Abstract
Epidemiological investigations suggest a link between exposure to indoor air chemicals and adverse health effects. Consumer products contain reactive chemicals which can form secondary pollutants which may contribute to these effects. The reaction of limonene and ozone is a well characterized example of this type of indoor air chemistry. The studies described here characterize an in vitro model using an epithelial cell line (A549) or differentiated epithelial tissue (MucilAir™). The model is used to investigate adverse effects following exposure to combinations of limonene and ozone. In A549 cells, exposure to both the parent compounds and reaction products resulted in alterations in inflammatory cytokine production. A one hour exposure to limonene + ozone resulted in decreased proliferation when compared to cells exposed to limonene alone. Repeated dose exposures of limonene or limonene + ozone were conducted on MucilAir™ tissue. No change in proliferation was observed but increases in cytokine production were observed for both the parent compounds and reaction products. Factors such as exposure duration, chemical concentration, and sampling time point were identified to influence result outcome. These findings suggest that exposure to reaction products may produce more severe effects compared to the parent compound.
Keywords: Indoor air, Limonene, Ozone, Secondary oxidation products
1. Introduction
Exposure to the indoor air environment has the potential for a wide range of effects on human health and it has been estimated that indoor air quality-related health issues cost businesses $20–70 billion annually due to lost productivity, decreased performance, and sick absences (Mendell et al., 2002). Investigations have ascribed these effects (Arif and Shah, 2007; Jang et al., 2007), in part, to volatile organic compounds (VOCs) emitted from building materials and furnishings and application of chemicals (paints, cleaners, pesticides, glues and adhesives). (Singer et al., 2006; Weschler, 2004). Research has associated VOC exposure with allergic airway inflammation, airway hyperresponsiveness, asthma, and loss of pulmonary function in animals and/or humans (Arif and Shah, 2007; Jang et al., 2007; Yoon et al., 2010; Bonisch et al., 2012). In addition, the secondary pollutants resulting from reactive indoor air chemistry (e.g. ozonolysis of VOCs) may also be responsible for some of the health effects associated with indoor air exposures. Consumer cleaning products and air fresheners contain large amounts of VOCs which can react with OH• (hydroxyl radicals), ozone, and/or NO3• (nitrate radicals) to form secondary oxidation products or secondary pollutants not detected with conventional sampling methods. These secondary pollutants include oxygenated organic chemicals, such as aldehydes, ketones, carboxylic acids and dicarbonyls (Forester et al., 2007; Ham et al., 2006; Harrison et al., 2007; Wells, 2005) which can be formed into thousands of chemical compounds. The potential toxicity of these secondary pollutants, either individually or as mixtures, is poorly understood and because of the lack of research in this area, associations with adverse health effects have yet to be made. Although many of these secondary pollutants have been observed from simulated indoor air chemistry, they are not routinely detected with conventional sampling methods which may lead to inaccurate exposure assessments of indoor environments.
The respiratory tract plays a protective role against xenobiotics and invading microorganisms and also plays a significant role in immune surveillance. Epithelial cells are a major contact point for atmospheric pollutants since they are needed for gaseous exchange, mucous secretion, and protection. Disorders of the respiratory tract following chemical exposure include: disruption of the barrier functions including the mucociliary clearance, irritation, coughing, acute injury, altered gas exchange and decreased immune function. Due to the complexity of chemical-respiratory tract interactions, several in vitro methods using relevant airway cells, or tissues and implementation of target specific endpoints have been developed for toxicity assessment (Lambre et al., 1996). However, a lack of standardization among methods has made data interpretation and extrapolation challenging (Ritter et al., 2001). Complicating factors include: lack of complexity, differences in exposure method, chemical exposure concentration, flow and duration of exposure, experimental model and endpoints selected for analysis (Bakand et al., 2005). More primitive exposure systems include the addition of the chemical or compound of interest directly to the media in a closed flask (static environment). While the main benefits of these types of exposure studies include reduced costs and large sample number, they do have limited sensitivity and provide an unrealistic environment due to chemical-media interactions. (Fischader et al., 2008). Recent advances in the field include the development of air/cell interface exposure systems such as those produced by companies including Vitrocell® Systems (Waldkirch, Germany) and Cultex Laboratories (Hannover, Germany). These exposure systems allow for direct exposure (flowing system) of the apical surface of the cell line or tissue with the aerosolized compound of interest, eliminating the potential for chemical/media interactions (Anderson et al., 2010; Persoz et al., 2010; Schmalz et al., 2011). While these systems are highly efficient and sensitive they are often expensive and most do not easily allow for dose response studies.
