Abstract
Background
The potential for aerosol transmission of infectious influenza virus (ie, in healthcare facilities) is controversial. We constructed a simulated patient examination room that contained coughing and breathing manikins to determine whether coughed influenza was infectious and assessed the effectiveness of an N95 respirator and surgical mask in blocking transmission.
Methods
National Institute for Occupational Safety and Health aerosol samplers collected size-fractionated aerosols for 60 minutes at the mouth of the breathing manikin, beside the mouth, and at 3 other locations in the room. Total recovered virus was quantitated by quantitative polymerase chain reaction and infectivity was determined by the viral plaque assay and an enhanced infectivity assay.
Results
Infectious influenza was recovered in all aerosol fractions (5.0% in >4 µm aerodynamic diameter, 75.5% in 1–4 µm, and 19.5% in <1 µm; n = 5). Tightly sealing a mask to the face blocked entry of 94.5% of total virus and 94.8% of infectious virus (n = 3). A tightly sealed respirator blocked 99.8% of total virus and 99.6% of infectious virus (n = 3). A poorly fitted respirator blocked 64.5% of total virus and 66.5% of infectious virus (n = 3). A mask documented to be loosely fitting by a PortaCount fit tester, to simulate how masks are worn by healthcare workers, blocked entry of 68.5% of total virus and 56.6% of infectious virus (n = 2).
Conclusions
These results support a role for aerosol transmission and represent the first reported laboratory study of the efficacy of masks and respirators in blocking inhalation of influenza in aerosols. The results indicate that a poorly fitted respirator performs no better than a loosely fitting mask.
Current evidence indicates that influenza can be transmitted by direct and indirect contact, droplet spray, and aerosol particles in the inhalable size range (≤10 µm) [1]. Transmission via respirable particles (≤4 µm), which can remain airborne for long periods and be inhaled into the lung alveoli, has been particularly controversial [2–5]. As early as 1941, aerosol transmission was demonstrated between ferrets that were separated by up to 2.75 m [6]. More recent studies in ferrets [7–9] and guinea pigs [10–14] support airborne transmission (ie, by aerosol and/or large droplets and droplet nuclei) over considerably shorter distances (5–107 cm), although this transmission was strain dependent [9, 10, 14, 15]. Findings of studies in which influenza was administered experimentally by aerosol or intranasal inoculation provide indirect evidence that transmission of influenza in communities can occur by the aerosol route [16–18].
Transmission of influenza on respirable particles potentially generated during coughing, sneezing, and breathing is a concern in healthcare facilities because these particles may remain airborne for prolonged periods. Several studies have detected influenza RNA in the exhaled breath and coughs of patients with influenza [19–23]. In 1 study, patients shed about 33 viral copies/min in aerosol particles ≥5 µm and 187 viral copies/min in particles <5 µm, and infectious virus was detected in the breath from 2 patients [22]. In another study, cough aerosols from 81% of the influenza-positive patients contained influenza RNA and 65% of the viral RNA was contained in particles <4 µm [23]. Two clinical studies showed that the highest concentrations of influenza RNA were detected in locations where the number of patients with influenza was highest and that 42%–53% of the viral RNA was contained in particles ≤4 µm [24, 25].
If it were known that infectious influenza virus is present on these small particles, the risk of infection could be properly assessed, and appropriate guidelines for prevention could then be established. To address this issue, a patient examination room containing a coughing manikin that “coughs” influenza virus into the room to simulate a patient with influenza and a breathing manikin to simulate a healthcare worker was constructed. National Institute for Occupational Safety and Health (NIOSH) aerosol samplers positioned within the breathing manikin and at various locations throughout the room were used to collect and size-fractionate the airborne particles. In this study, we show that infectious virus is present on a range of collected particles and we examine the effectiveness of surgical masks and N95 respirators in blocking virus inhalation.
MATERIALS AND METHODS
Cells and Virus
Madin-Darby canine kidney (MDCK) cells (American Type Culture Collection [ATCC] CCL-34) and influenza strain A/WS/33 (H1N1, ATCC VR-825, lot 58023547 at 1.58 × 108 50% chicken embryo infectious dose [CEID50]/mL, and lot 58772128 at 2.8 × 106 CEID50/mL) were purchased from the ATCC and maintained as described elsewhere [26].
