Abstract
The investigation of C. elegans males and the male-specific sensory neurons required for mating behaviors has provided insight into the molecular function of polycystins and mechanisms that are needed for polycystin ciliary localization. In humans, polycystin 1 and polycystin 2 are needed for kidney function; loss of polycystin function leads to autosomal dominant polycystic kidney disease (ADPKD). Polycystins localize to cilia in C. elegans and mammals, a finding that has guided research into ADPKD. The discovery that the polycystins form ciliary receptors in male-specific neurons needed for mating behaviors has also helped to unlock insights into two additional exciting new areas: the secretion of extracellular vesicles; and mechanisms of ciliary specialization.
First, we will summarize the studies done in C. elegans regarding the expression, localization, and function of the polycystin 1 and 2 homologs, LOV-1 and PKD-2, and discuss insights gained from this basic research. Molecules that are co-expressed with the polycystins in the male-specific neurons may identify evolutionarily conserved molecular mechanisms for polycystin function and localization.
We will discuss the finding that polycystins are secreted in extracellular vesicles that evoke behavioral change in males, suggesting that such vesicles provide a novel form of communication to conspecifics in the environment. In humans, polycystin-containing extracellular vesicles are secreted in urine and can be taken up by cilia, and quickly internalized. Therefore, communication by polycystin-containing extracellular vesicles may also use mechanisms that are evolutionarily conserved from nematode to human.
Lastly, different cilia display structural and functional differences that specialize them for particular tasks, despite the fact that virtually all cilia are built by a conserved Intraflagellar Transport (IFT) mechanism and share some basic structural features. Comparative analysis of the male-specific cilia with the well-studied cilia of the amphid and phasmid neurons has allowed identification of molecules that specialize the male cilia. We will discuss the molecules that shape the male-specific cilia. The cell biology of cilia in male-specific neurons demonstrates that C. elegans can provide an excellent model of ciliary specialization.
Keywords: polycystins, TRP channel, cilia, ciliopathies, mating behavior, sensory biology
Introduction
Cilia are antenna-like organelles that protrude from many eukaryotic cells and sense the environment [1]. Cilia also provide motile functions, and are often called flagella when they are essential to the motility of a cell or organism, such as in algae and spermatozoa. Virtually every cilium in the eukaryotic world is built by the same mechanism, known as Intraflagellar Transport or IFT [1], in which conserved kinesin-2 and dynein motors both move upon and build the microtubules that define the ciliary structure. Because cilia are built by IFT, cilia share basic cytoskeletal structures, such as the “9 + 2” (9 outer doublets surrounding a pair of inner singlets) formation and the “9 + 0” (9 outer doublets with no inner singlets) formation [2]. Research of IFT processes over the past decade has suggested that primary cilia—small, hair-like immotile organelles that protrude from virtually every cell in the human body—are essential for organizing the development of tissues and organs and maintaining homeostasis in mammals [3].
Cilia also serve a wide variety of sensory functions, and even come in a variety of shapes and sizes. For example, in contrast to the simple structure of most primary cilia, the retinal rods and cones in which phototransduction occurs are large and elaborately-shaped cilia [4]. The cilia of the olfactory epithelium, in which odor transduction takes place, have a simple morphology, but each cell has a multitude of cilia [5]. Such differences in form and functions of cilia in different cells and tissues suggest that cilia are not all alike, despite the fact that ciliogenesis is mediated by the conserved process of IFT [1]. Therefore, other processes must be overlaid upon IFT to specialize cilia for diverse functions [2].
In addition to sensing the outside environment that surrounds an organism, cilia may also monitor the internal environment. Internal sensing includes reception of signaling from nearby cells [3] as well as other stimuli. For example, cilia that protrude from kidney epithelia are proposed to sense urine flow through the kidneys [6, 7]. Loss of the ability to sense urine flow is proposed to lead to polycystic kidney disease [6, 7].
ADPKD is a common genetic disease that affects 1:1000 individuals [8, 9]. Mutations in genes encoding polycystin 1 are responsible for 85% or cases of ADPKD, and mutations in polycystin 2 account for the remaining 15% of ADPKD cases [9].
Polycystins reside in sensory cilia in both C. elegans neurons and mammalian kidneys and are involved in sensation needed for male mating in nematodes and kidney function in humans. Although C. elegans has no kidneys, studies using the nematode have helped to demonstrate that ADPKD is a “ciliopathy” or disease of the cilia [10]. The realization that a ciliary defect underlies ADPKD also touched off an exciting series of discoveries that many human diseases, such as Bardet-Biedl, Meckel-Gruber, Nephronophthisis, and Joubert syndromes are also ciliopathic in nature [10, 11]. Many ciliopathies are found to be syndromic, which likely reflects the fact that many post-mitotic cells in the human body are ciliated [10, 11]. Various ciliopathies affect different tissues, or have differing severity in various tissues, illustrating that cilia are not all identical, and must have differing molecular requirements.
This review will focus on how studies of the C. elegans male have helped to illuminate ADPKD and ciliopathies, secretion of ECVs, and specialization of cilia for diverse roles.
Genetic Analysis Of Male Vulva Location Behavior in Mating Identified Polycystins In C. elegans
Possessing only 383 neurons, the C. elegans male can perform a complex series of behavioral steps needed for mating with the C. elegans hermaphrodite. Mating behavior is robust and stereotyped from one individual male to another, which has enabled forward genetic screening for location of vulva defective (Lov) mutants and the identification of lov-1 [12]. The lov-1 gene encodes the protein LOV-1, one of two polycystins in C. elegans [12]. LOV-1 is expressed in 21 male-specific ciliated sensory neurons that help the male detect potential hermaphrodite mates, respond to contact (“response” behavior), and locate the mate's vulva.
The second C. elegans polycystin, called PKD-2, was found by database search for homologs of another human polycystin PKD2 [12] [13]. pkd-2 is also needed for response and location of vulva mating behaviors, and is co-expressed with lov-1. Moreover, both LOV-1 and PKD-2 localize to sensory cilia, where they may function in a sensory capacity [12, 13].
Additional Mating Behaviors In C. elegans That Require The Polycystins
Male mating behavior is a complex, yet stereotyped, behavior that can be dissected into different substeps that comprise simple locomotory patterns [14]. First, males detect mates both at a distance and locally by multiple cues provided by hermaphrodites (Barrios review in this issue; Srinivasan review in this issue). Then, upon encountering the hermaphrodite, a male exhibits response behavior: he pushes his tail fan against the hermaphrodite and starts to move backward and scan the hermaphrodite's body for the vulva ([14]; Lints Review in this issue). Subsequently, when he encounters the vulva, he ceases backward locomotion (location of vulva behavior [14]). Males that have successfully performed response and location of vulva behavioral steps continue male mating behavior inserting their spicules into the vulva, transferring sperm, and retracting their spicules (Garcia review in this issue).
