Abstract
Chronic hepatitis C virus infection is now curable by antiviral therapy but the global burden of liver disease is unlikely to diminish without a vaccine to prevent transmission. The objective of HCV vaccination is not to induce sterilizing immunity, but instead to prevent persistent infection. One vaccine that incorporates only non-structural HCV proteins is now in phase I/II efficacy trials to test the novel concept that T cell priming alone is sufficient for protection. Evidence also suggests that antibodies contribute to infection resolution. Vaccines comprised of recombinant envelope glycoproteins targeted by neutralizing antibodies have been assessed in humans for immunogenicity. Here, we discuss current concepts in protective immunity and divergent approaches to vaccination against a highly mutable RNA virus.
The need for a vaccine to prevent persistent HCV infection
The hepatitis C virus (HCV) is a small, positive-stranded RNA virus discovered in 1989 as the cause of most transfusion and community-acquired non-A, non-B hepatitis [1]. Globally, an estimated 180 million people have been exposed to the virus [2]. An estimated 70% of infections persist for life [3]. Introduction of effective blood screening approximately 20 years ago resulted in a precipitous drop in new HCV infections. This early progress towards reducing HCV transmission has reversed in the last decade because of a sharp increase in injection drug use amongst adolescents and young adults. Recent studies in the United States documented an increased incidence of new HCV infections, particularly in suburban and rural populations [4•,5]. HCV is also still transmitted in some developing countries through unsafe medical practices and so effective strategies to interrupt transmission globally are still needed.
Direct acting antiviral (DAA) regimens that do not contain type I interferon can now safely cure most chronic HCV infections [6]. At least conceptually, widespread adoption of DAA therapy could also reduce HCV transmission by shrinking the pool of virus donors with chronic hepatitis C [6]. However, implementation of this approach is complicated by the cost of antivirals and surveillance programs to detect new, largely asymptomatic HCV infections in at-risk populations [6]. A vaccine to prevent HCV infection would not have the same limitations and would be useful in two settings. Most obvious is prevention of primary HCV infection in those not yet been exposed to the virus. A more unique and targeted use for a vaccine is prevention of reinfection after cure of chronic hepatitis C with costly DAA. This second use may be of critical importance in extending antiviral therapy to individuals with ongoing risk for exposure to the virus.
Feasibility and objectives of preventive HCV vaccination
There is compelling evidence that spontaneous resolution of HCV infection, observed in 30% of cases, protects against persistence upon re-exposure to the virus. Rechallenge of immune chimpanzees with HCV results in viremia, but of much shorter duration and peak magnitude than in primary infections [7•]. Most importantly, the rate of persistence is much lower in second versus first HCV infections, even when rechallenge was undertaken years later [7•]. A protective effect of a prior resolved infection is also apparent in humans; prospective studies in injection drug users revealed that 80 percent of primary HCV infections persist, compared with only 20 percent of secondary infections in those who cleared an earlier infection [8,9].
These observations suggested that prevention of persistence, rather than infection, would be an acceptable objective for HCV vaccination. Sterilizing immunity is also less important because acute hepatitis C is often clinically silent, and there is no apparent latency or long-lived cellular reservoir that can lead to resurgence of replication [3]. At the same time, there are also scientific challenges for vaccine development. Globally, HCV exists as seven distinct genotypes with nucleotide sequences that differ by at least 70 percent [10]. The virus is also highly mutable and can readily escape selection pressure by antibodies and CD8+ T cells. More practically, the lack of a tractable, fully immunocompetent animal model or HCV infection has limited progress to identify and refine promising vaccine candidates.
Protective immune responses and divergent approaches to HCV vaccination
Many candidate HCV vaccines have been assessed for immunogenicity in rodents over the past two decades (Figure 1). They span the spectrum from synthetic peptides, proteins, and virus-like particles to recombinant viruses and DNA plasmids [11]. The potential for a whole inactivated or even a live attenuated HCV vaccine has also recently emerged with development of cell culture models that support virus replication [12]. Very few of these candidate vaccines have been assessed for protection of chimpanzees from persistent HCV infection [7•] and represents a bottleneck in vaccine development. Of those HCV vaccines that showed promise in protecting chimpanzees, only two have been assessed for immunogenicity in humans. One vaccine developed by Chiron (now Novartis) is comprised of recombinant envelope glycoproteins E1 and E2 that are the target of neutralizing antibodies [13]. The other, developed by Okairos (now GlaxoS-mithKline), relies on expression of HCV non-structural proteins from recombinant viruses for induction of CD8+ T cell immunity [14••]. Here, we review progress in development of these two vaccines. These very different approaches to antigen selection and delivery are summarized in Figure 2. They reflect an unsettled debate about the role of antibodies versus T cells in protection from chronic hepatitis C.
