Abstract
Granulomatous amebic encephalitis (GAE) from Balamuthia mandrillaris, a free-living ameba, has a case fatality rate exceeding 90% among recognized cases in the United States. In August 2010, a GAE cluster occurred following transplantation of infected organs from a previously healthy landscaper in Tucson, Arizona, USA, who died from a suspected stroke. As B. mandrillaris is thought to be transmitted through soil, a serologic survey of landscapers and a comparison group of blood donors in southern Arizona was performed. Three (3.6%) of 83 serum samples from landscapers and 11 (2.5%) of 441 serum samples from blood donors were seropositive (p = 0.47). On multivariable analysis, county of residence was associated with seropositivity, whereas age, sex, and ethnicity were not. Exposure to B. mandrillaris, previously unexamined in North America, appears to be far more common than GAE in Southern Arizona. Risk factors for disease progression and the ameba's geographic range should be examined.
Keywords: Balamuthia mandrillaris, Fluorescent Antibody Technique, Encephalitis, Amoeba, Arizona
Introduction
Balamuthia mandrillaris, a free-living ameba, is a rare and usually fatal cause of encephalitis. Between 1986, when the organism was discovered (Visvesvara et al. 1990), through 2009, only 70 cases of B. mandrillaris granulomatous amebic encephalitis (GAE) were identified in the United States, though the illness is likely underdiagnosed (Schuster et al. 2009). B. mandrillaris has been found in soil and dust (Dunnebacke et al. 2004; Schuster et al. 2003; Niyyati et al. 2009), and might also live in water, as do other free-living amebae such as Naegleria fowleri and Acanthamoeba spp. (Schuster et al. 2009). Isolation of B. mandrillaris from the environment is difficult due to strict growth requirements in vitro and a prolonged doubling time (Dunnebacke et al. 2004; Schuster et al. 2003; Ahmad et al. 2011). Exposure to B. mandrillaris is thought to occur primarily through disrupted skin causing skin infections, or via inhalation causing pulmonary infections (Siddiqui and Khan), though other exposure routes are possible (Kiderlen et al. 2007). The amebae may later disseminate hematogenously to the central nervous system causing encephalitis (Visvesvara et al. 2007). The incubation period for GAE is unclear, but intervals of two months to two years have been reported between onsets of skin lesions and encephalitis (Visvesvara et al. 2007).
Because of the small number of recognized cases, epidemiological data about B. mandrillaris infection are limited. In case reviews, males were more commonly affected than females, and the proportion of patients with reported Hispanic ethnicity is higher than the proportion of Hispanics in the general US population (Schuster et al. 2004). Both immunocompromised and immunocompetent individuals have been affected (Visvesvara et al. 1990). A preponderance of cases in hot, dry climates has been suggested (Seas et al. 2004), though cases have been reported from a variety of regions and climates (Matin et al. 2008).
Because most cases of B. mandrillaris infection are not diagnosed until after death, little is known about early stages of infection or frequency of asymptomatic or self-limited infections. Studies in West Africa using a fluorescence-activated cell sorter (FACS) method found elevated B. mandrillaris antibody levels among a traditional farming population with high levels of soil exposure, suggesting that B. mandrillaris exposure without disease might be common in certain groups (Kiderlen et al. 2009; Kiderlen et al. 2010). Serum from young children and adults in Australia had higher levels of antibodies than sera from umbilical cord blood, suggesting that widespread, low-level, environmental exposure is common (Huang et al. 1999). No serologic surveys for B. mandrillaris exposure among asymptomatic individuals in the Western Hemisphere have been performed.
