is a new promising strategy to assess axonal loss in MS, as the visual pathway is frequently involved in this disease and retinal axons have no myelin sheath.[28,29]
Other studies reported that many patients with MS and even clinically isolated syndrome (CIS) had a lower RNFL thickness compared to the control healthy group,[30,31] suggesting that this method may be an effective biomarker of global axonal loss, and does not only reflect the changes caused by optic neuritis.[28]
On the other hand, it has been shown that OCT correlates with cognition in patients with MS.[32,33] We found a significant correlation between the average RNFL thickness of the macula and total volume of the macula with verbal IQ and full IQ. However, the optic nerve head RNFL thickness only had significant correlation with the vocabulary subtest and had no significant correlation with the other intelligence parameters. Thus, our results suggest that the RNFL measurements, especially average RNFL thickness at the macula, are able to identify tissue changes associated with cognitive and intelligence impairment.
Some studies demonstrated significant severe axonal loss at the temporal quadrants.[34,35] However, in our studies, like some other studies,[8,33] we did not find preferential involvement in the superior or inferior quadrant.
Our study has some clear limitations. There are no detailed data comparing the ophthalmological and neuropsychological evaluations between patients and the healthy group of controls, therefore, it could be argued that decreased visual acuity or other parameters influenced the performance in cognitive tests among patients.
In this study we have evaluated the cognition of patients with WAIS-R; it would have been better if we done some additional tests like PASAT) paced auditory serial addition test) 2 and 3 seconds for evaluation of cognition changes in MS patients, but unfortunately these tests were not validated for use in Iranian patients. In our center a new MRI method, such as, the volumetric MRI was not available, so we used the traditional MRI method.
CONCLUSION
The RNFL thickness had significant correlation with some domains of intelligence that was most affected in multiple sclerosis patients. Therefore, the OCT technique can be used for evaluation and monitoring of MS patients with cognition and intelligence problems.
Footnotes
Source of Support: Financial support obtained from the Isfahan Medical Science School
Conflict of Interest: None declared.
REFERENCES
- 1.Ferreira ML. Cognitive deficits in multiple sclerosis: A systematic review. Arq Neuropsiquiatr. 2010;68:632–41. doi: 10.1590/s0004-282x2010000400029. [DOI] [PubMed] [Google Scholar]
- 2.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 3.McNalley TE, Haselkorn JK. Disorders of mobility in multiple sclerosis. In: Giesser BS, editor. Primer on multiple sclerosis. New York, NY: Oxford University Press; 2011. pp. 189–96. [Google Scholar]
- 4.Ryan JJ, Gontkovsky ST, Kreiner DS, Tree HA. Wechsler adult intelligence scale-fourth edition performance in relapsing-remitting multiple sclerosis. J Clin Exp Neuropsychol. 2012;34:571–9. doi: 10.1080/13803395.2012.666229. [DOI] [PubMed] [Google Scholar]
- 5.Filippi M, Paty DW, Kappos L, Barkhof F, Compston DA, Thompson AJ, et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: A follow-up study. A follow up study. Neurology. 1995;45:255–60. doi: 10.1212/wnl.45.2.255. [DOI] [PubMed] [Google Scholar]
- 6.Amato MP, Portaccio E, Stromillo ML, Goretti B, Zipoli V, Siracusa G, et al. Cognitive assessment and quantitative magnetic resonance metrics can help to identify benign multiple sclerosis. Neurology. 2008;71:632–8. doi: 10.1212/01.wnl.0000324621.58447.00. [DOI] [PubMed] [Google Scholar]
- 7.Benedict RH, Bruce JM, Dwyer MG, Abdelrahman N, Hussein S, Weinstock-Guttman B, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol. 2006;63:1301–6. doi: 10.1001/archneur.63.9.1301. [DOI] [PubMed] [Google Scholar]
- 8.Amato MP, Portaccio E, Goretti B, Zipoli V, Battaglini M, Bartolozzi ML, et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol. 2007;64:1157–61. doi: 10.1001/archneur.64.8.1157. [DOI] [PubMed] [Google Scholar]
- 9.Tekok-Kilic A, Benedict RH, Weinstock-Guttman B, Dwyer MG, Carone D, Srinivasaraghavan B, et al. Independent contributions of cortical gray matter atrophy and ventricle enlargement for predicting neuropsychological impairment in multiple sclerosis. Neuroimage. 2007;36:1294–300. doi: 10.1016/j.neuroimage.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7. [PubMed] [Google Scholar]
- 11.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P, et al. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–94. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- 13.Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B, et al. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler. 2008;14:906–12. doi: 10.1177/1352458508090221. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wechsler D. New York: Psychological cooperation; 1981. Mannual for the Wechsler intelligence scale-revised, 1981. [Google Scholar]