Figure 1.
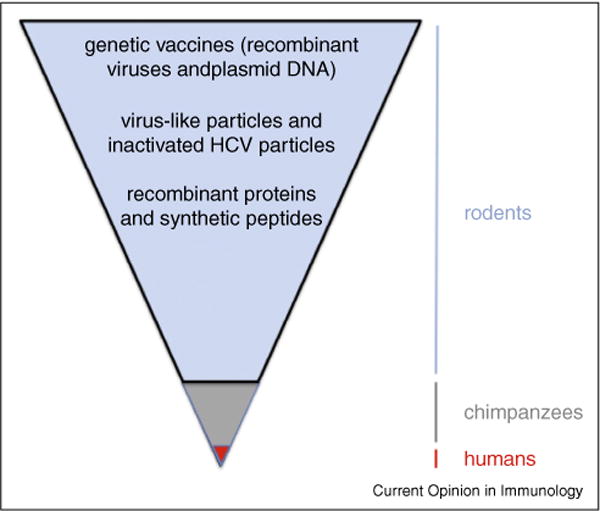
Bottlenecks in HCV vaccine development. HCV was discovered approximately 25 years ago when there was rapid progress in understanding pathways of antigen processing and presentation, as well as development of recombinant proteins and vectors as vaccines. These advances provided a rich pipeline of ideas for strategies to vaccinate against HCV. Many candidate vaccines were tested for immunogenicity in rodents or other species not susceptible to HCV infection. Very few vaccines were assessed for protection of chimpanzees, the only immunocompetent animal model for human HCV infection. Approximately half of chimpanzees infected with HCV resolve the infection spontaneously. Because chimpanzee vaccine studies necessarily involve very few individuals, it is difficult to detect an increased frequency of resolved infections compared to mock vaccinated controls. The lack of a more tractable animal model is a significant bottleneck in vaccine development. To date, only two vaccine approaches have been assessed for immunogenicity in humans. The difficulty in designing phase II and III human vaccine studies in populations who are at risk for infection, with protection from persistence rather than protection as an endpoint, is also a barrier to progress.
Figure 2.
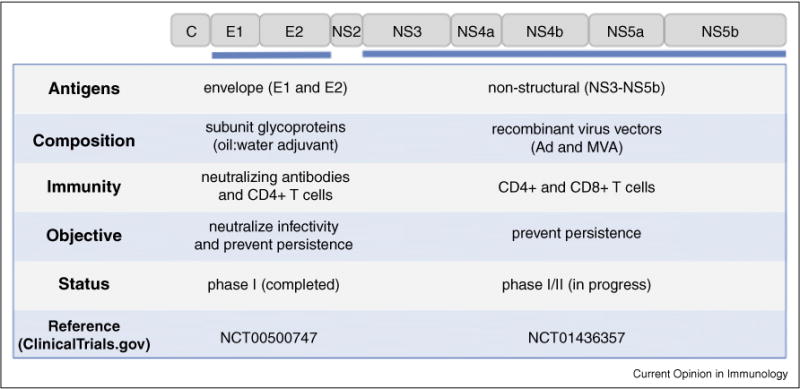
Summary of two HCV vaccines that advanced to human clinical trials. Very different approaches to vaccine antigen selection and delivery reflect uncertainty about mechanisms of protective immunity against HCV. The Chiron vaccine is comprised of adjuvanted recombinant envelope glycoproteins E1 and E2. The Okairos vaccine is comprised of recombinant viruses expressing the non-structural 3 to 5 genes as indicated in the figure.