Bioaerosol Samplers
NIOSH samplers, which collect and size-fractionate aerosols into 3 fractions (>4-, 1–4-, and <1-µm aerodynamic diameters), were used to collect influenza-containing aerosols [24, 27].
Extraction of Virus From Surgical Gloves, Masks, and Respirators
Virus was eluted by overnight incubation at 4°C in 1 mL of supplemented [26] Hank’s balanced salt solution.
Real-Time Quantitative Polymerase Chain Reaction
Matrix gene copies were detected by real-time quantitative polymerase chain reaction analysis, as described elsewhere [26].
Viral Plaque Assay
For viral plaque analysis, aerosol samples containing infectious influenza were inoculated onto a confluent lawn of MDCK cells and plaque-forming units were calculated, as described elsewhere [26].
Viral Replication Assay
To enhance the ability to detect infectious virus, the copy number of infectious virus was amplified before detection by a modified 50% tissue culture infectious dose (TCID50) assay, that is, the viral replication assay (VRA), as described elsewhere [26].
Aerosol Exposure Simulation Chamber
The simulated examination room was 2.75 m × 2.75 m × 2.40 m and included a high-efficiency particulate air filter and a UV lamp [28] to disinfect the room. Influenza was aerosolized with an Aeroneb 2.5–4-µm micropump nebulizer (Aerogen), as described elsewhere [26], and loaded into the cough simulator remotely for a total of 5 coughs at approximately 2-minute intervals, also as described elsewhere [28]. The coughing simulator uses a metal bellows driven by a computer-controlled linear motor (Model STA2506; Copley Controls) to reproduce the flow and aerosol pattern of a human cough. The cough had a 4.2-L volume with a peak flow of 16.9 L/s and a mean flow of 5.28 L/s. The digital breathing simulator (Warwick Technologies) was equipped with a standard medium-sized head form (Sheffield model 189003; ISI). The breathing waveform was sinusoidal with a flow rate of 32 L/min (ISO standard for an adult 1.88 m tall with a mass of 85 kg engaged in moderate work) [29]. The coughing and breathing simulators were synchronized so that each cough was initiated at the start of an inhalation.
A surgical mask (Kimberly Clark 47625) or N95 respirator (3MM1860) was either tightly sealed over the mouth of the breathing simulator using silicone sealant or attached using the tie straps or elastic headbands of the mask or respirator. The fit factor of each mask or respirator was measured using a standard respirator fit-testing device (Model 8038 PortaCount Pro Plus; TSI). The fit factor is defined as 1/fraction of particles that pass through the mask.
RESULTS
Detection of Infectious Influenza on Aerosolized Particles
To determine whether infectious influenza could be recovered from airborne particles, influenza expelled by the coughing simulator was collected for 60 minutes by 5 NIOSH samplers. The samplers drew aerosol samples from a port located approximately 1 mm above the mouth (through mouth) of the breathing simulator, 10 cm to the right of the mouth (beside mouth), and at 3 other positions (P1, P2, P3) within the simulation chamber (Figure 1). Approximately 3.49 × 106 total virus was coughed into the simulation chamber (202 virus per liter of chamber air). The average total recovered virus per liter of collected air from each of the 5 samplers in 5 independent experiments was 1.35 × 104 (standard error, 1.74 × 103) (Figure 2A). Most of the virus was recovered in the 1–4-µm aerosol fraction (75.5%) and <1-µm fraction (19.5%); the remainder was detected in the >4-µm fraction (5.0%) (Figure 2A). Infectious influenza, assessed by the viral plaque analysis, was recovered in all 3 fractions and from all NIOSH samplers regardless of their position within the simulation chamber (Figure 2B). The presence of infectious influenza was confirmed using an enhanced infectivity assay, the VRA (Figure 2C). There was no statistically significant difference in the percentages of virus that remained infectious in the 3 fractions or the 5 samplers.
Figure 1.
Three-dimensional view of the aerosol exposure chamber. National Institute for Occupational Safety and Health samplers collected aerosols through the mouth (depicted as black oval in breathing mannequin's head), 10 cm beside the mouth of the breathing simulator, and in 3 other positions (P1, P2, P3), as shown. The mouths of the coughing and breathing simulators and sampler inlets at P1, P2, and P3 were located 152 cm above the floor (approximate height of a sitting patient and healthcare worker). For 3 experiments, fingertips from medical gloves were also placed on the manikin's forehead and alongside 3 of the aerosol samplers. All dimensions adjacent to white arrows within chamber are in centimeters.