In addition to exposure system, selection of the experimental model is another potential for variability. Different models can be utilized depending on the health effect of interest (Verstraelen et al., 2008a). Inflammation and irritation of the lower respiratory tract is often evaluated in bronchial epithelial cells (NHBE, BEAS-2B) (Pichavant et al., 2005; Persoz et al., 2012) or alveolar epithelial cells (A549) (Krakauer, 2000) while respiratory sensitization is often evaluated in monocyte/macrophage (Mono-Mac-6, THP-1) cell lines (Elms et al., 2001; Verstraelen et al., 2008b). Other advances in the field also include the use of primary cell lines and the development of highly differentiated three dimensional human airway tissue samples, such as (EpiAirway™ Tissue Model (Mattek, Ashland, MA) and MucilAir™ Epithelix (Geneva, Switzerland). To a lesser extent, cellular co-cultures consisting of epithelial cells, human blood monocyte-derived macrophages and dendritic cells have been used for investigational purposes (Lehmann et al., 2011). The selection of relevant endpoints is often based on the cell line or tissue selected for use and include but are not limited to: inflammatory cytokines [Interleukin 8 (IL-8), Interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP-1)], cell proliferation, cytotoxicity (measurements of metabolic activity and cell membrane integrity), oxidative stress [glutathione (GSH), cellular markers (HO-1, SOD-1, GSTP1, PTGS2, DUSP1)], reactive oxygen species (ROS), signaling pathways (NF-kB and MAP kinase), and genotoxicity (DNA damage). Differences in cell culture technique, use of cell stimulation with agents such as tumor necrosis factor alpha (THF-α) as surrogates for cellular signaling, and time point for experimental sampling are also potential sources of variability.
The majority of research in the field of indoor air has focused on the parent compounds, or the chemicals most widely recognized as indoor air pollutants including chlorobenzene, styrene, m-xylene, formaldehyde, toluene, terpenes, and aldehydes. Research has shown that exposure of TNF-α stimulated A549 cells (Static/20 h) to chlorobenzene, styrene or m-xylene (within the indoor relevant concentration range 1–25,000 mg/m3) increased MCP-1 production while higher concentrations increased IL-8 production (Fischader et al., 2008). Mixtures of the three VOCs produced similar results. In addition to alternations in IL-8 and MCP-1 production, increased IL-13 levels were observed when supernatants of chlorobenzene exposed A549 cells (Static/20 h) were incubated with human peripheral blood mononuclear cells (Lehmann et al., 2008). Expression of cellular markers for oxidative stress, such as HO-1, GSTP1, SOD-1, prostaglandin-PTGS2 and DUSP1, were also found to be elevated in the presence of chlorobenzene (102–104 mg/m3 for 24 h) along with intracellular ROS. However, in the presence of antioxidants chlorobenzene-induced alterations were suppressed (Feltens et al., 2010). Exposure of A549 cells (0.2 ppmv for 1 h/Cultex®) to toluene and benzene, but not formaldehyde, increased IL-8 production and cytotoxicity following exposure. The ratio of reduced to oxidized glutathione was increased for benzene treated cells and decreased for formaldehyde treated cells (Pariselli et al., 2009). However, cells pre-stimulated with TNF-α prior to formaldehyde (50 mg/m3 for 30 min) exposure, resulted in enhanced IL-8 expression (Persoz et al., 2010). Gminski et al., 2010 demonstrated that the aldehydes 2-heptenal and 2-octenal (main VOC constituents emitted from pine wood) caused genotoxic effects in A549 cells following exposure (15–65 ppm; Vitrocell® for 1 h) to concentrations exceeding 100 mg/m3 and 40 mg/m3, respectively (Gminski et al., 2010). In vitro investigations into the specific health effects associated with exposure to secondary pollutants in the indoor environment are limited. One study conducted by Anderson et al. (2010) demonstrated that exposure of A549 cells (Vitrocell® for 4 h) to structurally similar terpene ozonolysis reaction products (dicarbonyl compounds) resulted in an increased pro-inflammatory response suggesting the potential for toxicity of secondary pollutants. The differences in exposure techniques and endpoints among the above mentioned studies emphasize the need for the standardization of this type of model.
The ozone-initiated reaction of limonene, an abundant VOC that provides a citrus smell to many cleaning supplies and personal care products, is a well characterized chemistry model for the identification of secondary pollutants and the evaluation of indoor air mixtures. While in vivo studies suggest the potential for more severe health effects following exposure to ozone/limonene reaction products compared to the parent compounds (Wolkoff et al., 2012), currently no in vitro work has been conducted. Therefore, this study used the prototypical indoor air reaction of limonene + ozone to begin to characterize if secondary products are more toxic than their parent compounds and to emphasize the importance of method development and validation for these types of in vitro exposure models.