Neutralizing antibodies and vaccine-mediated protection from HCV persistence
Seroconversion against envelope glycoproteins E1 and E2 usually occurs several weeks after infection with HCV infection, regardless of whether the virus is cleared or persists [3]. Progress in understanding serum neutralization patterns was slow until about 10 years ago when HCV pseudoparticle (HCVpp) and cell culture (HCVcc) models of virus entry and replication were established [15]. Recent identification of multiple receptors for HCV also led to development of rodent models for measuring antibody-mediated blockade of virus entry into hepatocytes [16], including one genetically humanized mouse that facilitates measurement of vaccine-induced humoral responses [17]. The molecular structure of the E2 ectodomain has been solved [18,19] and key antibody epitopes have been identified [15], providing new structural models to explain HCV entry into cells, mechanisms of neutralization, and evasion through mutational escape or generation of inhibitory antibodies.
Despite this progress, there is still uncertainty about the capacity of anti-envelope antibodies to protect from persistent infection. Neutralization of HCV infectivity was demonstrated first in chimpanzees [20,21] and later in mice with humanized livers [22,23]. In these studies, incubation of the HCV inoculum with anti-HCV antibodies prevented infection [20], as did passive transfer of the antibodies before challenge in humanized mice [22,23] and some chimpanzees [21]. Several observations from acutely infected animals and humans suggest that antibodies can also inhibit replication of HCV once infection is established. Passive transfer of immune serum [24] or an anti-E2 monoclonal antibody [21] to chimpanzees after virus challenge at least transiently suppressed HCV replication. Additionally, one very recent study has demonstrated that neutralizing antibodies expressed from a recombinant adeno-associated virus can cure an established HCV infection in human liver chimeric mice [25]. In humans, broadly neutralizing activity developed more rapidly in individuals with acute resolving versus persisting infections and the virus was less prone to mutational escape in key E2 epitopes [26–28]. Higher neutralization titers were also associated with lower viremia [29] or more frequent resolution of acute infection in two single source HCV outbreaks [30]. Recall of broad neutralizing antibody responses has been observed in human subjects re-infected with HCV after resolution of a prior infection. Although the number of subjects studied was small, some individuals with this broadly neutralizing response did develop persistent infection after reinfection, suggesting that humoral immunity provides incomplete protection [31]. As noted above, control of HCV infection by antibodies can be compromised by rapid mutational escape of key epitopes in the E2 envelope glycoprotein [26] and by direct cell to cell spread of the virus so that it is not susceptible to neutralization [32–34].
Development of the prototypical vaccine for generation of neutralizing anti-HCV antibodies began at Chiron almost 25 years ago [13]. The recombinant hepatitis B virus (HBV) surface antigen vaccine developed a short time before discovery of HCV provided conceptual support for the use of envelope glycoproteins to prevent persistent virus infection. Recombinant E1 and E2 envelope glycoproteins from a genotype 1a HCV strain elicited high-titer antibody responses in chimpanzees when formulated with an oil:water emulsion adjuvant [13]. Retrospective studies conducted several years later with the HCVpp and HCVcc cell assay documented serum neutralization of several HCV genotypes [35]. A subset of vaccinated animals had no virological or immunological evidence of infection after challenge with related HCV genotype 1a or 1b viruses [13], suggesting the potential for sterilizing immunity. Importantly, the majority of vaccinated animals developed viremia despite the presence of serum anti-E1 and E2 antibodies, but had an attenuated course of infection compared to unvaccinated controls [13]. One meta-analyses demonstrated that vaccination of chimpanzees with subunit envelope proteins provided protection equivalent to spontaneous resolution of infection when peak viremia, infection duration, and rate of persistence were compared [7•]. These studies provided the first evidence that a vaccine designed to elicit antibodies might provide protection even when breakthrough virus replication is observed.
Humans vaccinated with the recombinant E1 and E2 vaccine also developed pan-genotypic neutralizing antibodies [36]. It is important to note that that vaccine also elicits a strong CD4+ T cell response against the envelope proteins [37••]. As described below, CD4+ T cells are essential for control of acute hepatitis C. At present, the relative contribution of this cellular immune response versus neutralizing antibodies in protection from persistence cannot be determined. No follow-up trials of the Chiron/Novartis vaccine have been described and plans for further development are unknown.