Since C. elegans is a hermaphroditic species, males (which arise spontaneously only rarely) are not essential for reproduction [15, 16]. Therefore, the male is motivated to search for mates (Barrios Review in this issue). In the absence of potential mates, males exhibit mate-searching behavior: males leave a food source, while hermaphrodites do not [17]. lov-1 and pkd-2 mutant males are defective in mate-searching behavior [18].
When mates are present in the local environment, males must first chemotax to hermaphrodites, likely by detection of pheromones, requiring function of the cephalic male-specific (CEM) ciliated sensory neurons in the head [19, 20]. Males with lov-1 or pkd-2 mutations are impaired in chemotaxis to a conditioned spot containing raw pheromone extracts from C. remanei females [19].
For response and location of vulva behaviors, the behavioral defects in lov-1;pkd-2 double mutants are no more severe than in the single mutants, suggesting the LOV-1 and PKD-2 may act in the same genetic pathway for mating behavior [13]. Males with lov-1 mutations are not defective in spicule insertion or sperm transfer [12].
It is interesting to note that response behavior defects in pkd-2 mutants can be suppressed by sperm-depleted hermaphrodite mates [21]. Since hermaphrodites continually produce oocytes, production of sperm is the limiting factor in producing self-progeny [22]. Hermaphrodites may increase their sex appeal when sperm-depleted to attract males to provide more sperm by mating, and thereby maximize the number of offspring [21]. Hermaphrodites also seek to maximize their progeny when depleted of sperm by suppressing “sprinting” behavior, a locomotory avoidance of males [23]. Since mating behaviors are so robust in wild-type males, the increased sex appeal of sperm-depleted hermaphrodites is apparent in the increased mating success of pkd-2 males, but wild-type males also display a preference for sperm-depleted hermaphrodite mates [21]. The ability of sperm-depleted hermaphrodites to overcome the loss of pkd-2 does not require known acaroside pheromone attractants; daf-22 mutants, which are defective in ascaroside production [20], still show increased sex appeal to pkd-2 mutant males [21]. Therefore, wild-type C. elegans males can use both polycystin-dependent and independent pathways for sensation and mating with hermaphrodites.
Expression And Localization Of The Polycystins
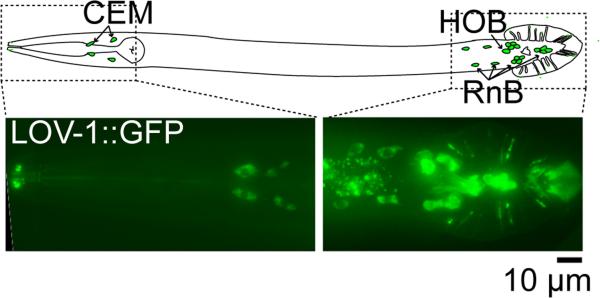
In C. elegans, LOV-1 and PKD-2 are expressed identically in male-specific sensory neurons required for chemotaxis to mates, response to mates, and location of vulva behaviors (see Lints review). The 21 male-specific neurons that express polycystins are: the four CEM neurons in the head, with ciliated sensory endings in the nose; the ray B-type neurons (RnBs, where n=1 - 9 excluding 6) in the tail, with ciliated sensory endings that tip the ray structures in the male's tail fan; and the HOB neuron, with a sensory cilium in the hook structure of the male tail (Fig. 1; [12, 13]). The CEM neurons are needed for chemotaxis to hermaphrodites [19, 20]; the RnB neurons are needed for response behavior when contacting hermaphrodites [14]; and the HOB neuron is needed for location of vulva behavior [14]. The sensory cilia of all of these neurons are exposed to the environment through pores in the cuticle [24, 25]. Within these neurons, LOV-1 and PKD-2 a share similar subcellular localization: they localize to the cell body, specifically the ER, the distal dendrite, and cilia [13, 26].
Fig. 1. A Diagram Of The Polycystin-Expressing Male-Specific Neurons In C. elegans.
(Top panel) Bilateral pairs of dorsal and ventral CEM neurons have cell bodies anterior to the terminal bulb of the pharynx, and extend dendrites to the tip of the nose. The approximate locations of the HOB neuron and the 16 polycystin expressing RnB neurons of the tail are shown. The HOB extends a dendrite to a hook structure anterior to the tail fan. The RnB neurons extend dendrites into the rays of the tail fan. (Bottom Panels) Expression of LOV-1::GFP under the endogenous promoter illuminates the cell bodies of CEM in the head (left), and the HOB and RnB neurons in the tail (right). LOV-1::GFP fluorescence is faintly visible in the sensory cilia.
In humans, polycystin 1 and 2 have distinct but overlapping expression patterns and subcellular localization, suggesting that they may act both as a single receptor ion channel complex, as well as independently in different signal transduction pathways [27]. Consistent with its adhesive molecular structure, polycystin 1 protein is found to localize to multiple cell-cell junctions, including tight junctions, adherent junctions, desmosomes, and apical junctions, while the polycystin 2 is found most abundantly in endoplasmic reticulum membrane and plasma membrane [28]. Polycystin 1 and 2 appear to colocalize and form a single complex in the primary cilia membrane [29]. Interestingly, polycystin 2 is found localize to cell body plasma membrane and ciliary membrane via different routes, moving through the cis-Golgi compartment for somatic plasma membrane localization, and through the trans-Golgi compartment for ciliary membrane localization [28]. Since the trans-Golgi compartment is specialized for post-translational modifications (such as glycosylation), such modifications may facilitate assembly of the polycystin 1 and 2 complex for ciliary localization. The similarities in subcellular localization of C. elegans polycystins and mammalian polycystins suggests that they may play conserved roles in both ER and cilia.
What Are The Polycystins?
The ciliary localization and function of polycystins in conserved from nematodes to humans: human polycystins PKD1 and PKD2 also localize to cilia, where they are needed for sensation of fluid flowing through the kidneys [6, 30]. The evolutionary conservation of the ciliary localization and sensory function of polycystins from nematodes to humans was very important because it provided compelling evidence that loss of either PKD1 or PKD2 in humans caused defective sensory function of renal epithelial cilia. C. elegans has provided a useful genetic model for polycystin localization and function [12].