2. Experimental methods
2.1. Teflon chamber preparation
Teflon chambers (FEP 500, American Durafilm, Hollston, MA) were constructed and filled with treated air (described below) to facilitate cell exposure to gas-phase chemicals via the Vitrocell® apparatus. Compressed air from the National Institute for Occupational Safety and Health (NIOSH) facility was passed through anhydrous CaSO4 and molecular sieves (Drierite, Xenia, OH) to remove both moisture and organic contaminants. The resultant dry air (less than 5% relative humidity) was humidified to 50% relative humidity to simulate average indoor environment conditions. R(+)-Limonene (99% purity) was injected into a 50% relative humidity air stream through a heated ¼ inch stainless steel tee into the 60 liter Teflon chambers. Lower target concentrations (500 ppb (1.2 × 1013 molecule cm−3)) of limonene in both limonene and limonene/ozone chambers were used for the MucilAir™ exposures while higher concentrations of approximately 20 ppm (5 × 1014 molecule cm−3) was used for A549 exposures. For the reaction product experiments, ozone was produced by photolyzing air with a mercury pen lamp (Jelight, Irvine, CA) in a separate Teflon chamber. Ozone concentrations were measured with a UV photometric ozone analyzer (model 49C or 49i, Thermo Fisher Scientific, Inc., Waltham, MA). Ozone concentrations of either 100 ppb (2.5 × 1012 molecule cm−3 for MucilAir™ exposure) or 4 ppm (1 × 1014 molecule cm−3 for A549 exposure) were achieved by transferring large volumes (2 liters) from the separate high concentration (~120 ppm) ozone chamber using a gas-tight syringe or an additional smaller Teflon chamber. Ozone was injected into the respective Teflon chamber containing ~500 ppb (1.2 × 1013 molecule cm−3) or ~20 ppm (5 × 1014 molecule cm−3) limonene 15 to 30 min prior to the Vitrocell® exposure. The high concentrations of ozone (4 ppm) and limonene (20 ppm) were based on preliminary concentration range finding studies where the highest dose that did not induce cellular toxicity was selected for the initial hazard identification studies. In an attempt to mimic in door environments, lower realistic concentrations of ozone (100 ppb) and limonene (500 ppm) were selected (Weschler, 2004) for the subsequent studies. Previous gas-phase VOC experiments indicated the sample preparation method above provides multi-hour concentration stability (Forester and Wells, 2009).
2.2. Chemical characterization
Sampling for monitoring chamber contents was performed using a 65 μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) solid phase micro-extraction (SPME) fiber (Supelco, Milwaukee, WI) assembly which was inserted into a 6.4-mm Swagelok (Solon, OH) fitting attached to the Teflon®-film chambers (described above). The chamber contents were sampled for 5 min then the SPME was inserted through a Merlin Microseal (Half Moon Bay, CA) and into the heated injector of an Agilent (Wilmington, DE) 6890 gas chromatograph with a 5975 mass selective detector (GC/MS) and Agilent ChemStation software. Compound separation was achieved by a J&W Scientific (Folsom, CA) HP-5MS (0.25 mm i.d., 30-m long, 0.25 μm film thickness) column and the following GC oven parameters: injection port was set to 250 °C, and oven temperature began at 40 °C for 2 min and was ramped 20 °C min−1 to 130 °C then ramped 40 °C min−1 to 240 °C and held for 2 min.
2.3. A549 cell culture
Human alveolar epithelial cells (A549) were purchased from American Type Culture Collection (ATCC No.CCL-185). For each set of experiments cell culture was initiated from an A549 stock (1 × 106 cells/ml) prepared from early passages. Cells were incubated at 37 °C with 5% CO2 in F12 K medium (Kaighn’s Modification of Ham’s F-12 with L-Glutamine, ATCC, VA, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS) and 0.05 mg/ml of Gentamycin. Cells were propagated in sterile and vented 75 cm2 cell culture flasks until desired number of cells was reached then harvested, counted and seeded on Costar 24 mm (0.4 μm) transwell inserts and placed in 6-well tissue culture treated plates. To determine the optimal growth of A549 cells on inserts, a range of 1.25 × 105 to 5 × 105 cells per insert and incubation times of 24 to 48 h were tested. During this incubation period complete culture medium (with 10% FBS) was added to the apical (1.5 ml) and basolateral (2.5 ml) tissue surfaces. Twenty-four hours prior to exposure the complete culture medium was removed and replaced with serum-free medium to synchronize the cells. Recombinant human TNF-α (Invivogen, San Diego, CA) at a final concentration of 2 ng/ml was added to serum-free medium 24 h prior to exposure for pre-stimulation/activation of cells prior to exposure in select experiments. Inserts containing unexposed cells (n = 3) were included in every experiment to evaluate cellular integrity. These controls were treated exactly the same as the experimental cells except they remained in the incubator while the other cells were exposed in the Vitrocell® chambers.
2.4. Mucilair™ tissue culture
MucilAir™ tissue samples are 3D models of highly differential human airway epithelium consisting of primary human cells isolated from the nasal cavity, the trachea and the bronchus. The manufacturer claims that these samples are functional for more than 1 year and can therefore be used for long term and/or repeated dose exposures. Commercially available transwell inserts with MucilAir™ epithelium were purchased from Epithelix (Geneva, Switzerland). Upon arrival inserts were transferred into 24-well tissue culture plates containing 0.8 ml of serum free MucilAir™ Culture Medium (Geneva, Switzerland) which did not exceed the air/liquid interface. Cultures were maintained at 37 °C in a humidified 5% CO2 incubator. Media were changed every 2–3 days. Unexposed inserts (n = 3) were included in the 28-day exposure experiment to evaluate cellular integrity. These controls were treated exactly the same as the experimental cells except they remained in the incubator while the other inserts were exposed in the Vitrocell® chambers.