CD8+ T cells and vaccine-mediated protection from HCV persistence
Detailed characterization of immunity during acute primary hepatitis C in humans and chimpanzees over the past 2 decades documented a temporal relationship between the T cell response and infection outcome [38,39]. A rapid drop in viremia typical of most acute human and chimpanzee infections is kinetically associated with the onset of virus-specific T cell immunity [38,39]. Importantly, failure to sustain this cellular immune response is perhaps the most reliable predictor of a persistent outcome. Long-lived memory CD4+ and CD8+ T cell responses that develop after successful resolution of a primary infection are rapidly recalled after re-exposure to the virus [40,41•,42]. These responses are temporally associated with accelerated control of the second infection. Moreover, antibody-mediated depletion of CD4+ T cells from immune chimpanzees just before rechallenge with HCV resulted in persistent infection [42]. HCV-specific CD8+ T cells were compromised by CD4+ T cell depletion; they were functionally exhausted and selected for immune escape variants [42]. Depletion of CD8+ T cells by administration of antibodies resulted in prolonged HCV replication until recovery of the response in liver [41•].
These observations provided the rationale for development of the Okairos/GSK genetic vaccine encoding HCV genotype 1b non-structural HCV proteins, from the NS3 protease/helicase to the NS5b polymerase, that are dominant targets of CD4+ and CD8+ T cells [14••]. Immunization of five chimpanzees with recombinant adenovirus vectors (Ad serotype 6 followed by serotype 24) primed strong, functional CD8+ T cell responses against the non-structural proteins [14••]. CD4+ T cell responses induced by the recombinant adenovirus vectors were somewhat weaker, but frequencies were substantially increased after boosting with a plasmid DNA vaccine that encoded the same HCV proteins. Challenge of the immunized animals with a heterologous (genotype 1a) HCV strain resulted in accelerated T cell immunity and sharply reduced peak HCV viremia when compared to mock-vaccinated controls [14••]. HCV infection persisted in only 1 vaccinated animal. HCV RNA was detected for several weeks or months in the other 4, but these infections eventually cleared. Persistent infection was established in 3 of the 5 mock-vaccinated controls [14••]. A follow-up study revealed that reduced HCV replication in the vaccinated animals was associated with rapid and sustained multifunctional CD8+ T cell activity. They also transitioned rapidly to a memory phenotype, defined by increased expression of CD127, a component of the IL-7 receptor required for memory cell homeostasis, and decreased expression of programmed cell death-1 (PD-1), an inhibitory receptor that can contribute to CD8+ T cell exhaustion [43].
This vaccine strategy, with some modification, has been assessed for immunogenicity in humans at low risk of exposure to HCV. In an initial study, priming with a recombinant human adenovirus (serotype Ad6) followed by boosting with a chimpanzee adenovirus (serotype chAd63), resulted in vigorous CD4+ and CD8+ T cell immunity against the encoded HCV genotype 1b NS3-NS5b proteins [44••]. The T cells were multifunctional and cross-reactivity for HCV genotype 3 non-structural proteins was reported. Phenotypic analysis of the CD8+ T cells revealed expression of activation markers (CD38 and HLA-DR) at the earliest time points, and a transition to long-term effector and central memory populations distinguished by expression of CD45RA and the chemokine receptor CCR7 [44••]. A second modification of the vaccine regimen involving priming with a chimpanzee adenovirus (serotype chAd3) and boosting with modified vaccinia virus Ankara was recently evaluated [45••]. When compared with the heterologous adenovirus boost used in the first study, MVA boosting provided a very substantial increase in CD4+ and CD8+ T cell frequencies and broadening of the response to non-structural proteins of multiple HCV genotypes [45••]. Multiparameter CyTof analysis revealed that more of the CD8+ T cells responding to the MVA boost had a central or effector memory versus a naïve phenotype at the end of the study [45••]. A phase I/IIclinical trial of this vaccine is now underway in individuals at risk for HCV infection because of intravenous drug use (see Clinical Trials.gov NCT01436357 for details). From a practical perspective, it will establish the feasibility of preventive vaccine trials in humans at greatest risk for HCV infection, where the objective is to prevent persistence rather than infection.
While immunogenicity data for this vaccine are impressive, a cautious approach to development of vaccines that prime CD8+ T cells with the goal of preventing HCV persistence is warranted. Some vaccines designed to induce CD8+ T cell immunity against non-structural HCV proteins may not protect chimpanzees from persistent infection despite a similar sharp reduction in HCV replication after challenge [13]. The significance of prolonged, low-level persistence of HCV in vaccinated animals, and conditions under which it might result in chronic infection, are poorly understood. Even adaptive immunity that is naturally acquired by resolution of infection can fail in chimpanzees and humans. In these cases, CD8+ T cell expansion after reinfection is weaker, they express more PD-1 and less CD127, and do not broaden to recognize new epitopes for reasons that are not yet understood. Perhaps most importantly, how HCV silences CD4+ T cells during primary and sometimes secondary infection is not yet understood. The possibility that some vaccines prime a T cell response that is more susceptible to failure or mutational escape after HCV infection [46] cannot be excluded. Combining T cell and antibody vaccines may optimize protection against HCV. Recent immunogenicity studies in monkeys have demonstrated the potential for combining the Okairos/GSK genetic vaccine with adjuvanted subunit envelope proteins in generating humoral and cellular immunity [47].