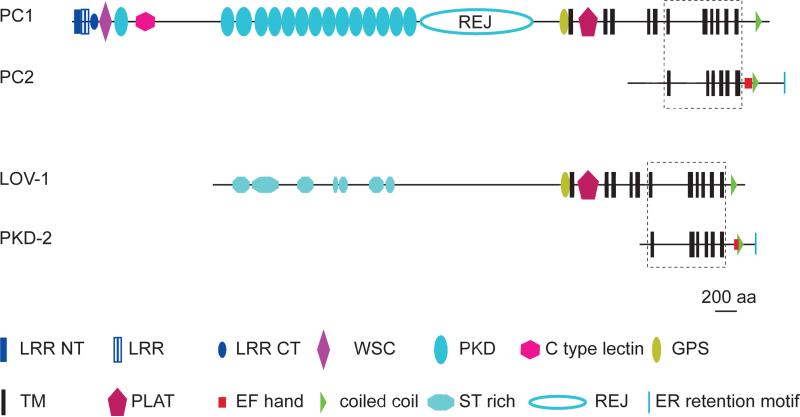
LOV-1 is the sole C. elegans homolog of human Polycystin 1(PKD1/PC1) [12]. There are five polycystin 1 family proteins encoded in the human genome: polycystin 1, PKDL1, PKDL2, PKDL3 and PKDREJ [31]. Polycystin 1 proteins are characterized by a long adhesive N-terminal extracellular domain containing PKD domain repeats, 11 transmembrane (TM) domains, a GAIN domain including a GPCR proteolysis site (GPS), an intracellular PLAT (polycystin/lipoxygenase/α-toxin) domain between TM1 and TM2, and a coiled-coil region in the carboxy-terminus (thought to interact directly with polycystin 2), that are either predicted or experimentally confirmed (Fig. 2; [32-36]). The region spanning the last six transmembrane domains is homologous with polycystin 2 [37]. LOV-1 shares many of the same structural features; however, LOV-1 contains stretches of mucin-like serine/threonine-enriched adhesive domains, instead of the immunoglobulin-like PKD repeats in the large N-terminal extracellular domain in Polycystin 1 (Fig. 2; [12]; SMART [38-40]). A recent analysis predicts (COILS Server [39-41]) a 16 amino acid coiled-coil in the C terminus of LOV-1 that had not previously been detected.
Fig. 2. The functional Domains Of Human And C. elegans Polycystins.
Human polycystin 1 (PC1) and polycystin 2 (PC2) are shown on top. Domains of C. elegans polycystin 1 homolog LOV-1 and polycystin 2 homolog PKD-2 are shown on bottom. All diagrams are of equal scale, shown bottom right. Boxed areas indicate regions homologous to TRPP ion channel proteins. Note that all four contain homology to TRP ion channels, but only polycystin 2 and PKD-2 possess a voltage sensitive 4th TM domain. (Abbreviations: LRR: Leucine-Rich Repeat; WSC: cell wall integrity and stress response component; PKD: polycystic kidney disease domain; GPS: GPCR proteolytic site; TM: transmembrane; PLAT: polycystin/lipoxygenase/α-toxin; ST rich: serine-threonine rich.)
A lov-1(sy582) deletion allele, which lacks the polycystin/ion channel homology region is predicted to be a recessive genetic null allele [12]. Loss of 58 C-terminal amino acids appears to eliminate function of LOV-1 [12]. A transgene encoding the first 991 amino acids of LOV-1 fused to GFP (Green Fluorescent Protein; [42]) is sufficient for ciliary localization, but acts as a dominant negative for vulva location behavior [12].
PKD-2 is the C. elegans homolog of human polycystin 2 (PKD2/PC2), an ion channel of the TRP (Transient Receptor Potential) superfamily, which has homologs throughout the eukaryotes [43]. There are 27 TRP ion channel proteins encoded in the human genome, and 17 in the C. elegans genome [43]. TRPs form non-selective cation channels with six transmembrane domains and are involved in virtually all types of sensation, such as vision, hearing, touch, taste, olfaction, and thermosensation [43]. TRP channels are gated by a variety of stimuli, reflecting their diverse roles [43]. PKD-2 encodes the sole TRPP (TRP-polycystin) sub-family member in the nematode [12, 13]. PKD-2 contains six membrane-spanning domains, a C-terminal coiled-coil domain, and the EF hand Ca2+-binding domain found in human polycystin 2 [12, 44]. pkd-2(sy606) encodes a protein truncated from the middle of the first TM domain onward, and is likely a null allele [13]. Both PKD-2 and human polycystin 2 localize to the endoplasmic reticulum (ER) and plasma membrane [45]. Although a human PKD2 transgene can partially rescue pkd-2 mating behavior defects, suggesting that polycystin 2 and PKD-2 are functional homologs, human polycystin 2 does not localize to cilia [45]. This is consistent with C. elegans PKD-2 requiring its putative partner LOV-1 for efficient ciliary localization [26].
LOV-1 and PKD-2 are thought to form a ciliary TRPP ion channel receptor complex, like human polycystins 1 and 2 [29]. However, both polycystin 1 and 2 family members are now known to form functional complexes with other molecules, making assignment of function complicated [29]. Therefore, many open questions remain regarding the function of polycystins 1 and 2, such as: what stimuli are they sensing, and how are they activated?
Functions Of Polycystins
Studies of mammalian polycystins expressed heterologously in cells such as Xenopus oocytes, CHO cells, HEK cells, and cultured sympathetic neurons have uncovered many functions of the polycystins [7, 29, 46]. The functions demonstrated in these systems are often in conflict with one another, possibly reflecting the different cellular environments of each expression system [29]. However, it is generally accepted that polycystin 1 and 2 form a ciliary ion channel receptor complex in renal epithelial cilia that may be activated (either directly or indirectly) in response to fluid flow and conducts non-selective cationic currents [7, 29, 46, 47].
Sensation of fluid flow was proposed to occur by polycystin-mediated mechanotransduction [6, 30]. In cultured mammalian kidney cells, Ca2+ influx in response to bending of the primary cilium by either fluid flow requires polycystin 1 and 2 [6]. However, several experimental details weaken the conclusion that the polycystins act as mechanotransduction ion channels. Ca2+ influx was undetectable when ryanodine receptors—ER Ca2+-release channels—were inhibited. Antibodies against intracellular domains of polycystin 2 could also partially block the response [6]. Additionally, the time-course of activation (peaking 10-20 sec after stimulation; [6]) is far slower than has been demonstrated for direct mechanotransduction by an ion channel (sub-millisecond; [48, 49]).
A model in which polycystins act as ciliary mechanotransduction channels, proposed by Nauli et al, has been pervasive in polycystin literature, and the LOV-1/PKD-2 complex has occasionally been described as a sensory mechanotransduction ion channel in C. elegans male neuronal cilia. However, the fact that C. elegans males require polycystins for at least three mating-related behaviors—mate-searching motivation [18], response to hermaphrodite contact [13], and location of vulva [12])—which can not all rely on the same mechanosensory cue, suggests that the polycystins may instead amplify or potentiate sensory transduction events mediated by other molecules. Alternatively, as other TRP ion channels can be activated by multiple sensory modalities, a PKD-2-based TRPP channel could be activated by mechanical and chemical cues. Thus far, activation of polycystin-expressing HOB or RnB neurons in vivo by mechanical stimuli has not been observed in electrophysiology experiments (O'Hagan and Barr, unpublished data). Therefore, available data neither support nor rule out mechanotransduction by the LOV-1 and PKD-2 polycystin complex.