2.5. Vitrocell exposures
For A549 cells, immediately before exposures culture medium was completely removed from the apical side of the inserts (n = 3), cells were washed twice with sterile phosphate buffered saline then transferred into the Vitrocell® PT-CF exposure system (Vitrocell, Waldkirch, Germany). For the exposures of MucilAir™ tissue, the inserts (n = 3) were transferred directly into the Vitrocell® PT-CF exposure system. Once a week, a washing step (three times within 1 h using MucilAir™ culture medium) was performed to remove accumulated mucus produced by fully differentiated and functional MucilAir™ tissue. Exposures were conducted as previously described (Anderson et al., 2010). In brief, two separate exposure modules, each accommodating three inserts were used for parallel exposures to control (n = 3) and test atmospheres (n = 3). During exposure cells were immersed in serum-free medium on the basal surface, allowing cells to be nourished from the bottom while being exposed to gas on air/liquid interface from the top. To minimize mechanical stress and maintain cell viability, the test atmosphere was delivered via trumpets raised 0.5 cm above the cell layer at an optimal constant air flow of 3 ml/minute (A549 cells) or 2 ml/minute (MucilAir). A single exposure (1–4 h) was tested for A549 cells. MucilAir™ inserts were exposed for 1 h per day, 5 days a week for a total of 4 weeks. Immediately after the exposure, inserts were transferred to regular 6-well (A549) or 24-well (MucilAir™) plates. Complete medium with 10% FBS (A549) or MucilAir™ Culture Medium was added on both (apical and basolateral) or basolateral side respectively. Cells were allowed to recover in a 37 °C, 5% CO2 incubator. Culture supernatants were collected at 10–12 h post-exposure and then again at 24 h (A549) or at 72 h after the last exposure of each week for 4 weeks (MucilAir™). Supernatants were stored at −20 °C for subsequent analysis. Following exposure, cells and tissues were analyzed for cell proliferation and supernatants were evaluated for cytokine production.
2.6. XTT proliferation assay
Cell proliferation of A549 cells and MucilAir™ tissue samples was determined using Cell Proliferation Kit II–XTT, (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocol with slight modifications. In brief, in order to minimize the loss of cells during the trypsinization process the reaction was performed directly on the transwell inserts in a 6-well (A549) or a 24-well plate (MucilAir™). For A549 cells 24 h post exposure both top and bottom culture supernatants were removed. Cells were washed once with F-12 K Medium supplemented with 10% of heat inactivated fetal bovine serum. Fresh culture medium (1 ml) pre warmed to 37 °C was added to each insert. For MucilAir™ samples basal supernatants were removed after the last exposure (at the end of 4 weeks). Inserts were rinsed once with MucilAir™ Culture Medium and 125 μl of fresh warm medium was added on top of each insert. Reaction reagents were thawed immediately prior to use. A XTT labeling mixture was prepared by mixing 5 ml of XTT labeling reagent and 100 μl of electron-coupling reagent. The mixture was then added to each insert (0.5 ml per A549 or 125 μl per MucilAir™ insert) to obtain a final concentration of XTT 0.3 mg/ml. To ensure even distribution of the dye on top of the inserts, the plate was swirled in a circular motion and incubated for 2 h in a humidified atmosphere (37 °C, 5% CO2 incubator). Following the 2 h incubation period, 100 μl aliquots from each sample were transferred into a 96-well, flat bottom plate and the absorbance was determined using a Spectramax Vmax plate reader (Molecular Devices, Sunnyvale, CA) at 492 nm.
2.7. Cytokine detection
Levels of IL-8 and MCP-1 were measured in the combined apical and basal culture supernatants of A549 cells (10–24 h post exposure) and IL-8, IL-6, MCP-1, and granulocyte–macrophage colony-stimulating factor (GM-CSF) were measured from basal supernatants of MucilAir™ tissues collected post exposure using commercially available ELISA kits (OptEIA™, BD Biosciences, San Jose, CA) according to the manufacturer’s instructions.
2.8. Statistics
To determine statistically significant differences in cell proliferation or concentrations of inflammatory proteins, a 2-tailed unpaired t-test was used to compare clean air or limonene exposed to limonene or limonene + ozone exposed samples for each specified time point. All data is based on three independent biological replicates (n = 3) per exposure group. Cytokine levels are based on the mean of triplicate samples from each biological replicate at each time point. Analysis of cell proliferation is based on the mean for each biological replicate sample for each treatment group. Linear trend analysis was performed to determine if the test articles had exposure duration-related effects for the specified endpoints. Significant differences between control and experimental groups are designated with **(p ≤ 0.01) or *(p ≤ 0.05).