Figure 2.
Detection of infectious influenza on aerosolized particles. National Institute for Occupational Safety and Health (NIOSH) samplers drew aerosol samples from a port located ~1 mm above the mouth (through mouth) of the breathing simulator, 10 cm to the right of the mouth (beside mouth), and at 3 other positions (P1, P2, P3) within the environmental chamber. The amount of influenza virus detected in each fraction (>4, 1–4, and <1 µm) collected by the NIOSH sampler per liter of air collected is shown. A, B, Amounts of total virus (infectious and noninfectious) collected in each fraction was determined by quantification of the matrix gene by quantitative polymerase chain reaction (qPCR) (A) and by the plaque-forming unit assay (B). C, Viral replication assay (VRA) demonstrated the amount of infectious virus collected after amplification in Madin-Darby canine kidney cells to increase the sensitivity of detection. Data are means ± standard errors (n = 5).
Tightly Fitted (Sealed) Surgical Masks and N95 Respirators and Exposure to Airborne Infectious Influenza
To examine the extent to which personal protective equipment (PPE) can effectively protect against aerosol exposure to influenza, surgical masks and N95 respirators were sealed to the breathing manikin’s face to prevent aerosols from circumventing the PPE. Fit factors were determined to be 135 for the surgical masks and 200+ for the N95 respirators (for the sealed PPE, the fit factor measurement reflects the penetration of particles through the PPE, because face seal leakage was prevented). The total virus collected through the manikin’s mouth by a NIOSH sampler compared with that collected beside the mouth revealed that 99.8% was blocked from entering the mouth by a tightly fitted respirator (Figure 3A). Furthermore, ≥99.5% of viral entry was blocked for all aerosol fractions. Similarly, 99.6% of the infectious virus was blocked from entering the mouth, with ≥99.4% of virus from each aerosol fraction blocked from entry (Figure 3B). The VRA confirmed these results and showed that 99.8% of the total infectious virus was blocked by the sealed respirator (Figure 3C).
Figure 3.
Tightly fitting (sealed) N95 respirators efficiently block exposure to airborne infectious influenza. An N95 respirator was sealed over the mouth of the breathing mannequin with silicone caulk. Amounts of infectious and noninfectious virus collected are as described for Figure 2. Data are means ± standard errors (n = 3); qPCR, quantitative polymerase chain reaction; VRA, viral replication assay.
A tightly fitted mask blocked 94.5% of the total virus and ≥91.8% was blocked regardless of which aerosol fraction was tested (Figure 4A). Similarly, 94.8% of the total infectious virus was blocked, with ≥92.7% being blocked regardless of which aerosol fraction was tested (Figure 4B). The VRA showed that 92.9% of the total infectious virus was blocked by the sealed mask (Figure 4C).
Figure 4.
Tightly fitting (sealed) surgical masks efficiently block exposure to airborne infectious influenza. A surgical mask was sealed over the mouth of the breathing manikin with silicone caulk. The amount of infectious and noninfectious virus collected is as described for Figure 2. Data are means ± standard errors (n = 3); qPCR, quantitative polymerase chain reaction; VRA, viral replication assay.