Mammalian polycystins can also function independently of one another. Polycystin 1 has been reported to form a functional ion channel on it's own [50], to alter the function of other channels such as N-type Ca2+ channels and GIRK (G-protein activated inwardly rectifying K+ channels) via activity as a GPCR [51], and to activate phospholipase C via G-protein activation [52]. Polycystin 1 proteins, like adhesion GPCRs, contain a GAIN domain, which can activate G-protein second messengers [27, 35], endowing them with GPCR-like activity.
Delmas 04 found that polycystin 2 binding inhibits the ability of polycystin 1 to activate G-proteins, and that, independently of G-proteins, polycystin 1 positively regulates gating of polycystin 2 [27]. Polycystin 2 has also been reported to function as a homomeric ion channel [53], to form heteromeric channels with other TRP ion channel subunits [54], and to activate BK potassium channels by polycystin 2-mediated Ca2+ entry [55].
Therefore, although it is generally believed that LOV-1 and PKD-2, like mammalian polycystin 1 and 2, form a ciliary receptor ion channel complex, they might also perform independent functions that are currently unknown. This conclusion is supported by findings that mating behavior defects in males harboring both lov-1 and pkd-2 mutations are slightly, but consistently, less severe than in lov-1 or pkd-2 single mutants [13, 56, 57]. Additionally, loss of the single-TM lectin and mucin protein CWP-5 (Co-expressed With Polycystins) can enhance the mating behavior defects of lov-1 or pkd-2 single mutants, but not the lov-1;pkd-2 double mutant, suggesting that polycystins have a latent ability to repress mating behavior [57]. In the model of polycystin function presented in Delmas 04, although polycystin 1 and 2 work together as an ion channel complex, each inhibits the independent activity of the other by binding. It is currently unknown if such a model might accurately describe the functions of LOV-1 and PKD-2 in male neuronal cilia. LOV-1 possesses a GPS domain (GPCR proteolytic site; SMART website prediction: [39, 40]), suggesting that it might act as an adhesion GPCR in C. elegans.
An apt summary of what is known about polycystin function may be that, while many functions have been discovered for polycystin 1 and 2, both in complex and independently, the contributions of these diverse functions in vivo still remain unclear.
Molecules That Regulate Polycystin Localization Or Abundance
The conserved ciliary localization of polycystins in nematodes and mammals suggests that this site is important for polycystin function. PKD-2 ciliary localization is regulated by both cell-specific and general molecules [26]. For example, LOV-1, expressed only in the polycystin-expressing neurons, regulates abundance of PKD-2::GFP in the cell body, and may stabilize ciliary localization of PKD-2 [26]. General factors include unc-101, a widely-expressed gene encoding the clathrin adaptor AP-1 μ1 subunit, is required for restricting PKD-2 to the somatodendritic compartment and cilia [26]. Many genes that encode IFT (intraflagellar transport) components provide all or most cilia with the main microtubule-based motor mechanism needed for ciliogenesis and ciliary transport, and are also necessary for proper localization, abundance, or ECV release of PKD-2::GFP [58]. Mutations in IFT genes usually cause abnormal accumulation of PKD-2::GFP in the distal dendrite or ciliary base [26]. Table 1 includes a listing of known regulators of polycystin localization or abundance.
Table 1.
Regulators of PKD-2 localization or function.
| Molecule | Function | Reference |
|---|---|---|
| Broadly expressed | ||
| UNC-101 | AP-1 clathrin adaptor μ1 subunit | [Bae 06] |
| CIL-1 | phosphoinositide-5 phosphatase | [Bae 09] |
| STAM-1 | signal transduction adaptor molecule | [Hu 07] |
| TAX-6 | Calcineurin phosphatase | [Hu 06] |
| Pan ciliary expression | ||
| DAF-10 | IFT Complex A | [Bae 06] |
| OSM-5 | IFT Complex B | Qin 2001 |
| CHE-13 | IFT Complex B | [Bae 06] |
| OSM-3 | kinesin-2/KIF17 | [Bae 06] |
| KLP-11 | kinesin-II subunit | [Bae 06] |
| CHE-3 | dynein motor | [Bae 06] |
| XBX-1 | dynein subunit | [Bae 08] |
| CCPP-1 | tubulin carboxypeptidase | [O'Hagan 11] |
| IL2 and male B-type neuronal expression (PKD gene battery) | ||
| DAF-19M | Mating isoform of RFX transcription factor | [Wang 10] |
| KLP-6 | Kinesin-3 | [Peden 05; Morsci 11] |
| CWP-1 | Coexpressed with polycystin | [Portman 04] |
| TBA-6 | alpha-tubulin | [Hurd 10] |
| Male B-type neuronal expression (PKD gene battery) | ||
| CWP-2 to -4 | Coexpressed with polycystin | [Portman 04] |
| CWP-5 | Coexpressed with polycystin, single TM mucin protein | [Miller 10] |
PKD-2::GFP motility is not visible in cilia [26, 59], so polycystins may not be directly transported within cilia by IFT motors. However, PKD-2::GFP movement is visible in male-specific CEM and RnB neuron dendrites; anterograde and retrograde movement velocities differ, which is expected if PKD-2 dendritic transport is mediated by a kinesin and a dynein [26]. Dendritic transport rates of PKD-2 in male-specific neurons and the ODR-10 GPCR in AWB [60] are similar, suggesting these ciliary receptors are carried by a similar dendritic transport system. However, the motors responsible are currently unknown.