3. Results
3.1. Generation of limonene and ozone reaction products
Fig. 1 shows the overlaid chromatograms following SPME sampling of the chamber contents connected to the Vitrocell® apparatus used for the exposures in the above mentioned studies. The dashed lines show the limonene peak before and after addition of ozone in the limonene + ozone chamber while the solid line (shifted by +0.05 min) shows the limonene peak for the limonene alone chamber. Because the peak areas are proportional to concentration it can be observed that prior to ozone addition the two chambers contained the same limonene concentration. After addition of ozone to the limonene + ozone chamber, the decrease in the limonene chromatographic peak area demonstrates the reaction of limonene with ozone. The ozone is consumed completely by the limonene + ozone reaction because there is significantly more limonene than ozone (data not shown).
Fig. 1.
GC/MS chromatogram of SPME sampled limonene and limonene/ozone chambers. Peak areas are proportional to concentration and all peaks are on the same y-axis scale. Solid line is the chromatographic peak of limonene only (20 ppm) chamber (shifted by +0.05 min for clarity). Gray dashed line is the chromatographic peak of limonene (20 ppm) in limonene/ozone chamber prior to addition of ozone while black dashed line is limonene peak in limonene/ozone chamber after ozone (4 ppm) addition.
3.2. A 4 h exposure to limonene and ozone reaction products augments pro inflammatory cytokine production in A549 cells
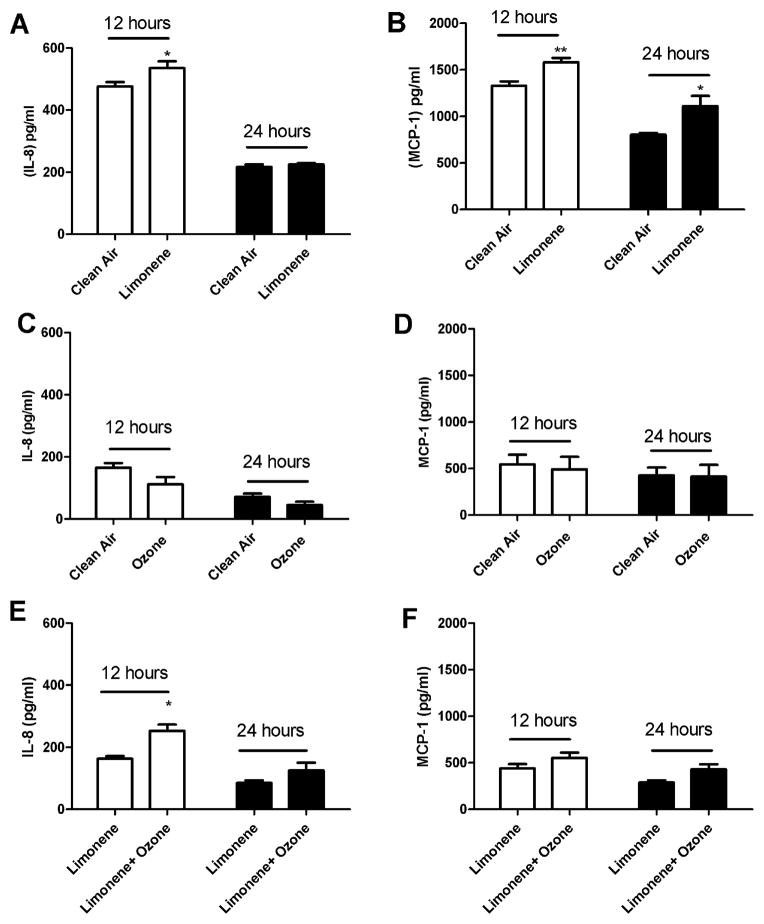
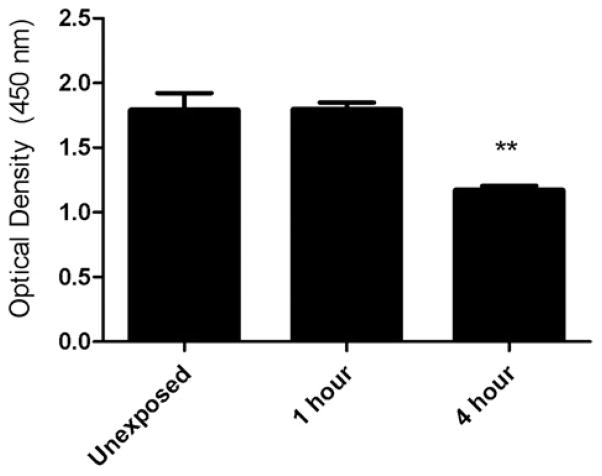
To determine if exposure to indoor air reaction products alters the pro inflammatory response, pulmonary epithelial cells were exposed to clean air, ozone (4 ppm), limonene (20 ppm), or limonene (20 ppm) + ozone (4 ppm) (Fig. 2) for 4 h. Statistically significant increases in IL-8 (12 h post-exposure) and MCP-1 (12 and 24 h post-exposure) were observed following exposure to limonene when compared to clean air (Fig. 2A and B). No significant differences in cytokine production were observed following exposure to ozone when compared to clean air (Fig. 2C and D) which suggests that ozone alone does not influence the inflammatory cytokine response. However, a significant increase in IL-8 cytokine production was observed following exposure to limonene + ozone (12 h post-exposure) when compared to limonene alone (Fig. 2E). Although not statistically significant, a modest increase in cytokine production was observed at 24 h post-exposure. There were no statistically significant increases for MCP-1 cytokine production at 12 or 24 h post-exposure (Fig. 2F). The data presented are the best representation of three separate studies. Literature searches have identified that exposure times vary for research utilizing similar types of exposure models. To mimic a realistic indoor air environment an extended exposure duration is desired. To determine if exposure duration influences cell proliferation, A549 cells were exposed to clean air for durations of one and 4 h (Fig. 3). A statistically significant reduction in the metabolic state of the cells, indicating decreased proliferation, was observed following a 4 h exposure to clean air when compared to unexposed controls. This result was not observed following the 1 h exposure to clean air.