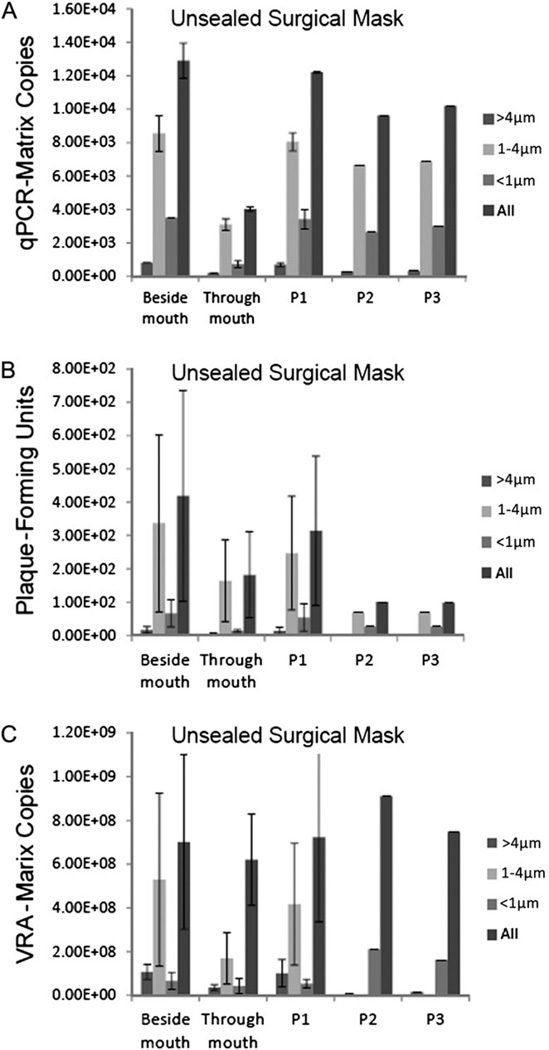
Loosely Fitting (Unsealed) Surgical Masks and Poorly Fitting (Unsealed) N95 Respirators and Exposure to Airborne Infectious Influenza
Surgical masks typically have low fit factors owing to gaps and leaks between the mask and face. N95 respirators that are poorly fitted or improperly worn can also have a dramatically reduced fit factor [30–32]. To simulate low fit factors, masks and respirators were attached to the face using the tie strings or elastic headbands but without using sealant. Fit factors ranged from 2.3 to 4.6 (100 is considered passing). The total virus collected through the manikin’s mouth compared with that collected by a sampler beside the mouth showed that 69.9% was blocked from entering the mouth by a poorly fitting respirator (Figure 5A). Furthermore, ≥64.5% of virus from all 3 aerosol fractions was blocked from entering. Approximately 66.5% of the total infectious virus was blocked by the unsealed, poorly fitting respirator (≥59.2% of the blocked infectious virus was in the ≤4 µm fractions; Figure 5B). Similarly, the VRA showed that 66.5% of the total infectious virus was blocked from entry (Figure 5C).
Figure 5.
Poorly fitting (unsealed) N95 respirators are less efficient at blocking exposure to airborne infectious influenza. An N95 respirator was fitted over the mouth of the breathing manikin with the mask's tie straps. The amount of infectious and noninfectious virus collected is as described for Figure 2. Data are means ± standard errors (n = 3); qPCR, quantitative polymerase chain reaction; VRA, viral replication assay.
Similarly, a poorly fitting mask blocked 68.9% of the total virus (Figure 6A), and entry of 56.6% of the total infectious virus was blocked (≥51.2% of the blocked infectious virus was in the ≤4-µm fractions) (Figure 6B). In contrast, the VRA indicated that only 11.6% of the total infectious virus was blocked (Figure 6C).
Figure 6.
Loosely fitting (unsealed) surgical masks are less efficient at blocking exposure to airborne infectious influenza. A surgical mask was fitted over the mouth of the breathing manikin with the mask's tie straps. The amount of infectious and noninfectious virus collected is as described for Figure 2. Data are means ± standard errors (n = 2); qPCR, quantitative polymerase chain reaction; VRA, viral replication assay.
Recovery of Infectious Influenza Virus From PPE
Significant amounts of influenza were recovered from a 25-mm–diameter coupon punched out from the center of masks and respirators worn by the breathing simulator (Table 1). The amount of virus recovered on the mask and respirator coupons were 5.6%–5.8% and 8.2%–11.0%, respectively, of the total amount recovered by NIOSH samplers positioned 10 cm beside the manikin’s mouth. Infectious influenza was present on all mask and respirator coupons, regardless of whether or not they were sealed to the manikin’s head, and infectivity of the recovered virus was reduced approximately 4–8 fold from that of the viral preparation before aerosolization.
Table 1.