Inappropriate localization of PKD-2::GFP is known as the Cil (Ciliary localization defective) phenotype. Individual mutations in a plethora of genes have been found to produce the Cil phenotype in male-specific neurons which affect diverse processes such as microtubule-based motor traffic, microtubule stability, and IFT complex components, membrane dynamics, modifiers of phosphorylation, and ubiquitination (Table 1). Microtubule-based motor Cil genes include the IFT kinesins osm-3 and klp-11 [26], the novel ciliary kinesin-3 motor, klp-6 [56], and the dynein genes che-3 [26] and xbx-1 [61]. IFT complex Cil genes include osm-5 and daf-10 [26, 62]. Loss of ccpp-1, which encodes a tubulin deglutamylase involved in tubulin stability and regulation of kinesins OSM-3 and KLP-6, also causes a Cil phenotype [63]. Mutation of cil-1, encoding a PtdIns 5-phosphatase involved in membrane composition and dynamics, also causes the Cil phenotype [64]. A loss-of-function mutation of tax-6, encoding the calcium-activated phosphatase calcineurin causes abnormally low levels of PKD-2::GFP in male-specific cilia [65]. Regulation of PKD-2 phosphorylation at serine residue 534, which is conserved in human polycystin 2, was found to be important for it's ciliary localization [65]. Dynamic (and antagonistic) functions of TAX-6/calcineurin phosphatase and casein kinase II, encoded by kin-3 and kin-10, regulate both serine 534 phosphorylation and ciliary localization of PKD-2 [65]. Casein kinase II interacts with LOV-1 and human polycystin 1 via their PLAT domains, suggesting that LOV-1 recruits casein kinase II to PKD-2. Mammalian polycystin 1 has been shown to activate calcineurin via G-protein mediated activation of phospholipase C [52], suggesting that functional interactions among these proteins are conserved. Mutation of stam-1, which along with hgrs-1 encode proteins involved in ubiquitination pathways, are also needed to downregulate LOV-1 and PKD-2 for appropriate ciliary polycystin levels [66].
The Kinesin-3 KLP-6 Is Required For Polycystin Localization
Among the Cil genes, klp-6 is especially notable. The kinesin-3 KLP-6 is expressed in the polycystin-expressing neurons and the IL2 neurons and localizes diffusely throughout neurons, including the cilia but excluding the nucleus [56]. Like lov-1, a klp-6 mutant was identified by the genetic screen for Location of Vulva deficient males [56]. In fact, klp-6 males, like lov-1 or pkd-2 mutants, are defective in both response and location of vulva behaviors [56]. Since KLP-6 is needed for appropriate ciliary localization of PKD-2, it is reasonable to conclude that klp-6 defects in mating result from abnormal function of PKD-2. However, it is interesting to note that lov-1 and pkd-2 mutants display more severe mating behavior defects than klp-6, but that the double mutants lov-1;klp-6 and klp-6;pkd-2, as well as the triple mutant lov-1;klp-6;pkd-2, display behavioral defects indistiguishable from klp-6 alone [56]. This suggests that klp-6 is epistatic to both lov-1 and pkd-2 for mating behavior, and that the products of these three genes may have separable functions in male-specific cilia.
Although not essential for ciliogenesis, KLP-6 does display motility in CEM cilia [67]. The ciliary kinesins OSM-3 and heterotrimeric kinesin-II (comprised of KLP-11, KLP-20, and KAP-1) have well described roles in IFT and ciliogenesis of the amphid and phasmid cilia [1]. However, deeper investigation into the function of KLP-6 in male-specific cilia led to the surprising finding that neither KLP-6, nor OSM-3, nor heterotrimeric kinesin-II alone is essential for ciliogenesis in CEM cilia [67]. Although all three motors are motile in CEM cilia, loss of the canonical IFT kinesin-II causes increased ciliary length, which is suppressed by loss of OSM-3 and KLP-6 [67]. Therefore, OSM-3 and KLP-6 appear to play a role in positive regulation of ciliary length, while heterotrimeric kinesin-II plays a role in restricting length [67].
The Polycystins LOV-1 And PKD-2 Are Shed In Extracellular Vesicles (ECVs)
The ECV-Shedding Neurons
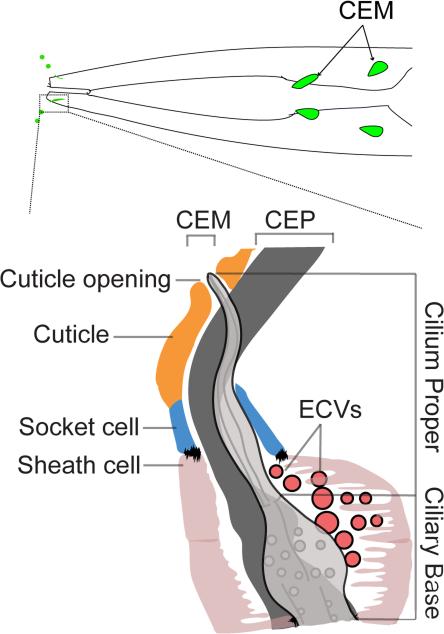
The study of C. elegans males has elucidated another common feature between mammalian polycystin-expressing kidney epithelia and male-specific ciliated sensory neurons: the ability to shed ECVs that contain polycystins. Among C. elegans ciliated sensory neurons, a subset comprising the six IL2 neurons (present in both male and hermaphrodite) and 21 polycystin-expressing neurons (including four CEM neurons in the head, one HOB and 16 RnB neurons; n=1-9 except 6, bilateral pairs) can release ECVs into the environment (Fig. 3; [58]). The cilia of these cells are exposed to the environment directly through a cuticular pore, which is the exit route of ECVs (Fig. 3). The cuticular pore is continuous with an extracellular lumen formed by glial socket and sheath cells, which envelop the cilia base and cilia. Recently, transmission electron microscopy (TEM) and electronic tomography have shown extracellular vesicles inside the extracellular lumen formed by the glial cells that surround the IL2 and CEM neurons [58, 68]. However, access to the environment via a cuticular pore is insufficient for shedding of ECVs, as other chemosensory cilia exposed to the environment through pores do not secrete ECVs [58].
Fig. 3. Polycystin-Containing ECVs Are Secreted By Ciliated Neurons Into The Environment.
Top Panel shows a diagram of CEM neurons in the head secreting tiny vesicles out of the ciliary pore. Bottom Panel is a diagram of the structure of CEM cilia, surrounded by glial sheath and socket cells. ECVs are visible in the space between the CEM ciliary base and the sheath cell. ECVs are thought to travel along the cilium to be released into the environment through the ciliary pore. Modified from Wang et al. 2014.
GFP reporters, immunostaining, and TEM data showed that LOV-1 and PKD-2 are cargoes of ECVs from C. elegans males [58]. The IL2 neurons in both males and hermaphrodites also shed ECVs, as shown by a CWP-1::GFP reporter visible in ECVs [58]. The IL2 and the male-specific polycystin- expressing neurons are unique in C. elegans in that they shed ECVs to the environment.
The IL2 neurons and male-specific neurons that shed ECVs display some differences and similarities. For example, the IL2 neurons represent a neuronal type that is present in both males and hermaphrodites and arises from different lineages than the 21 polycystin-expressing neurons [56]. The CEM male-specific neurons are born during embryogenesis in both genders, and undergo apoptosis in the hermaphrodite [69]. The HOB and RnB neurons are born post-embryonically, during early L4 larval stage [70]. The ECV-shedding neurons are patterned by different transcription factors, and produce different neurotransmitters [71]. Their similarities include: 1) they are all members of neuronal sensory organs composed of neuronal pairs of an A-type (cilium not exposed to environment) neuron and a B-type (cilium exposed to environment) neuron; 2) all have cilia that protrude through the cuticle out into the environment; and 3) express the DAF-19m transcription factor.