Key questions for the future of HCV research and vaccine development
The Okairos/GSK vaccine is the first to enter phase I/II trials to prevent persistent HCV infection. The study is groundbreaking for vaccine science because it will test the very novel hypothesis that a vaccine designed to prime T cell immunity alone can prevent a serious human viral disease. Whether a reduction in the rate of HCV persistence, but not necessarily infection, is a viable endpoint for a vaccine clinical trial will also be established. The vaccine is being assessed in individuals who have no evidence of prior HCV infection, but who are at risk because of needle sharing associated with intravenous drug use (see Clinical Trials.gov NCT01436357 for details). However, preventing reinfection after DAA cure of chronic hepatitis C may be the most pragmatic and targeted use of a preventive HCV vaccine. Whether a vaccine that prevents infection in HCV naïve humans will also afford protection in this population is uncertain. During chronic infection, CD8+ T cells are highly localized to the liver, functionally exhausted and/or target escaped epitopes. CD4+ T cells are difficult to detect in blood or liver during persistent HCV infection. Loss of antigen-specific CD4+ T cell activity occurs during the acute phase of infection and has not yet been explained.
There may be multiple layers of negative regulation involving inhibitory signaling through receptors like PD-1 and possibly regulatory T cell activity [38]. Whether these defects are restored after cure of chronic hepatitis C with DAA is one of the most pressing questions when strategies to control HCV transmission using antiviral therapy or vaccines are considered. Two recent studies of CD8+ T cell immunity after DAA cure of chronic hepatitis C have addressed this question. A comparison of all CD8+ T cell responses in DAA treated humans revealed enhanced antigen-driven proliferation after successful therapy, especially for those populations targeting invariant HCV epitopes thought to be more exhausted [48••]. Detailed longitudinal study of two patients before and after cure documented increased CD127 expression on CD8+ T cells targeting an invariant epitope. PD-1 expression decreased slightly, suggesting at least partial recovery of circulating CD8+ T cells from exhaustion [48••]. Another recent study in a chimpanzee cured of chronic hepatitis C directly assessed recovery of CD8+ T cells and protection from re-infection [49••]. Intrahepatic CD8+ T cells targeting intact epitopes retained an exhausted phenotype characterized by high PD-1 and low CD127 for up to 2 years after cure [49••]. They did not expand in liver after rechallenge with HCV. Transient control of the second infection was associated with expansion of CD8+ T cells targeting class I epitopes prone to escape. Persistence was kinetically linked to the rapid appearance of escape mutations in these epitopes [49••]. Because the impact of DAA therapy on phenotype and effector function was studied in very few CD8+ T cell populations from a small number of humans and chimpanzees, the need for a vaccine to prevent reinfection remains uncertain.
Summary and prospects for a preventive HCV vaccine
Paradoxically, the development of highly effective DAA to treat chronic hepatitis C should intensify efforts to identify defects in T cell immunity before and after cure of persistent infection. It is not yet known if infections that occur after DAA cure will resolve at the same high rate as primary HCV infections. If not, it will be important to determine if vaccines designed to prevent HCV persistence in naïve individuals will also restore immunity in those successfully treated with DAA. Success will depend on the ability of the vaccine to restore potentially exhausted CD8+ T cell responses against epitopes that are not prone to escape, and to broaden the response to new epitopes not targeted during HCV infection. The possibility that very low levels of persistent virus [50] or antigen can interfere with induction of T cell immunity by vaccines for a period of time after DAA cure cannot be excluded. Under these circumstances, the distinction between preventive and therapeutic vaccination may begin to blur for HCV infection.