Recovery of Infectious Virus From Surgical Masks and N95 Respirators
| Mask Type | Total Matrix Copies Collected Beside Mouth |
Total Matrix Copies on Mask Coupon |
Total PFUs on Mask | Infectious Virus, % | |
|---|---|---|---|---|---|
| Before Aerosolization |
On Mask Coupon |
||||
| Unsealed SM | 2.70 × 106 ± 2.20 × 105 | 1.52 × 105 ± 1.28 × 105 | 3.56 × 103 ± 2.81 × 103 | 9.8 ± 2.7 | 2.6 ± 0.4 |
| Unsealed N95 | 1.33 × 106 ± 7.10 × 105 | 1.46 × 105 ± 4.26 × 104 | 2.28 × 104 ± 2.01 × 104 | 5.6 ± 1.9 | 1.1 ± 0.7 |
| Sealed SM | 1.89 × 106 ± 3.78 × 105 | 1.1 × 105 ± 9.8 × 104 | 1.7 × 103 ± 1.7 × 103 | 7.1 ± 2.1 | 0.9 ± 0.8 |
| Sealed N95 | 3.11 × 106 ± 3.95 × 105 | 2.54 × 105 ± 1.12 × 105 | 2.70 × 103 ± 2.53 × 103 | 5.8 ± 4.1 | 0.8 ± 0.7 |
A 25-mm diameter coupon was punched out from the center of the mask and respirator (mouth area) and assayed for the presence of influenza. Data are the means ± standard errors of 2 experiments each for studies with unsealed surgical masks, sealed surgical masks, and sealed N95 respirators, or 3 experiments for studies with unsealed N95 respirators.
Abbreviations: N95, N95 respirator; PFUs, plaque-forming units; SM, surgical mask.
The location of virus within the coupons was also assessed. Coupons of 19-mm diameter were punched out from the center and side sections of a sealed mask and respirator, and the 3 layers of each coupon (outer water-repellent cover, middle filtering layer, and inner hydrophilic lining) were then separately processed. Most of the virus was located in the middle and outer layers of each coupon (Table 2). The inner layers of the coupons from the center and side sections of the mask contained only 2.3% and 0.8%, respectively, of the total virus recovered. The inner layers of the coupons from the center and side sections of the respirator contained only 0.4% and 0.2%, respectively, of the total virus recovered.
Table 2.
Penetration of Influenza Into Layers of Mask and Respirator
| Center Coupon | Side Coupon | |||||
|---|---|---|---|---|---|---|
| Mask Layer | Total Matrix Copies | PFUs | Infectious Virus on Mask, % |
Total Matrix Copies | PFUs | Infectious Virus on Mask, % |
| Sealed SM | ||||||
| Outer | 1.66 × 103 | 1.25 × 101 | 0.8 | 3.42 × 103 | 1.25 × 101 | 0.4 |
| Middle | 4.61 × 103 | 0.0 | 0.0 | 1.55 × 104 | 5.00 × 101 | 0.3 |
| Inner | 1.47 × 102 | 0.0 | 0.0 | 1.56 × 102 | 0.0 | 0.0 |
| Total | 6.42 × 103 | 1.25 × 101 | 1.91 × 104 | 6.25 × 101 | ||
| Sealed N95 | ||||||
| Outer | 1.46 × 104 | 3.13 × 102 | 2.1 | 4.14 × 104 | 6.25 × 102 | 1.5 |
| Middle | 3.45 × 104 | 5.38 × 102 | 1.6 | 1.50 × 105 | 1.24 × 103 | 0.8 |
| Inner | 2.04 × 102 | 0.0 | 0.0 | 3.13 × 102 | 0.0 | 0.0 |
| Total | 4.93 × 104 | 8.51 × 102 | 1.92 × 105 | |||
A 19-mm-diameter coupon was punched out from the center and side of each mask and respirator. The 3 layers of each coupon were separated and assayed for the presence of influenza virus.
Abbreviations: N95, N95 respirator; PFUs, plaque-forming unit; SM, surgical mask.
The tips (~20 mm) of surgical gloves were attached to the manikin’s forehead and to NIOSH samplers located at positions P1, P2, and P3 during 3 simulated examinations (Figure 1). Total influenza was recovered from the glove tips placed at all positions, and infectious virus was recovered from glove tips located on the manikin’s forehead and at P1 (Table 3).
Table 3.
Recovery of Infectious Virus From Surgical Gloves
| Glove Location Within Environmental Chamber |
Virus on Glove Tip | Infectious Virus, % | ||
|---|---|---|---|---|
| Total Matrix Copies | Total PFUs | Before Aerosolization | On Glove | |
| On forehead | 2.93 × 103 ± 2.27 × 103 | 2.5 ± 2.0 | 9.2 ± 2.1 | 0.1 ± 0.1 |
| P1 | 6.58 × 102 ± 1.54 × 102 | 6.3 ± 5.1 | 9.2 ± 2.1 | 2.7 ± 1.1 |
| P2 | 3.13 × 103 ± 2.00 × 103 | 0 | 9.2 ± 2.1 | 0 |
| P3 | 3.08 × 103 ± 2.16 × 103 | 0 | 9.2 ± 2.1 | 0 |
The ~20-mm tip of the index finger of a surgical glove was assayed for the presence of influenza virus. Data are means ± standard errors of 3 experiments. A total of 1.18 × 104 ± 1.73 × 103 matrix copies per liter of air were collected from a National Institute for Occupational Safety and Health sampler positioned beside the mouth.