DAF-19m (for function in mating) is an isoform of DAF-19, the sole RFX-type transcription factor in C. elegans, which is an evolutionarily conserved master regulator of ciliogenesis genes [69, 72]. In daf-19 null mutants, cilia are entirely eliminated [73]. However, the DAF-19m isoform that is expressed exclusively in the IL2 and polycystin-expressing neurons, and is required for response and location of vulva behavior [69]. Expression of lov-1, pkd-2 and klp-6 genes, but not general ciliogenesis genes, requires the DAF-19m Isoform [69]. Since DAF-19m is expressed in all the neurons that secrete ECVs, this transcription factor isoform may activate expression of important unknown ECV-secretion pathway genes.
The Function Of The Polycystin-Containing ECVs
Extracellular vesicles, either in the form of microvesicles that bud from plasma membrane directly, or in the form of exosomes that are released to extracellular space via fusion of multi-vesicular body (MVB) to plasma membrane, play an important role in intercellular communication in normal and pathological states [74]. Although several mRNAs encoding TRP channels have been found in exosomes [75], polycystin 2 is the only TRP channel protein (along with polycystin 1) found in extracellular vesicles [76, 77]. In the Hogan (2009) studies, the presence of both polycystin 1 and 2 as cargoes was confirmed in the exosome-like vesicles from human urine samples. Intriguingly, these vesicles are taken up only by particular cilia types, not by all cilia. In a Pkhd1/fibrocystin knock-out mouse model of autosomal recessive polycystic kidney disease, vesicles accumulate along the primary cilia of cholangiocytes in dilated bile ducts, indicating that fibrocystin, is required for primary cilia to take up the ECVs [76]. Urinary ECVs shed by the renal epithelium can interact with the primary cilia of distant epithelial cells of the nephron [78].
C. elegans ECVs collected from klp-6 mutants lack PKD-2::GFP [58]. Transmission electron microscopy showed that klp-6 mutants instead accumulate excessive ECVs in the extracellular lumen surrounding CEM neuronal cilia [58]. Thus, in addition to roles in polycystin localization and modulation of IFT kinesins in cilia length regulation, KLP-6 also functions in the release of polycystin-containing ECVs into the environment.
We found that the ECVs isolated from wild-type C. elegans males are able to elicit a male tail-chasing behavior, indicating that the ECVs function in communication with conspecifics [58]. However, ECVs isolated from klp-6 mutants, which are defective in releasing polycystin-containing ECVs into the environment, fail to elicit tail-chasing behavior [58].
Further studies of the C. elegans male may illuminate the function of polycystin-containing ECVs such as those found in human urine, which are currently of unknown function. The evolutionary conservation of polycystin secretion in ECVs in both humans and nematodes suggests that export of polycystins in the ECV is functionally important, and may play a role in facilitating ECV-mediated intercellular and inter-organismal communication. There also appears to be a close relationship between ECVs and cilia, suggesting that cilia may be essential in ECV-mediated communication. We envision at least three advantages of ECV-mediated communication: 1) effectiveness—ECVs basically allow two cells exchange membrane, protein, and mRNA components; 2) selectivity—only cells with certain receptors allow fusion of the ECVs; 3) long distance signaling—the vesicles can travel long distance within a lumen, as in the case of epithelial primary cilia.
C. elegans Cilia Are Specialized In Form And Function
The polycystin-expressing male neurons display many functional and structural differences from the previously studied amphid and phasmid ciliated sensory neurons, which are present in both hermaphrodites and males. The most obvious difference is the lack of expression of polycystins in ciliated neurons of the hermaphrodite [12] (which coincidentally demonstrates that study only of C. elegans hermaphrodites, to the exclusion of males, would not possibly have provided many of the insights into the ciliopathic nature of a plethora of human diseases).
Differences in Ciliary Motors and Ciliogenesis
The molecular requirements for IFT and ciliogenesis in male-specific cilia are different versus amphid and phasmid cilia [67]. In amphid and phasmid cilia, the IFT motors kinesin-II (formed by KLP-11, KLP-20, and KAP-1) and OSM-3 are coupled in middle segments, but only OSM-3 continues into the distal singlet region [79]. In CEM neurons, kinesin-II and OSM-3 are partially uncoupled throughout the cilium [67]. Loss of OSM-3 alone in amphid and phasmid cilia results in loss of distal segments, but in CEM cilia, has no effect on cilia length [67].
KLP-6 is not expressed in amphid and phasmid neurons, but in CEM cilia, acts an accessory motor and negatively regulates the velocity of OSM-3 and kinesin-II, but positively regulates ciliary length [67]. This finding was the first demonstration that the activity of a kinesin-3 motor could specialize cilia by modifying the activities of the canonical IFT motors.
Differences in Microtubules in Polycystin-Expressing Neurons
Differences in microtubules might underlie the morphological specializations of cilia. The C. elegans genome encodes nine α and six β tubulins [80, 81]. The α-tubulin TBA-6 is expressed in the IL2 neurons and polycystin-expressing neurons, but absent from amphid and phasmid ciliated neurons [81].
Post-translational glutamylation of microtubules also differs between the polycystin-expressing cilia and the amphid and phasmid cilia. Glutamylation of microtubules is a post-translational modification that has long been recognized as a marker of stable microtubules [82]. Loss of ccpp-1, which encodes a cytosolic carboxypeptidase that acts as a tubulin deglutamylase to negatively regulate glutamylation, causes progressive ciliary degeneration of amphid and phasmid cilia but not of male-specific cilia [63]. However, loss of ccpp-1 does cause abnormal accumulation of PKD-2::GFP in polycystin-expressing neurons [63]. Loss of ttll-4, which encodes a tyrosine-ligase-like protein that adds glutamylation to microtubules [83], can suppress the ciliary degeneration of the amphid and phasmid cilia in ccpp-1 mutants, but does not suppress the PKD-2::GFP aggregation phenotype in polycystin-expressing neurons [63]. These results suggest that hyperglutamylation, caused by loss of CCPP-1, a negative regulator of microtubule glutamylation, has different effects in amphid and phasmid neurons. The function of TTLL-4, which opposes CCPP-1 in regulation of microtubule glutamylation, also appears to play differing roles in male-specific versus amphid and phasmid cilia [63].
Such comparative studies of cilia in C. elegans may identify some of the processes that give rise to distinct ciliary types in humans, such as the highly specialized rods and cones of the retina, and the simple primary cilia that decorate almost every cell in the human body. Mechanisms that create these ciliary forms are almost completely unknown.