In 2009, B. mandrillaris GAE emerged as a risk to organ transplant recipients following a disease cluster in the US state of Mississippi (CDC 2010a). In August 2010, a second transplant-associated cluster was identified in the US state of Arizona in which two patients, a liver recipient and a pancreas and kidney recipient, died of confirmed B. mandrillaris GAE (CDC 2010b). Stored serum from a common organ donor later tested positive for B. mandrillaris antibodies by immunofluorescence assay (IFA) at a titer of 1:64. Titers of 1:64 have been observed in patients with histologically-confirmed B. mandrillaris infection (G. Visvesvara, pers. comm.). Two other organ recipients, who received the heart and the other kidney from the same donor, developed IFA titers for B. mandrillaris of 1:32 and 1:64, respectively. Both were treated with experimental chemotherapy and remained asymptomatic. Antibody titers for both recipients declined to 1:16 by 7 months after beginning therapy.
In response to the Arizona GAE transplant cluster, we sought to determine risk factors for B. mandrillaris infection in the donor patient to improve epidemiological knowledge and to guide potential prevention strategies. Because the donor worked in Pima County, Arizona, as a landscaper, an occupation expected to have frequent soil exposures and therefore potentially greater exposure to B. mandrillaris, we performed a cross-sectional serologic survey of landscaping workers to evaluate whether B. mandrillaris exposure, measured by antibody seropositivity, was present in this group. We also assessed landscaping occupation and other soil exposures as risk factors for B. mandrillaris exposure using blood donors in Southern Arizona as a comparison group, one that would likely represent a wider range of occupations and exposures more similar to the general population.
Methods
Organ Donor Investigation
To assess the donor's exposures and health history, we interviewed his family members and co-workers and reviewed his medical records.
Serologic Survey Participant Selection and Serum Collection
From September 21 through 28, 2010, we recruited landscaping workers in Pima County, Arizona by contacting all publicly-listed landscaping companies by telephone and electronic mail. At companies that permitted us to visit, we surveyed all landscapers willing to participate using a one-time, in-person questionnaire, available in both English and Spanish, and collected a single blood specimen for serum antibody testing. The survey included questions about demographics, work duties, and outdoor recreational activities. No personal identifiers were collected. To quantify work duties, we asked whether participants worked primarily in installation landscaping (e.g., installing irrigation systems and building retaining walls) versus primarily in maintenance landscaping (e.g., mowing lawns and trimming bushes).
Two blood donation agencies serving southern Arizona provided 1 ml anonymized serum samples (American Red Cross, 241 samples; Creative Testing Solutions, 200 samples) collected during November 5 to December 11, 2010 from Arizona blood donors in Pima, Pinal, and Yuma Counties with available remainder serum. Demographic information for each blood donor was supplied. Occupations of blood donors were not available.
B. mandrillaris Culture
To grow amebae for antibody testing, we cultured B. mandrillaris (CDC:V619; isolated from the CSF of a GAE patient from Mississippi in 2010) on monolayers of monkey kidney (E6) cells as described before (Kucerova et al. 2011) and harvested cultures after they cleared the monolayer by ingesting all of the tissue culture cells. We then chilled the flasks on ice for 2 to 5 minutes, shook them to dislodge the amebae, and washed them three times in Hanks’ balanced salt solution (HBSS; Gibco catalog no. 14 025, Invitrogen). We then killed the amebae with formalin and washed them five times with phosphate-buffered saline.
ELISA
We performed a screening ELISA on serum specimens for B. mandrillaris IgG antibodies, and we followed with confirmatory IFA for specimens with positive and equivocal tests on ELISA. To make ELISA plates, we used the B. mandrillaris cultures described above and added 100 μl containing 2×103 killed amebae in phosphate-buffered saline (PBS) to Immulon 2 microtiter plate wells (Dynex Technologies, Inc., Chantilly, Virginia, USA) and dried them overnight at 37°C. We then blocked each plate with 5% milk in PBS for 30 minutes at room temperature. We diluted each serum specimen at a 1:800 dilution in PBS with 0.05% Tween 20 (PBST) and added 100 μl to the ELISA plates, which we incubated at room temperature for one hour and washed five times with PBST. We then diluted peroxidase-conjugated goat anti-human immunoglobulin (Biosource Internatinonal, Camarillo, California, USA) at 1:7,000 in PBST and added 100 μl to each well and incubated the plates at room temperature for one hour. Plates were again washed five times with PBST. We developed the wells using tetramethylbenzadine (TMB) substrate solution (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA) and stopped the reaction using 1:20 phosphoric acid (Fisher, Fair Lawn, New Jersey, USA). We read absorbance values for each well using a Molecular Devices Vmax Microplate Reader (Menlo Park, California) at 450 nm and SOFTmax version 2.35, 1993 software. On each plate, we also ran positive control sera at serial dilutions of 1:100 to 1:3,200 and negative control sera at a dilution of 1:800. We constructed standard curves using positive controls for each plate. Serum specimens with absorbance values ≥ mean + 2 standard deviations of negative control sera were selected for further testing with IFA; 26 randomly-selected specimens with absorbance values below this level were also tested by IFA.