Abbreviations: P1, P2, P3, position 1, position 2, position 3; PFUs, plaque-forming units.
DISCUSSION
To maintain the availability of healthcare workers during an influenza pandemic, it is imperative to assess the nature of the risk of transmission in healthcare settings, such as during patient examinations, and to develop appropriate mitigation measures. To address this, Lindsley et al [28] constructed a simulated examination room and showed that coughed aerosol particles of potassium chloride disperse within minutes throughout the room. The present study with influenza supports that finding and suggests that anyone present in a room with a patient who has influenza might be at risk of exposure.
Before aerosolization of the virus in the 16 simulated exposure experiments, an average 6.3% was infectious (the stock presumably contained >93% defective [noninfectious] virus) and 2.2% of the virus remained infectious after collection by the NIOSH samplers. However, the final infectivity varied considerably among the individual experiments. As reported by Cao et al [33], some of the losses were probably due to the use of the NIOSH sampler, which fractionates aerosolized virus on the dry walls of a collection tube and Teflon filter. In addition, humidity may have influenced the survival of infectious virus; 2 studies reported that maximal stability of influenza occurs at 20%–40% relative humidity, and minimal stability at 50% relative humidity [11, 34]. In our study, the relative humidity in the simulation chamber was 44%–63%.
In a real-world examination room, the actual number of aerosolized viral particles that a healthcare worker could potentially inhale would be dependent on the number of viral copies shed by infected individuals and the airflow in the room. In 1 study, naturally infected participants shed 33 copies/min in aerosol particles ≥5 µm and 187 viral copies/min in particles <5 µm [22]. Assuming an examination room is occupied by ≥1 infected patients for 60 minutes, up to 1.12 × 104 viral particles <5 µm in size may be shed, and 1.23 × 103 viral particles could potentially be inhaled by a healthcare worker. Teunis et al [35] developed a dose-response model for infectivity and pathogenicity of influenza A using 3 clinical studies in which influenza was administered via aerosol and 12 studies in which it was administered through intranasal droplet inoculation. They concluded that the probabilities of infection by either aerosol or droplet transmission are approximately equal and that the probability of infection is significant (Pinf = .2–.4) at low doses (101–2 TCID50 infectious units). They also noted that most of the freshly shed viruses are potentially infectious and that environmental conditions may rapidly decrease the fraction of infectious viruses.
Two systematic reviews on the use of surgical masks and N95 respirators came to different conclusions. One review was based primarily on data from severe acute respiratory syndrome outbreaks, and these authors concluded that interventions, including the use of masks or respirators, could reduce the spread of respiratory infections [36]. The other review found few data showing that masks are effective against influenza [37]. Similarly, a Canadian prospective randomized controlled trial of respirator and mask use by nurses found that use of a mask resulted in similar rates of laboratory-confirmed influenza, mostly documented by serological changes [38]. A recent large cluster randomized clinical trial conducted in China, where mask acceptance is high, compared the effectiveness of masks and respirators (fit tested and non–fit tested) in protecting healthcare workers from respiratory infection [39]. Their conclusion was that a benefit of respirators was suggested but would need to be confirmed by a larger trial because the study was underpowered. Rates of fit test failure were very low, perhaps accounting for the study’s finding that fit testing did not improve the efficacy of respirators. Thus, data from clinical settings has thus far failed to resolve uncertainty about the relative importance of aerosol transmission and the necessity for use of N95 respirators to prevent it.
In our study, we evaluated the effectiveness of surgical masks and N95 respirators when the masks and respirators were sealed to a manikin’s face or unsealed to a manikin’s face and documented to fit poorly (approximating how masks normally perform and how poorly fitting respirators might perform in the field). Sealed masks were not as effective as sealed respirators at blocking total influenza (94.5% vs 99.8% blocked) or at blocking infectious virus (95.8% vs 99.6% blocked). Rengasamy et al [40] examined the filtration efficiency of 5 models of masks using a standard filter tester and found penetration values ranging from <0.2% to 63% at 30 L/min. Our results were comparable to those for the mid-range masks in their study. Because filtration efficiencies of masks vary considerably, protection afforded by even a sealed mask would be further reduced. Unsealed masks and unsealed, poorly fitting respirators were not effective at blocking total influenza virus (68.5% vs 64.5% blocked) or infectious virus (56.6% vs 66.5%).