Identification Of Molecules That Function With Polycystins: The PKD Gene Battery
Further genetic studies of mating behavior and the polycystin-expressing male-specific neurons has uncovered genes that are co-expressed with and interact genetically with LOV-1 and/or PKD-2, which we refer to as the “PKD Gene Battery,” that is needed for the function or localization of the polycystins. The PKD Gene Battery includes genes with human homologs that may be candidate ciliopathy genes or polycystin regulators (Barr Laboratory, unpublished data).
A microarray-based search for male-enriched genes identified six genes that are co-expressed with polycystins, (cwp-1, cwp-2, cwp-3, cwp-4, cwp-5 plus the α-tubulin gene tba-6) [57, 81, 84]. The cwp genes all encode proteins with signal peptides, and both cwp-4 and cwp-5 contain predicted extracellular mucin domains [57, 84]. Fluorescent reporters for these genes confirm that they are indeed co-expressed with polycystins in C. elegans (Table 1). Of the cwp genes, only cwp-5 has been characterized. cwp-5 mutant males are defective in the response and location of vulva behavioral steps [57]. CWP-5 is proposed to function analogously to Pkhd1/fibrocystin, which is thought to interact directly with the polycystin complex in renal epithelial cilia [57]. Although no homology is apparent from their primary sequences, both CWP-5 and fibrocystin are single-pass TM proteins. Mutation of tba-6 also causes a slight defect in response behavior [81].
Conclusion and Outlook
Elucidation Of The Pathways Of The Polycystins, ECVs, And Ciliary Specialization, By Finding Genes Co-Expressed With The Polycystins
Identification of new members of the PKD Gene Battery in C. elegans males could define a polycystin expression signature, which might be useful to find new therapeutic targets for ADPKD. Genes that are co-expressed with polycystins in male-specific C. elegans neurons might regulate the function or localization of polycystins, and therefore identify pathways that are physiologically relevant for the etiology of ADPKD. Transcriptome or proteome analysis of the polycystin-expressing neurons or polycystin-containing ECVs may reveal key signatures of these neurons and the partners that act with polycystins in different cell types. Furthermore, genes that are co-expressed with the polycystins might reveal mechanisms for signaling by ECV release or the specialization of the form and function of cilia. New expression profiling experiments are under way, with the promise of identifying new members of the PKD gene battery (Barr Laboratory, manuscript in preparation).
Acknowledgements
R.O. was supported by NJCSCR Fellowship 10– 2951-SCR-E-0. Work in the Barr laboratory is supported by NIH DK074746 and NIH DK059418.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell's antenna. The Journal of Cell Biology. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–316. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 4.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menco BP, Morrison EE. Morphology of the mammalian olfactory epithelium: Form, fine structure, function, and pathology. In: Doty R, editor. Handbook of Olfaction and Gustation. Marcel Dekker; New York: 2003. pp. 17–49. [Google Scholar]
- 6.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 7.Nauli SM, Jin X, AbouAlaiwi WA, El-Jouni W, Su X, Zhou J. Non-motile primary cilia as fluid shear stress mechanosensors. Methods Enzymol. 2013;525:1–20. doi: 10.1016/B978-0-12-397944-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. Journal of the American Society of Nephrology : JASN. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 9.Patel A, Honore E. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol. 2010;6:530–538. doi: 10.1038/nrneph.2010.97. [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest. 2005;85:452–463. doi: 10.1038/labinvest.3700253. [DOI] [PubMed] [Google Scholar]
- 11.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 13.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Current Biology : CB. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 15.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chasnov JR. The evolutionary role of males in C. elegans. Worm. 2013;2:e21146. doi: 10.4161/worm.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrios A, Ghosh R, Fang C, Emmons SW, Barr MM. PDF-1 neuropeptide signaling modulates a neural circuit for mate-searching behavior in C. elegans. Nat Neurosci. 2012;15:1675–1682. doi: 10.1038/nn.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrios A, Nurrish S, Emmons SW. Sensory regulation of C. elegans male mate-searching behavior. Current Biology : CB. 2008;18:1865–1871. doi: 10.1016/j.cub.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Natl Acad Sci U S A. 2007;104:6730–6735. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morsci NS, Haas LA, Barr MM. Sperm Status Regulates Sexual Attraction in Caenorhabditis elegans. Genetics. 2011;189:1341–1346. doi: 10.1534/genetics.111.133603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argon Y, Ward S. Caenorhabditis elegans fertilization-defective mutants with abnormal sperm. Genetics. 1980;96:413–433. doi: 10.1093/genetics/96.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleemann G, Basolo A. Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Animal Behaviour. 2007;74:1339–1347. [Google Scholar]
- 24.Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Developmental Biology. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- 25.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 26.Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 27.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB Journal. 2004;18:740–742. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- 28.Kottgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflugers Arch. 2005;451:286–293. doi: 10.1007/s00424-005-1417-3. [DOI] [PubMed] [Google Scholar]
- 29.Delmas P. The gating of polycystin signaling complex. Biol Res. 2004;37:681–691. doi: 10.4067/s0716-97602004000400026. [DOI] [PubMed] [Google Scholar]
- 30.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 31.Semmo M, Kottgen M, Hofherr A. The TRPP Subfamily and Polycystin-1 Proteins. Handb Exp Pharmacol. 2014;222:675–711. doi: 10.1007/978-3-642-54215-2_27. [DOI] [PubMed] [Google Scholar]
- 32.Bateman A, Sandford R. The PLAT domain: a new piece in the PKD1 puzzle. Current Biology : CB. 1999;9:R588–590. doi: 10.1016/s0960-9822(99)80380-7. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Barr MM. ATP-2 interacts with the PLAT domain of LOV-1 and is involved in Caenorhabditis elegans polycystin signaling. Molecular Biology of the Cell. 2005;16:458–469. doi: 10.1091/mbc.E04-09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaletta T, Van der Craen M, Van Geel A, Dewulf N, Bogaert T, Branden M, King KV, Buechner M, Barstead R, Hyink D, et al. Towards understanding the polycystins. Nephron Exp Nephrol. 2003;93:e9–17. doi: 10.1159/000066650. [DOI] [PubMed] [Google Scholar]
- 35.Promel S, Langenhan T, Arac D. Matching structure with function: the GAIN domain of Adhesion-GPCR and PKD1-like proteins. Trends Pharmacol Sci. 2013;34:470–478. doi: 10.1016/j.tips.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Retailleau K, Duprat F. Polycystins and partners: proposed role in mechanosensitivity. J Physiol. 2014 Apr; doi: 10.1113/jphysiol.2014.271346. 2014; published ahead of print March 31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney International. 2005;67:1234–1247. doi: 10.1111/j.1523-1755.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 38.SMART Server http://smart.embl-heidelberg.de/
- 39.Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letunic I, Doerks T, Bork P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:D302–305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.COILS Server http://embnet.vital-it.ch/software/COILS_form.html.