Immunofluorescence Assay
We added 10 μl of killed B. mandrillaris amebae in PBS to each well of a 12 well slide, at a concentration of 1×105/well, and dried the slide at room temperature. For selected serum specimens, described above, we added sera at serial dilutions of 1:2 to 1:4,096 in PBS to each well of a slide and incubated the slide in a moist chamber for 30 minutes at 37°C. We then washed slides three times with PBS. Next we added a 1:100 dilution of fluorescein isothiocyanate (FITC) labeled goat anti-human immunoglobulins (Cappel Laboratories, Cochraneville, Pennsylvania, USA) with Evans Blue to each well, which we incubated in a moist chamber at 37°C for 30 minutes and washed five times with PBST. We read fluorescence using an Olympus microscope; a specimen was considered positive, and a participant seropositive, at a previously-established cutoff of ≥1:64 (Schuster et al. 2001). To assess cross-reactivity of the IFA with antibodies to other pathogens, we tested stored sera from persons with Acanthamoeba spp. GAE, cysticercosis, and Entamoeba histolytica infection.
Statistical Analysis
We determined B. mandrillaris seropositivity, defined by an IFA titer of ≥1:64, for both landscapers and blood donors and evaluated the demographic differences between the seropositive and seronegative within groups using Fisher's exact test. We also used multiple logistic regression to examine the association of seropositivity with age, sex, ethnicity, geographic location (for blood donors), and work duties (for landscapers), when adjusting for each of these factors. Because few blood donors resided in Pinal County, and because the main comparison was to Pima County, we combined Pinal and Yuma blood donors in this analysis. We used the Hosmer-Lemeshow test to assess model fit; the Firth's penalized maximum likelihood method was used to address quasi-complete separation of data. A significance level of 0.05 was used for all statistical tests.
Results
Organ Donor Investigation
The organ donor was a 27-year-old previously healthy Hispanic man who resided in Pima County, Arizona, worked as a landscaper (mostly maintenance) in that county, and had a history of occasional alcohol and cocaine use. His death had been attributed to a stroke. His only outdoor exposures apart from landscaping were jogging and playing soccer. The donor's coworkers recalled that six months prior to hospitalization he reported an insect bite on his left shoulder while pulling weeds. They reported that the lesion subsequently became painful and grew larger, first outward in a circle, then developing finger-like projections. During hospitalization, an approximately 10–12 cm erythematous skin lesion with serpiginous borders was noted on the donor's left upper back.
Serologic Survey
For the serologic survey, we contacted all 65 publicly-listed landscaping companies in Pima County and 9 (14%) agreed to assist. Within these companies, we enrolled a convenience sample of 83 landscapers willing to participate; all lived in Pima County, 82 (99%) were male, 47 (57%) were Hispanic, and the median age was 44 years (range 20–68 years). Median number of years landscaping was 10 (range 3 months to 40 years).
Of 441 persons in our sample of blood donors from Southern Arizona, 288 (65%) resided in Pima County, 148 (34%) resided in Yuma County, and 5 (1%) resided in Pinal County; 242 (55%) were male, 175 (40%) were Hispanic, and the median age was 34 years (range 16–86 years). These donors represented a convenience sample taken from among 2,441 donors to both blood donation agencies during November 5 to December 11, 2010. Of the 2,441 donors from Pima, Yuma, and Pinal Counties, 50% were male, 26% were Hispanic, and the median age was 33 years.