Acknowledgments
This manuscript was supported by The National Institute of Allergy and Infectious Disease of the National Institutes of Health under award numbers R37 AI047367 and R01 AI96882 (CMW), R01 AI070101 and R21 AI11837 (AG), and P510D011132 to the Yerkes National Primate Center.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4•.Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, Hamdounia SB, Church DR, Barton K, Fisher C, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59:1411–1419. doi: 10.1093/cid/ciu643. An epidemiological study documenting increased transmission of HCV amongst adolescent and young adults in the United States. [DOI] [PubMed] [Google Scholar]
- 5.Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002–2009. Morb Mortal Wkly Rep. 2011;60:537–541. [PubMed] [Google Scholar]
- 6.Hagan LM, Wolpe PR, Schinazi RF. Treatment as prevention and cure towards global eradication of hepatitis C virus. Trends Microbiol. 2013;21:625–633. doi: 10.1016/j.tim.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Dahari H, Feinstone SM, Major ME. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology. 2010;139:965–974. doi: 10.1053/j.gastro.2010.05.077. Provides a thorough meta-analysis of chimpanzee vaccine studies providing statistical proof that vaccination provides the same protection from chronic hepatitis C as spontaneous resolution of primary HCV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grebely J, Prins M, Hellard M, Cox AL, Osburn WO, Lauer G, Page K, Lloyd AR, Dore GJ. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis. 2012;12:408–414. doi: 10.1016/S1473-3099(12)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2009;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C Virus into 7 genotypes and 67 subtypes: updated criteria and assignment web resource. Hepatology. 2014;59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang TJ. Current progress in development of hepatitis C virus vaccines. Nat Med. 2013;19:869–878. doi: 10.1038/nm.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akazawa D, Moriyama M, Yokokawa H, Omi N, Watanabe N, Date T, Morikawa K, Aizaki H, Ishii K, Kato T, et al. Neutralizing antibodies induced by cell culture-derived hepatitis C virus protect against infection in mice. Gastroenterology. 2013;145:447–455. e441–444. doi: 10.1053/j.gastro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Houghton M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol Rev. 2011;239:99–108. doi: 10.1111/j.1600-065X.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 14••.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. Provides the first proof in the chimpanzee model that a vaccine comprised of non-structural HCV proteins can provide persistence from HCV infection. [DOI] [PubMed] [Google Scholar]
- 15.Ball JK, Tarr AW, McKeating JA. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res. 2014;105:100–111. doi: 10.1016/j.antiviral.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billerbeck E, de Jong Y, Dorner M, de la Fuente C, Ploss A. Animal models for hepatitis C. Curr Top Microbiol Immunol. 2013;369:49–86. doi: 10.1007/978-3-642-27340-7_3. [DOI] [PubMed] [Google Scholar]
- 17.Dorner M, Horwitz JA, Donovan BM, Labitt RN, Budell WC, Friling T, Vogt A, Catanese MT, Satoh T, Kawai T, et al. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature. 2013;501:237–241. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan AG, Whidby J, Miller MT, Scarborough H, Zatorski AV, Cygan A, Price AA, Yost SA, Bohannon CD, Jacob J, et al. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature. 2014;509:381–384. doi: 10.1038/nature13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L, Giang E, Nieusma T, Kadam RU, Cogburn KE, Hua Y, Dai X, Stanfield RL, Burton DR, Ward AB, et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science. 2013;342:1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter HJ, Purcell RH. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci U S A. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morin TJ, Broering TJ, Leav BA, Blair BM, Rowley KJ, Boucher EN, Wang Y, Cheslock PS, Knauber M, Olsen DB, et al. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012;8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 23.Vanwolleghem T, Bukh J, Meuleman P, Desombere I, Meunier JC, Alter H, Purcell RH, Leroux-Roels G. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology. 2008;47:1846–1855. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 24.Krawczynski K, Alter MJ, Tankersley DL, Beach M, Robertson BH, Lambert S, Kuo G, Spelbring JE, Meeks E, Sinha S, et al. Effect of immune globulin on the prevention of experimental hepatitis C virus infection. J Infect Dis. 1996;173:822–828. doi: 10.1093/infdis/173.4.822. [DOI] [PubMed] [Google Scholar]