- 42.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 43.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koulen P, Duncan RS, Liu J, Cohen NE, Yannazzo JA, McClung N, Lockhart CL, Branden M, Buechner M. Polycystin-2 accelerates Ca2+ release from intracellular stores in Caenorhabditis elegans. Cell Calcium. 2005;37:593–601. doi: 10.1016/j.ceca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Knobel KM, Peden EM, Barr MM. Distinct protein domains regulate ciliary targeting and function of C. elegans PKD-2. Exp Cell Res. 2008;314:825–833. doi: 10.1016/j.yexcr.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalagiorgou G, Basdra EK, Papavassiliou AG. Polycystin-1: function as a mechanosensor. Int J Biochem Cell Biol. 2010;42:1610–1613. doi: 10.1016/j.biocel.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 48.O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 49.Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babich V, Zeng WZ, Yeh BI, Ibraghimov-Beskrovnaya O, Cai Y, Somlo S, Huang CL. The N-terminal extracellular domain is required for polycystin-1-dependent channel activity. J Biol Chem. 2004;279:25582–25589. doi: 10.1074/jbc.M402829200. [DOI] [PubMed] [Google Scholar]
- 51.Delmas P, Nomura H, Li X, Lakkis M, Luo Y, Segal Y, Fernandez-Fernandez JM, Harris P, Frischauf AM, Brown DA, et al. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J Biol Chem. 2002;277:11276–11283. doi: 10.1074/jbc.M110483200. [DOI] [PubMed] [Google Scholar]
- 52.Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, Wallace DP, Hempson SJ, Calvet JP. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem. 2004;279:55455–55464. doi: 10.1074/jbc.M402905200. [DOI] [PubMed] [Google Scholar]
- 53.Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye C, et al. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(2+) homeostasis in polycystic kidney disease. Biochem Biophys Res Commun. 2001;282:341–350. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- 54.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci U S A. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelucchi B, Aguiari G, Pignatelli A, Manzati E, Witzgall R, Del Senno L, Belluzzi O. Nonspecific cation current associated with native polycystin-2 in HEK-293 cells. Journal of the American Society of Nephrology : JASN. 2006;17:388–397. doi: 10.1681/ASN.2004121146. [DOI] [PubMed] [Google Scholar]
- 56.Peden EM, Barr MM. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Current biology : CB. 2005;15:394–404. doi: 10.1016/j.cub.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 57.Miller RM, Portman DS. A latent capacity of the C. elegans polycystins to disrupt sensory transduction is repressed by the single-pass ciliary membrane protein CWP-5. Dis Model Mech. 2010;3:441–450. doi: 10.1242/dmm.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Current Biology : CB. 2014;24:519–525. doi: 10.1016/j.cub.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Current Biology : CB. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 60.Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 61.Bae YK, Lyman-Gingerich J, Barr MM, Knobel KM. Identification of genes involved in the ciliary trafficking of C. elegans PKD-2. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:2021–2029. doi: 10.1002/dvdy.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Current Biology : CB. 2001;11:457–461. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- 63.O'Hagan R, Piasecki BP, Silva M, Phirke P, Nguyen KC, Hall DH, Swoboda P, Barr MM. The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans. Current Biology : CB. 2011;21:1685–1694. doi: 10.1016/j.cub.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bae YK, Kim E, L'Hernault S W, Barr MM. The CIL-1 PI 5-phosphatase localizes TRP Polycystins to cilia and activates sperm in C. elegans. Current Biology : CB. 2009;19:1599–1607. doi: 10.1016/j.cub.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu J, Bae YK, Knobel KM, Barr MM. Casein kinase II and calcineurin modulate TRPP function and ciliary localization. Molecular Biology of the Cell. 2006;17:2200–2211. doi: 10.1091/mbc.E05-10-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu J, Wittekind SG, Barr MM. STAM and Hrs down-regulate ciliary TRP receptors. Molecular Biology of the Cell. 2007;18:3277–3289. doi: 10.1091/mbc.E07-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Current Biology : CB. 2011;21:1239–1244. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doroquez DB, Berciu C, Anderson JR, Sengupta P, Nicastro D. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. Elife. 2014;3:e01948. doi: 10.7554/eLife.01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Schwartz HT, Barr MM. Functional specialization of sensory cilia by an RFX transcription factor isoform. Genetics. 2010;186:1295–1307. doi: 10.1534/genetics.110.122879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Developmental Biology. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 71.Emmons SW. Male development. In: T.C.e.R. Community, editor. WormBook : the online review of C. elegans biology. WormBook; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol Cell. 2000;5:411–421. doi: 10.1016/s1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- 73.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Developmental Biology. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 74.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Vesiclepedia http://microvesicles.org/index.html.
- 76.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, et al. Characterization of PKD protein-positive exosome-like vesicles. Journal of the American Society of Nephrology : JASN. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hurd T, Zhou W, Jenkins P, Liu CJ, Swaroop A, Khanna H, Martens J, Hildebrandt F, Margolis B. The retinitis pigmentosa protein RP2 interacts with polycystin 2 and regulates cilia-mediated vertebrate development. Hum Mol Genet. 2010;19:4330–4344. doi: 10.1093/hmg/ddq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CY, Hogan MC, Ward CJ. Purification of exosome-like vesicles from urine. Methods Enzymol. 2013;524:225–241. doi: 10.1016/B978-0-12-397945-2.00013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellartransport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 80.Gogonea CB, Gogonea V, Ali YM, Merz KM, Jr., Siddiqui SS. Computational prediction of the three-dimensional structures for the Caenorhabditis elegans tubulin family. J Mol Graph Model. 1999;17:90–100. 126–130. doi: 10.1016/s1093-3263(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 81.Hurd DD, Miller RM, Nunez L, Portman DS. Specific alpha- and beta-tubulin isotypes optimize the functions of sensory Cilia in Caenorhabditis elegans. Genetics. 2010;185:883–896. doi: 10.1534/genetics.110.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 83.Kimura Y, Kurabe N, Ikegami K, Tsutsumi K, Konishi Y, Kaplan OI, Kunitomo H, Iino Y, Blacque OE, Setou M. Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs). J Biol Chem. 2010;285:22936–22941. doi: 10.1074/jbc.C110.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Portman DS, Emmons SW. Identification of C. elegans sensory ray genes using whole-genome expression profiling. Developmental Biology. 2004;270:499–512. doi: 10.1016/j.ydbio.2004.02.020. [DOI] [PubMed] [Google Scholar]