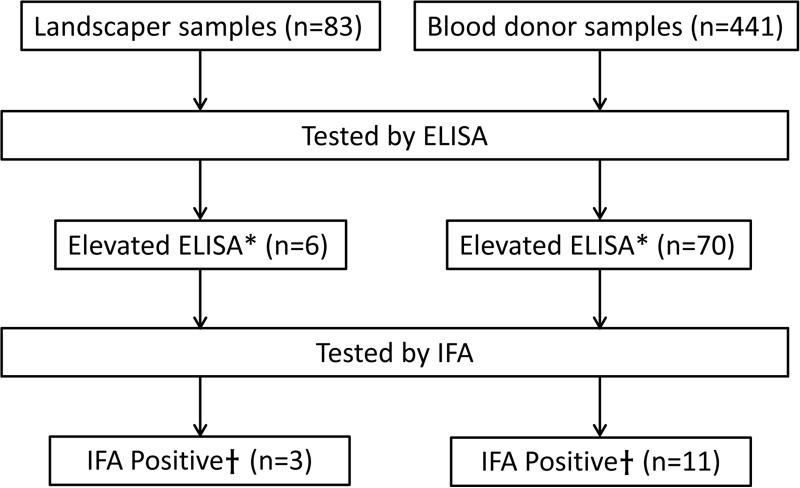
Of the 524 total serum specimens from both landscapers and blood donors, 76 (15%) had ELISA absorbance values ≥ mean +2 standard deviations of negative control sera and underwent testing by IFA; 14 of these specimens had positive IFA titers, all at the 1:64 dilution, and were considered seropositive (Fig. 1). In total, 3 of 83 (3.6%) landscapers were seropositive and 11 of 441 (2.5%) blood donors were seropositive (p = 0.47). All 26 specimens tested by IFA that had ELISA absorbance values below the cutoff had negative IFA titers (<1:64). Stored sera from persons with Acanthamoeba spp. GAE, cysticercosis, and Entamoeba histolytica infection also had negative IFA titers.
Fig. 1.
Flowchart of testing for serum antibodies to Balamuthia mandrillaris among landscapers and blood donors, Southern Arizona, USA, 2010.
aDefined as absorbance value ≥ mean + 2 standard deviations of negative control sera
bImmunofluorescence assay (IFA) titer ≥ 1:64 (designated as seropositive)
Among landscapers, there were no significant differences in seropositivity by sex, age, ethnicity, or work duties (Table 1), though all three seropositive landscapers were younger than age 40 years compared with 34% of seronegative landscapers (p = 0.06). Regarding work duties, two (67%) of three seropositive landscapers reported doing mostly or all installation landscaping compared with 13 (16%) of 80 seronegative controls (p = 0.08). On multivariable analysis of landscapers (Table 2), doing mostly or all installation landscaping remained a significant risk factor for seropositivity after adjusting for age and ethnicity (adjusted odds ratio 11.6, 95% CI 1.0–129.4). For this model, sex was excluded because nearly all landscapers were male. By the Hosmer-Lemeshow test, the model demonstrated reasonable overall fit to the data (p = 0.93).
Table 1.
Characteristics of Balamuthia mandrillaris seropositive and seronegative landscapers in Pima County, Arizona, USA, 2010
| Seropositive (N=3) | Seronegative (N=80) | p-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Male | 3 (100%) | 79 (99%) | >0.99 |
| Age < 40 years | 3 (100%) | 27 (34%) | 0.06 |
| Hispanic ethnicity | 2 (67%) | 45 (56%) | >0.99 |
| Most or all installation vs. maintenance landscaping | 2 (67%) | 13 (16%) | 0.08 |
Table 2.