- 16.Trnyen L, Gheuens J, Van de Vyver FL, Parizel PM, Peersmam GV, Martin JJ. Improved correlation of magnetic resonance imaging (MRI) with clinical status in multiple sclerosis (MS) by use of an extensive standardized imaging-protocol. J Neurol Sci. 1990;96:173–82. doi: 10.1016/0022-510x(90)90130-f. [DOI] [PubMed] [Google Scholar]
- 17.Abedi MR. Standardization of Wechsler adult intelligence test validity and reliability in Iranian patients. Thesis, Tehran University of Medical Sciences. 1995 [Google Scholar]
- 18.Miller DH. Biomarkers and surrogate outcomes in neurodegenerative disease: Lessons from multiple sclerosis. NeuroRx. 2004;1:284–94. doi: 10.1602/neurorx.1.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedict RH, Carone D, Bakshi R. Correlating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosis. J Neuroimaging. 2004;14(Suppl 3):36–45S. doi: 10.1177/1051228404266267. [DOI] [PubMed] [Google Scholar]
- 20.Bermel RA, Bakshi R, Tjoa C, Puli SR, Jacobs L. Bicaudate ratio as a magnetic resonance imaging marker of brain atrophy in multiple sclerosis. Arch Neurol. 2002;59:275–80. doi: 10.1001/archneur.59.2.275. [DOI] [PubMed] [Google Scholar]
- 21.Piras MR, Magnano I, Canu ED, Paulus KS, Satta WM, Soddu A, et al. Longitudinal study of cognitive dysfunction in multiple sclerosis: Neuropsychological, neuroradiological, and neurophysiological findings. J Neurol Neurosurg Psychiatry. 2003;74:878–85. doi: 10.1136/jnnp.74.7.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christodoulou C, Krupp LB, Liang Z, Huang W, Melville P, Roque C, et al. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology. 2003;60:1793–8. doi: 10.1212/01.wnl.0000072264.75989.b8. [DOI] [PubMed] [Google Scholar]
- 23.Rao SM, Bernardin L, Leo GJ, Ellington L, Ryan SB, Burg LS. Cerebral disconnection in multiple sclerosis. Relationship to atrophy of the corpus callosum. Arch Neurol. 1989;46:918–20. doi: 10.1001/archneur.1989.00520440114028. [DOI] [PubMed] [Google Scholar]
- 24.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–66. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 25.Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neuro. 2010;9:921–32. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 26.Grazioli E, Zivadinov R, Weinstock-Guttman B, Lincoff N, Baier M, Wong JR, et al. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J Neurol Sci. 2008;268:12–7. doi: 10.1016/j.jns.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Trip SA, Schlottmann PG, Jones SJ, Li WY, Garway-Heath DF, Thompson AJ, et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: Evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage. 2006;31:286–93. doi: 10.1016/j.neuroimage.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Frohman E, Costello F, Zivadinov R, Stuve O, Conger A, Winslow H, et al. Optical coherence tomography in multiple sclerosis. Lancet Neurol. 2006;5:853–63. doi: 10.1016/S1474-4422(06)70573-7. [DOI] [PubMed] [Google Scholar]
- 29.Sergott RC, Frohman E, Glanzman R, Al-Sabbagh A. OCT in MS Expert Panel. The role of optical coherence tomography in multiple sclerosis: Expert panel consensus. J Neurol Sci. 2007;263:3–14. doi: 10.1016/j.jns.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Noval S, Contreras I, Muñoz S, Oreja-Guevara C, Manzano B, Rebolleda G. Optical coherence tomography in multiple sclerosis and neuromyelitis optica: An update. Mult Scler Int 2011. 2011 doi: 10.1155/2011/472790. 472790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anhoque CF, Biccas-Neto L, Domingues SC, Teixeira AL, Domingues RB. Cognitive impairment and optic nerve axonal loss in patients with clinically isolated syndrome. Clin Neurol Neurosurg. 2013;115:1032–5. doi: 10.1016/j.clineuro.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Siger M, Dziegielewski K, Jasek L, Bieniek M, Nicpan A, Nawrocki J, et al. Optical coherence tomography in multiple sclerosis: Thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol. 2008;255:1555–60. doi: 10.1007/s00415-008-0985-5. [DOI] [PubMed] [Google Scholar]
- 33.Wieder L, Gäde G, Pech LM, Zimmermann H, Wernecke KD, Dörr JM, et al. Low contrast visual acuity testing is associated with cognitive performance in multiple sclerosis: A cross-sectional pilot study. BMC Neurol. 2013;13:167. doi: 10.1186/1471-2377-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–92. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- 35.Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, et al. An investigation of the retinal nerve fibre layers in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131:277–87. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]