Fig. 2.
The effect of limonene and limonene + ozone reaction products on A549 cells following a 4 h exposure. A549 cells (250,000) were incubated for 24 h on transwell inserts prior to exposure. Following exposure, cells were evaluated for IL-8 and MCP-1 protein production at 12 and 24 h post-exposure. Comparisons were made for (A and B) clean air vs. limonene (20 ppm), (C and D) clean air vs. ozone (4 ppm) and (E and F) limonene (20 ppm) vs. limonene (20 ppm)/ozone (4 ppm). Bars represent the mean ± SE from three independent biological replicates per exposure group. Significant differences are designated with *p < 0.05.
Fig. 3.
The effect of exposure duration on proliferation of A549 cells. A549 cells (250,000) were incubated for 48 h on transwell insert prior to exposure. Unexposed cells remained in incubator (37 °C, 5% CO2) while clean air was delivered to exposed cells for 1 or 4 h. Cell proliferation was evaluation 24 h post exposure. Bars represent the mean ± SE from three independent biological replicates per exposure group. Significant differences are designated with **p < 0.01.
3.3. A 1 h exposure to limonene and ozone reaction products augments pro inflammatory cytokine production in unstimulated A549 cells
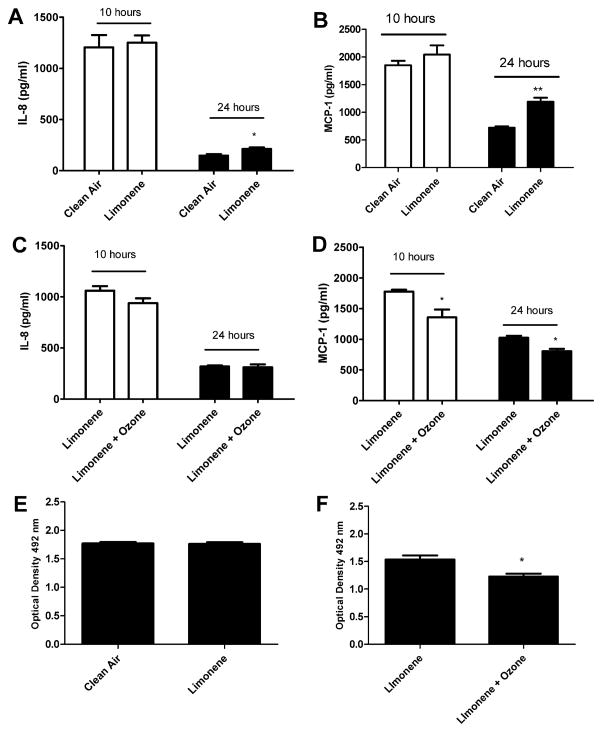
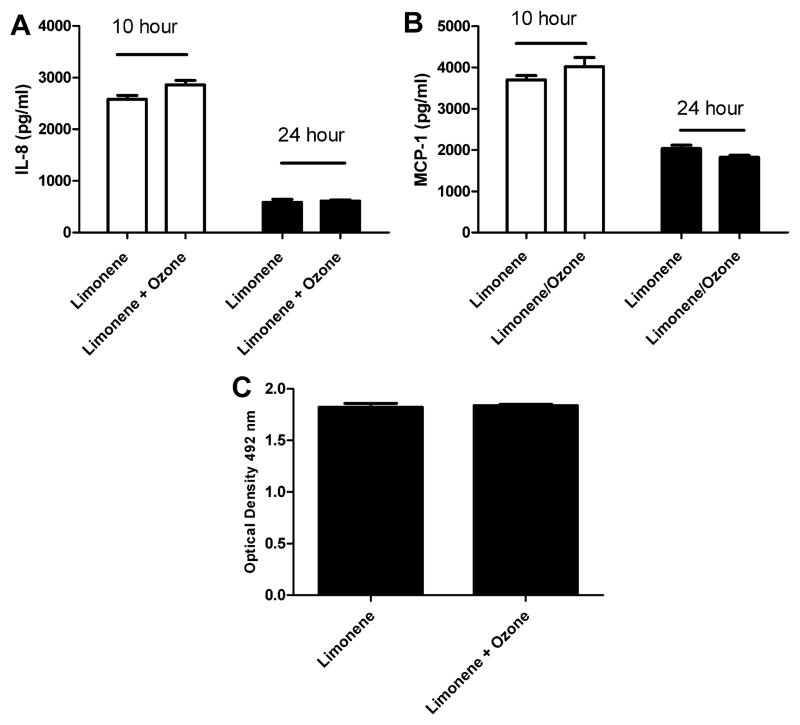
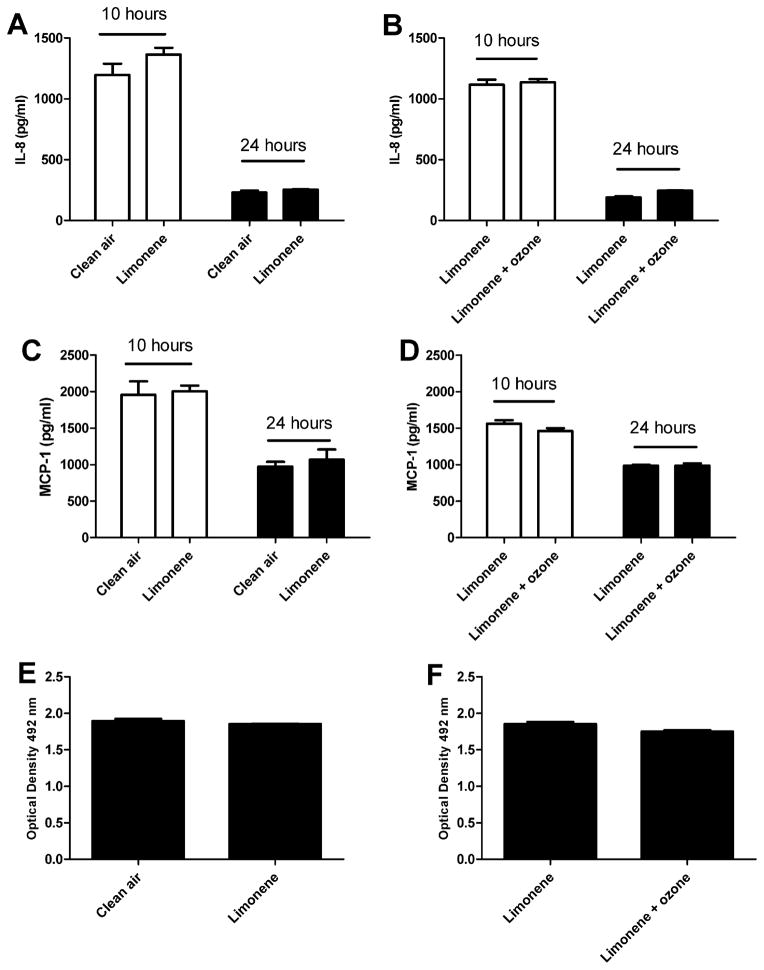
Due to the potential toxicity induced by exposure duration, subsequent exposures were reduced to 1 h. To determine if a 1 h exposure to indoor air reaction products alters cell proliferation or the pro inflammatory responses, pulmonary epithelial cells were exposed to clean air, limonene (20 ppm), or limonene (20 