Unadjusted and adjusted odds ratios for Balamuthia mandrillaris seropositivity among landscapers, Pima County, Arizona, USA, 2010
| Unadjusted OR | 95% CI | Adjusted ORa | 95% CI | |
|---|---|---|---|---|
| Age < 40 years | 11.6 | 0.6–239.8 | 11.5 | 0.7–187.0 |
| Hispanic ethnicity | 1.3 | 0.2–10.6 | 3.4 | 0.3–39.6 |
| Most or all installation vs. maintenance landscaping | 6.5 | 0.8–54.7 | 11.6 | 1.0–129.4* |
Calculated by multiple logistic regression, adjusted for other variables in the table
Among blood donors, no significant differences in seropositivity were observed for sex, age, or ethnicity (Table 3). Three (1.0%) of 288 blood donors residing in Pima County were seropositive, one (20.0%) of five blood donors residing in Pinal County was seropositive, and seven (4.7%) of 148 blood donors residing in Yuma County were seropositive; the difference among these three groups was significant (p = 0.02). The combined seroprevalence in Pinal and Yuma Counties (5.3%) was significantly higher than the seroprevalence in Pima County (p = 0.02). Among blood donors, residence in Yuma or Pinal Counties compared with Pima County remained significant after adjusting for sex, age, and ethnicity (Table 4) with an adjusted odds ratio of 5.2 (95% CI 1.4–19.7); p = 0.71 for the Hosmer-Lemeshow test.
Table 3.
Characteristics of Balamuthia mandrillaris seropositive and seronegative blood donors, Southern Arizona, USA, 2010
| Seropositive (N=11) | Seronegative (N=430) | p-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Male | 6 (55%) | 236 (55%) | >0.99 |
| Age < 40 years | 6 (55%) | 248 (58%) | >0.99 |
| Hispanic ethnicity | 6 (55%) | 169 (39%) | 0.53 |
Table 4.
Unadjusted and adjusted odds ratios for Balamuthia mandrillaris seropositivity among blood donors, Southern Arizona, USA, 2010
| Unadjusted OR | 95% CI | Adjusted ORa | 95% CI | |
|---|---|---|---|---|
| Yuma/Pinal County vs. Pima County | 4.8 | 1.3–16.9* | 5.2 | 1.4–19.7* |
| Male | 1.0 | 0.3–3.1 | 0.8 | 0.3–2.4 |
| Age <40 years | 0.9 | 0.3–2.7 | 0.7 | 0.2–2.5 |
| Hispanic ethnicity | 1.7 | 0.5–5.4 | 0.9 | 0.2–3.9 |
Calculated by multiple logistic regression, adjusted for other variables in the table
We also compared seropositivity between landscapers and the blood donors. The seroprevalence of 3.6% found for the Pima County landscapers was higher, but not significantly so, than the seroprevalence of 1.0% for blood donors also residing in Pima County (p = 0.13). The seroprevalence among landscapers (3.6%) was lower, also not significantly so, than the seroprevalence of 5.2% among blood donors from Pinal and Yuma Counties (p = 0.75).
Discussion
Our findings suggest that B. mandrillaris exposure occurs in Southern Arizona both among landscapers and blood donors. Although we do not know to what extent we can generalize these findings to the population of the area, the findings of seropositive participants among both landscapers and a diverse group of blood donors in various counties suggest that a small percentage of people in Southern Arizona have been exposed to B. mandrillaris. This proportion of seropositive individuals, approximately 3% among all participants, vastly exceeds the estimated incidence of B. mandrillaris GAE, supporting the hypothesis that exposure to B. mandrillaris is far more common than invasive B. mandrillaris infections resulting in GAE, of which there were only 70 known cases in the United States during 1974–2009 (CDC, unpub. data).
The reasons for the large difference observed between exposure and invasive disease are unclear. Host risk factors likely play a role in progression to illness among those exposed. However, many GAE patients have no known immunosuppressing conditions, suggesting that immune status might not be the only factor in disease progression. Variations in the pathogenicity of B. mandrillaris strains could exist and some strains could be more likely to lead to GAE, though little antigenic or genotypic variation has been seen across a range of B. mandrillaris isolates (Schuster et al. 2008; Booton et al. 2003).