This result shows that gaps between the wearer’s face and the PPE can have a tremendous impact on the protection offered. This is especially applicable for masks, which are not designed to seal to the wearer’s face. Typical fit factors from volunteers wearing these types of masks have been reported to range from 2.5 to 9.6 [29, 30]. In contrast, respirators are required to have fit factors ≥100, and measurements from volunteers wearing properly fitted respirators have shown much higher factors than from those wearing masks [30–32]. In our study, the fit factors for sealed respirators and masks were 200+ and 135, respectively, whereas unsealed masks and respirators had fit factors of 2.3 and 4.6. Thus, the sealed respirators obtained fit factors similar to well-fitting respirators, and the unsealed masks obtained fit factors comparable to those on human subjects during realistic use conditions. Therefore, our results support the use of properly fitted N95 respirators for maximal protection against infectious airborne influenza.
Finally, 2 important notes about our results should be made. First, the high fit factors seen with the sealed surgical masks in our study should not be interpreted to mean that surgical masks can be depended upon to provide respiratory protection. The filtration capacity of surgical masks varies tremendously from model to model, and large face seal leaks, which admit substantial amounts of aerosol particles, are normal even when surgical masks are tied tightly to the face. Second, the fit factor of respiratory PPE represents the protection offered by the PPE under ideal test conditions. In industrial hygiene, a distinction is made between this and the “assigned protection factor,” which is the amount of protection that would be expected from the PPE during real-world usage and can be considerably lower. For PPE to provide the needed protection to workers, they must be part of a respiratory protection program that includes training and fit testing of workers for the PPE they will use.
Acknowledgments
We thank David Edgell of National Institute for Occupational Safety and Health (NIOSH) for manufacturing the NIOSH samplers, Bean T. Chen of NIOSH for developing the original NIOSH cyclone sampler, Jeffrey S. Reynolds of NIOSH for help with software development for the cough simulator, and Kimberly S. Clough-Thomas of NIOSH for artwork. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by NIOSH and the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.IOM (Institute of Medicine) Preventing transmission of pandemic influenza and other viral respiratory diseases: personal protective equipment for healthcare personnel. Washington, DC: National Academies Press; 2011. Update 2010. [PubMed] [Google Scholar]
- 2.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2010;6:S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber TP, Stilianakis NI. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wein LM, Atkinson MP. Assessing infection control measures for pandemic influenza. Risk Anal. 2009;29:949–962. doi: 10.1111/j.1539-6924.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 5.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 6.Andrewes CH, Glover RE. Spread of infection from the respiratory tract of the ferret. 1. Transmission of influenza A virus. Br J Exp Pathol. 1941;22:91–97. [Google Scholar]
- 7.Munster VJ, de Wit E, van den Brand JM, et al. Pathogenesis and transmission of swine-origin 2009 A (H1N1) influenza virus in ferrets. Science. 2009;325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorrell EM, Wan H, Araya Y, Song H, Perez DR. Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc Natl Acad Sci USA. 2009;106:7565–7570. doi: 10.1073/pnas.0900877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hoeven N, Pappas C, Belser JA, et al. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc Natl Acad Sci USA. 2009;106:3366–3371. doi: 10.1073/pnas.0813172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouvier NM, Lowen AC, Palese P. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. J Virol. 2008;82:10052–10058. doi: 10.1128/JVI.01226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30 degrees C) blocks aerosol but not contact transmission of influenza virus. J Virol. 2008;82:5650–5655. doi: 10.1128/JVI.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mubareka S, Lowen AC, Steel J, Coates AL, Garcia-Sastre A, Palese P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J Infect Dis. 2009;199:858–865. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steel J, Lowen AC, Mubareka S, Palese P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 2009;5:e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maines TR, Chen LM, Matsuoka Y. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci USA. 2006;103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henle W, Henle G, Maris EP. Experimental exposure of human subjects to viruses of influenza. J Immunol. 1946;52:145–165. [PubMed] [Google Scholar]