- 25.de Jong YP, Dorner M, Mommersteeg MC, Xiao JW, Balazs AB, Robbins JB, Winer BY, Gerges S, Vega K, Labitt RN, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology. 2014;59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavillette D, Morice Y, Germanidis G, Donot P, Soulier A, Pagkalos E, Sakellariou G, Intrator L, Bartosch B, Pawlotsky JM, et al. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79:6023–6034. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brimacombe CL, Grove J, Meredith LW, Hu K, Syder AJ, Flores MV, Timpe JM, Krieger SE, Baumert TF, Tellinghuisen TL, et al. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol. 2011;85:596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, Schwarz A, Desombere I, Roels GL, Balfe P, et al. Hepatitis C virus cell–cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47:17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- 34.Witteveldt J, Evans MJ, Bitzegeio J, Koutsoudakis G, Owsianka AM, Angus AG, Keck ZY, Foung SK, Pietschmann T, Rice CM, et al. CD81 is dispensable for hepatitis C virus cell-to-cell transmission in hepatoma cells. J Gen Virol. 2009;90:48–58. doi: 10.1099/vir.0.006700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meunier JC, Gottwein JM, Houghton M, Russell RS, Emerson SU, Bukh J, Purcell RH. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J Infect Dis. 2011;204:1186–1190. doi: 10.1093/infdis/jir511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law JL, Chen C, Wong J, Hockman D, Santer DM, Frey SE, Belshe RB, Wakita T, Bukh J, Jones CT, et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS One. 2013;8:e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie A, Rinella P, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.06.084. This study provides detailed immunogenicity data for a recombinant E1/E2 ‘antibody’ vaccine in human subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Hakeem MS, Shoukry NH. Protective immunity against hepatitis C: many shades of gray. Front Immunol. 2014;5:274. doi: 10.3389/fimmu.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauer GM. Immune responses to hepatitis C virus (HCV) infection and the prospects for an effective HCV vaccine or immunotherapies. J Infect Dis. 2013;207(Suppl. 1):S7–S12. doi: 10.1093/infdis/jis762. [DOI] [PubMed] [Google Scholar]
- 40.Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586–6595. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. This study documents prolonged replication of HCV in immune chimpanzees after CD8+ T cell depletion. This study, and citation [43], provided a firm conceptual basis for development of vaccines that elicit T cells and not antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 43.Park SH, Shin EC, Capone S, Caggiari L, De Re V, Nicosia A, Folgori A, Rehermann B. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology. 2012;143:1048–1060. e1044. doi: 10.1053/j.gastro.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra111. doi: 10.1126/scitranslmed.3003155. A detailed characterization of immunogenicity in the first human recipients of and HCV T cell vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. Improved immunogenicity of a T cell vaccine involving priming and boosting with adenovirus vectors expressing HCV non-structural proteins. A very similar vaccine is now in Phase I/II trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puig M, Mihalik K, Tilton JC, Williams O, Merchlinsky M, Connors M, Feinstone SM, Major ME. CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology. 2006;44:736–745. doi: 10.1002/hep.21319. [DOI] [PubMed] [Google Scholar]
- 47.Chmielewska AM, Naddeo M, Capone S, Ammendola V, Hu K, Meredith L, Verhoye L, Rychlowska M, Rappuoli R, Ulmer JB, et al. Combined adenovirus vector and hepatitis C virus envelope protein prime-boost regimen elicits T cell and neutralizing antibody immune responses. J Virol. 2014;88:5502–5510. doi: 10.1128/JVI.03574-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, Bocher WO, Thimme R. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014;61:538–543. doi: 10.1016/j.jhep.2014.05.043. Restoration of T cell immunity was assessed in humans treated with an interferon-free regimen. The question of whether there is protective immunity after DAA cure will determine if a vaccine is needed to prevent reinfection. [DOI] [PubMed] [Google Scholar]
- 49••.Callendret B, Eccleston HB, Hall S, Satterfield W, Capone S, Folgori A, Cortese R, Nicosia A, Walker CM. T-cell immunity and hepatitis C virus reinfection after cure of chronic hepatitis C with an interferon-free antiviral regimen in a chimpanzee. Hepatology. 2014;60:1531–1540. doi: 10.1002/hep.27278. This study documents defects in the T cell response to reinfection in a chimpanzee that was cured of HCV with direct acting antivirals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SH, Veerapu NS, Shin EC, Biancotto A, McCoy JP, Capone S, Folgori A, Rehermann B. Subinfectious hepatitis C virus exposures suppress T cell responses against subsequent acute infection. Nat Med. 2013;19:1638–1642. doi: 10.1038/nm.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]