In our investigation, seropositivity was not significantly associated with age, sex, ethnicity, or landscaper status, but varied substantially by county of residence. The higher B. mandrillaris seroprevalence found for blood donors in Yuma and Pinal Counties compared with Pima County might be explained by varying occupations and soil exposures among blood donors in each county. We do not know the blood donors’ occupations, but county-level statistics suggest that population exposures to soil might differ. Agriculture, a potential source of soil exposure, is a much larger industry in Yuma and Pinal Counties than in Pima County. In 2007, Yuma County and Pinal County accounted for 30% and 25% of the state's agricultural sales, respectively, compared with only 2% for Pima County (U.S. Department of Agriculture 2013). Most Pima County residents live in the urban and suburban Tucson metropolitan area with a population of approximately one million, while Pinal and Yuma Counties each have lower population densities than Pima County (U.S. Census Bureau 2013).
Further supporting the soil exposure hypothesis, we found a trend towards higher B. mandrillaris seroprevalence among landscapers than blood donors from Pima County. Similarly, in the multivariable model, a greater proportion of landscapers with mostly or all installation duties were seropositive compared to those with mostly or all maintenance duties, suggesting that certain types of work might result in greater B. mandrillaris exposure. However, these findings must be interpreted with caution as they are based on only three seropositive landscapers and the confidence intervals are wide. Finally, a route of ameba entry following soil exposure was identified in the donor patient. The reported insect bite was a likely source of skin disruption, which could have allowed introduction of B. mandrillaris from soil.
As this was the first study of B. mandrillaris seroprevalence in the United States and the geographical habitat of B. mandrillaris is unknown, it is unclear to what extent residents of other regions might be similarly exposed. A report of B. mandrillaris GAE cases from California found that most cases occurred in the southern portion of the state (Schuster et al. 2006), which borders Arizona. Whether this finding represents a bias in detection, differences in exposure, or is a marker for a particularly suitable habitat for B. mandrillaris is unclear. Supporting the hypothesis that this region might provide particularly favorable conditions for B. mandrillaris, Ahmad et al found that 16 of 17 soil samples from Southern California were positive by PCR for B. mandrillaris compared with 0 of 44 from the United Kingdom (Ahmad et al. 2011).
In contrast to the higher proportion of B. mandrillaris GAE cases among males and Hispanic individuals previously reported, our investigation found no significant differences in exposure between the sexes or between Hispanics and other ethnic groups. It is possible that males and Hispanic persons are more likely to progress to GAE from exposure to B. mandrillaris. However, differences in sex and ethnicity among GAE cases might also be explained by occupation or county of residence, likely as markers of soil exposure. In our sample, 99% of landscapers were men and 57% were of Hispanic ethnicity, and there was a trend toward greater seropositivity among landscapers than among blood donors from the same county (of which only 55% were men and 25% were of Hispanic ethnicity). Similarly, Yuma County, which is more agricultural and 61% Hispanic, had a significantly higher B. mandrillaris seroprevalence than Pima County, which is less agricultural and 35% Hispanic (U.S. Census Bureau 2013).
The small sample size of the landscaper sample and lack of randomization limits the investigation's power and the ability to generalize our seroprevalence results to all landscapers in Pima County. However, despite non-randomized sampling, our sample still provides useful B. mandrillaris exposure information not otherwise available about a group of outdoor workers in the United States. Our investigation is also limited by non-randomized sampling of blood donors and lack of occupational information, as some blood donors could have worked as landscapers. However, landscapers likely compose a small percentage of blood donors, and landscaping is one of many occupations that involve soil exposure. Thus, we believe blood donors serve as a reasonable comparison group, much more similar to the general population than landscapers.