ppm) + ozone (4 ppm). Consistent with the 4 h exposure duration (Fig. 2), exposure to limonene resulted in significant increases in IL-8 and MCP-1 at 24 h post exposure when compared to the clean air control (Fig. 4A and B). Exposure to limonene + ozone resulted in a decreased production of MCP-1 at the 10 and 24 h post-exposure time points when compared to limonene (Fig. 3D). No change was observed in IL-8 production. No changes in cell viability were observed following exposure to limonene (Fig. 4E) when compared to the clean air control. However, a statistically significant decrease in cellular metabolism/proliferation was observed following limonene + ozone exposure (Fig. 4F). The data presented are the best representation of three separate studies. Similar exposure models described in the literature have elected to stimulate cells prior to exposure. Therefore, the effect of pre stimulation on cellular proliferation and pro inflammatory cytokine production was explored following exposure to limonene, or limonene + ozone (Fig. 5). In comparison to unstimulated cells, no statistically significant changes in cell proliferation or cytokine production were observed. Lower, exposure concentrations of limonene and ozone were used to explore the influence of exposure concentration on the pro inflammatory and proliferative responses of A549 cells. Pulmonary epithelial cells were exposed to clean air, limonene (500 ppb), or limonene (500 ppb) + ozone (100 ppb). In contrast to previous studies that used higher concentrations of limonene and ozone (Fig. 2), no changes in proliferation or cytokine production were observed following exposure (Fig. 6).
Fig. 4.