- 17.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;67:775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 18.Little JW, Douglas RG, Jr, Hall WJ, Roth FK. Attenuated influenza produced by experimental intranasal inoculation. J Med Virol. 1979;3:177–188. doi: 10.1002/jmv.1890030303. [DOI] [PubMed] [Google Scholar]
- 19.Fabian P, McDevitt J, DeHaan W. Influenza virus in human exhaled breath: an observational study. PLoS One. 2008;3:e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh KN, Oliver BG, Stelzer S, Rawlinson WD, Tovey ER. A new method for sampling and detection of exhaled respiratory virus aerosols. Clin Infect Dis. 2008;46:93–95. doi: 10.1086/523000. [DOI] [PubMed] [Google Scholar]
- 21.Stelzer-Braid S, Oliver BG, Blazey AJ, et al. Exhalation of respiratory viruses by breathing, coughing, and talking. J Med Virol. 2009;81:1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 22.Milton DK, Fabian P, Angel M, Perez DR, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks, April 18–20, 2010. Atlanta, Georgia: Emory Conference Center; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsley WG, Blachere FM, Thewlis RE, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PloS One. 2010;5:1–6. doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blachere FM, Lindsley WG, Pearce TA, et al. Measurement of airborne influenza in a hospital emergency department. Clin Infect Dis. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 25.Lindsley WG, Blachere FM, Davis KA, et al. Distribution of airborne influenza virus and respiratory synctial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50:693–698. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- 26.Blachere FM, Cao G, Lindsley WG, Noti JD, Beezhold DH. Enhanced detection of viable airborne influenza virus. J Virol Methods. 2011;176:120–124. doi: 10.1016/j.jviromet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Lindsley WG, Schmechel D, Chen BT. A two-stage cyclone using microcentrifuge tubes for personal bioaerosol sampling. J Environ Monit. 2006;8:1136–1142. doi: 10.1039/b609083d. [DOI] [PubMed] [Google Scholar]
- 28.Lindsley WG, King WP, Thewlis RE, Reynolds JS, Szalajda JV. Dispersion and exposure to a cough-generated aerosol in a simulated medical examination room. JOEH. doi: 10.1080/15459624.2012.725986. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ISO. Metabolic rates and respiratory flow rates. Geneva: ISO/TS; 2007. Respiratory protective devices—human factors. Part 1; pp. 16976–16971. [Google Scholar]
- 30.Lawrence RB, Duling MG, Calvert CA, Coffey CC. Comparison of performance of three different types of respiratory protection devices. J Occup Environ Hyg. 2006;3:465–474. doi: 10.1080/15459620600829211. [DOI] [PubMed] [Google Scholar]
- 31.Oberg T, Brosseau LM. Surgical mask filter and fit performance. Am J Infect Control. 2008;36:276–282. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SA, Grinshpun SA, Reponen T. Respiratory performance offered by N95 respirators and surgical masks: human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann Occup Hyg. 2008;52:177–185. doi: 10.1093/annhyg/men005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao G, Noti JD, Blachere FM, Lindsley WG, Beezhold DH. Development of an improved methodology to detect infectious airborne influenza virus using the NIOSH bioaerosol sampler. J Environ Monit. 2011;13:3321–3328. doi: 10.1039/c1em10607d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaffer FL, Soergel ME, Straube DC. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch Virol. 1976;51:263–273. doi: 10.1007/BF01317930. [DOI] [PubMed] [Google Scholar]
- 35.Teunis FM, Brienen N, Kretzschmar EE. High infectivity and pathogenicity of influenza A virus via aerosol and droplet transmission. Epidemics. 2010;2:215–222. doi: 10.1016/j.epidem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2008;336:77–80. doi: 10.1136/bmj.39393.510347.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowling BJ, Zhou Y, Ip DK, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138:449–456. doi: 10.1017/S0950268809991658. [DOI] [PubMed] [Google Scholar]
- 38.Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 39.MacIntyre CR, Wang Q, Cauchemez S, et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in healthcare workers. Influenza Other Respi Viruses. 2011;5:170–179. doi: 10.1111/j.1750-2659.2011.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rengasamy A, Miller AB, Eimer BC, Shaffer R. Filtration performance of FDA-cleared surgical masks. J Int Soc Respi Prot. 2009;26:54–70. [PMC free article] [PubMed] [Google Scholar]