Host antibodies to other pathogens are unlikely to explain positive B. mandrillaris IFA results. Studies of patients with encephalitis have found that IFA and ELISA testing appear to be relatively specific for B. mandrillaris infection, suggesting that they are good markers for exposure (Kucerova et al. 2011; Schuster et al. 2001; Schuster et al. 2008). Further, our findings confirm those from other studies showing that infections with other parasites, including infections with other amebae, do not yield false positives on B. mandrillaris antibody testing (Kiderlen et al. 2009; Kiderlen et al. 2010; Schuster et al. 2001).
Our findings of elevated B. mandrillaris antibody levels among presumably healthy Arizona residents are supported by previous research that found elevated B. mandrillaris antibody levels in other asymptomatic populations (Kiderlen et al. 2009; Huang et al. 1999), though these studies are not directly comparable to our investigation because of the use of differing testing methods. In contrast to the study of West African individuals with frequent soil exposures, which found a positive linear association between age and B. mandrillaris antibody levels, we found no association between age and seropositivity among landscapers or blood donors (Kiderlen et al. 2010). The lack of association between age and antibody status in our U.S. cohort, despite similar age distributions to the West African study, might be related to less frequent B. mandrillaris exposures compared with the African group, though little is known about the duration of elevated antibody levels following B. mandrillaris exposure.
Our investigation represents the first serologic survey for B. mandrillaris among asymptomatic individuals in the Americas. Based on antibody testing, B. mandrillaris exposure appears far more common than clinical disease, suggesting that exposure is relatively common and usually inconsequential. Risk factors for progression to GAE among exposed individuals should be examined. Data from this investigation on occupation and county of residence add to existing evidence that contact with soil is a likely risk factor for B. mandrillaris exposure; clinicians should consider B. mandrillaris infection as a potential cause of unexplained skin lesions or encephalitis, particularly among persons with frequent soil exposures.
Although B. mandrillaris exposure appears fairly common, disease is rare and no screening test is currently available for potential organ donors. Similarly, there is no screening test available for blood donors, although the risk for transfusion-transmitted disease is unknown since there have been no documented cases of transmission via this route. Greater emphasis is needed to promote early diagnosis of GAE pre-mortem before organ donation to protect potential organ recipients from exposure and potential disease. Furthermore, standardized serological methods using recombinant protein antigens are needed to better understand B. mandrillaris seroprevalence across populations and to improve exposure data and risk assessments.
Acknowledgements
We thank the landscapers for their participation and Chukwuma Mbaeyi, Eileen Farnon, Matthew Kuehnert, Brad McKinney, Michelle McDonald, Sherry Daniels, Ken Komatsu, Myrna Seiter, Orion McCotter, Michael Arrowood, and Anna Yaffee for contributing to this investigation.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Ethical Standards
The investigation described in this manuscript complied with the laws of the United States.
The authors declare that they have no conflict of interest.
References
- Ahmad AF, Andrew PW, Kilvington S. Development of a nested PCR for environmental detection of the pathogenic free-living amoeba Balamuthia mandrillaris. J Eukaryot Microbiol. 2011;58:269–71. doi: 10.1111/j.1550-7408.2011.00541.x. [DOI] [PubMed] [Google Scholar]
- Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg. 2003;68:65–9. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Balamuthia mandrillaris transmitted through organ transplantation—Mississippi, 2009. MMWR Morb Mortal Wkly Rep. 2010a;59:1165–70. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Notes from the field: transplant-transmitted Balamuthia mandrillaris—Arizona, 2010. Morbidity and Mortality Weekly Report. 2010b;59:1182. [PubMed] [Google Scholar]
- Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology. 2004;150:2837–42. doi: 10.1099/mic.0.27218-0. [DOI] [PubMed] [Google Scholar]
- Huang ZH, Ferrante A, Carter RF. Serum antibodies to Balamuthia mandrillaris, a free-living amoeba recently demonstrated to cause granulomatous amoebic encephalitis. J Infect Dis. 1999;179:1305–8. doi: 10.1086/314731. [DOI] [PubMed] [Google Scholar]
- Kiderlen AF, Laube U, Radam E, Tata PS. Oral infection of immunocompetent and immunodeficient mice with Balamuthia mandrillaris amebae. Parasitol Res. 2007;100:775–82. doi: 10.1007/s00436-006-0334-5. [DOI] [PubMed] [Google Scholar]
- Kiderlen AF, Radam E, Tata PS. Assessment of Balamuthia mandrillaris-specific serum antibody concentrations by flow cytometry. Parasitol Res. 2009;104:663–70. doi: 10.1007/s00436-008-1243-6. [DOI] [PubMed] [Google Scholar]
- Kiderlen AF, Radam E, Schuster FL, Adjogoua EV, Akoua-Koffi C, Leendertz FH. Balamuthia and Acanthamoeba-binding antibodies in West African human sera. Exp Parasitol. 2010;126:28–32. doi: 10.1016/j.exppara.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Kucerova Z, Sriram R, Wilkins PP, Visvesvara GS. Identification of antigenic targets for immunodetection of Balamuthia mandrillaris infection. Clin Vaccine Immunol. 2011;18:1297–301. doi: 10.1128/CVI.05082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A, Siddiqui R, Jayasekera S, Khan NA. Increasing importance of Balamuthia mandrillaris. Clin Microbiol Rev. 2008;21:435–48. doi: 10.1128/CMR.00056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, Maciver SK, Valladares B. Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res. 2009;106:279–81. doi: 10.1007/s00436-009-1592-9. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Glaser C, Gilliam S, Visvesvara GS. Survey of sera from encephalitis patients for Balamuthia mandrillaris antibody. J Eukaryot Microbiol. 2001;48:10s–2s. doi: 10.1111/j.1550-7408.2001.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, Vugia D, Bakardjiev A, Azimi P, Maddux-Gonzalez M, Martinez AJ, Visvesvara GS. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol. 2003;41:3175–80. doi: 10.1128/JCM.41.7.3175-3180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster FL, Glaser C, Honarmand S, Maguire JH, Visvesvara GS. Balamuthia amebic encephalitis risk, Hispanic Americans. Emerg Infect Dis. 2004;10:1510–2. doi: 10.3201/eid1008.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster FL, Honarmand S, Visvesvara GS, Glaser CA. Detection of antibodies against free-living amoebae Balamuthia mandrillaris and Acanthamoeba species in a population of patients with encephalitis. Clinical Infectious Diseases. 2006;42:1260–5. doi: 10.1086/503037. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Yagi S, Wilkins PP, Gavali S, Visvesvara GS, Glaser CA. Balamuthia mandrillaris, agent of amebic encephalitis: detection of serum antibodies and antigenic similarity of isolates by enzyme immunoassay. J Eukaryot Microbiol. 2008;55:313–20. doi: 10.1111/j.1550-7408.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Yagi S, Gavali S, Michelson D, Raghavan R, Blomquist I, Glastonbury C, Bollen AW, Scharnhorst D, Reed SL, Kuriyama S, Visvesvara GS, Glaser CA. Under the radar: Balamuthia amebic encephalitis. Clin Infect Dis. 2009;48:879–87. doi: 10.1086/597260. [DOI] [PubMed] [Google Scholar]
- Seas RC, Bravo PF. Amebic granulomatosis encephalitis due to Balamuthia mandrillaris: a fatal disease increasingly recognized in Latin America [in Spanish]. Rev Chilena Infectol. 2006;23:197–9. doi: 10.4067/s0716-10182006000300001. [DOI] [PubMed] [Google Scholar]
- Siddiqui R, Khan NA. Balamuthia amoebic encephalitis: an emerging disease with fatal consequences. Microb Pathog. 2008;44:89–97. doi: 10.1016/j.micpath.2007.06.008. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau [20 Aug 2013];State & County QuickFacts: Pima, Pinal, and Yuma Counties. 2013 http://quickfacts.census.gov/qfd/states/04000.html.
- U.S. Department of Agriculture [20 Aug 2013];State fact sheets: Arizona. 2013 http://www.ers.usda.gov/StateFacts/AZ.htm.
- Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28:2750–6. doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]