The effect of limonene and limonene + ozone reaction products on A549 cells following a 1 h exposure. A549 cells (250,000) were incubated for 48 h on transwell insert prior to exposure. Following exposure, cells were evaluated for IL-8 (A and B) and MCP-1 (C and D) protein production at 10 and 24 h post-exposure and cell proliferation (E and F) at 24 h post exposure. Comparisons were made for clean air vs. limonene (20 ppm) and limonene (20 ppm) vs. limonene (20 ppm)/ozone (4 ppm). Bars represent the mean ± SE from 3 independent biological replicates per exposure group. Significant differences are designated with **p < 0.01 or *p < 0.05.
Fig. 5.
The effect of limonene and limonene + ozone reaction products on stimulated A549 cells following a 1 h exposure. A549 cells (250,000) were incubated for 48 h on transwell insert prior to exposure. Following exposure, TNF-α stimulated cells (2 ng/ml) were evaluated for IL-8 (A) and MCP-1 (B) protein production at 10 and 24 h postexposure and cell proliferation (C) at 24 h post exposure. Comparisons were made between limonene (20 ppm) vs. limonene (20 ppm)/ozone (4 ppm). Bars represent the mean ± SE from three independent biological replicates per exposure group.
Fig. 6.
The effect of exposure concentration on A549 cells following a 1 h exposure. A549 cells (250,000) were incubated for 48 h on transwell insert prior to exposure. Following exposure, cells were evaluated for IL-8 (A and B) and MCP-1 (C and D) protein production at 10 and 24 h post-exposure and cell proliferation (E and F) at 24 h post exposure. Comparisons were made for clean air vs. limonene (500 ppb) and limonene (500 ppb) vs. limonene (500 ppb)/ozone (100 ppb). Bars represent the mean ± SE from three independent biological replicates per exposure group. Significant differences are designated with **p < 0.01 or *p < 0.05.
3.4. Repeated dose exposure to limonene and ozone reaction products augments pro inflammatory cytokine production in MucilAir™ tissue
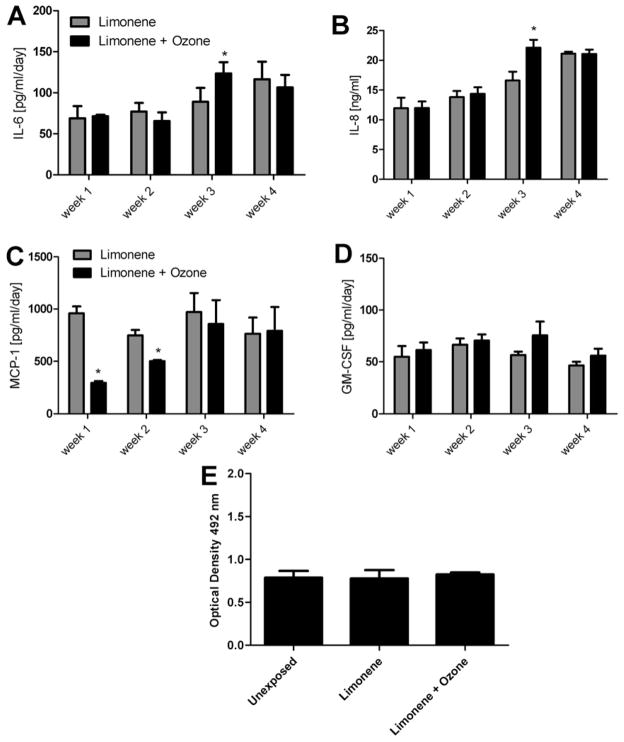
MucilAir™ tissue samples were tested in the Vitrocell® system to evaluate the effects of repeated dose exposure (1 h per day/5 days per week/4 weeks) on pro inflammatory and proliferative responses. MucilAir™ samples were exposed to limonene (500 ppb), or limonene (500 ppb) + ozone (100 ppb). Increases (Linear Trend Test p < 0.05) in cytokine production were observed for limonene (IL-6) and limonene + ozone (IL-6 and IL-8) over the 4 week exposure period. Statistically significant increases in IL-8 and IL-6 cytokine production were observed for the limonene + ozone exposure group when compared to the limonene exposure group at week three (Fig. 7A and B). Although at week three it appears there is an increase in GM-CSF production for limonene + ozone compared to limonene, it was not statistically significant. Similar to the A549 exposures, statistically significant decreases in MCP-1 were observed for limonene + ozone when compared to limonene at weeks one and two (Fig. 7C). There were no modulations in cytokine levels [IL-6 (70 ± 8 pg/ml), IL-8 (15 ± 1 pg/ml), MCP-1 (837 ± 263 pg/ml), and GM-CSF (59 ± 9 pg/ml)] at 1 week for limonene compared to unexposed tissues. No differences in metabolic activity for the limonene or limonene + ozone exposure groups compared to the unexposed tissues were observed following the 4 week exposure period (Fig. 7E).
Fig. 7.
The effect of limonene and limonene + ozone reaction products on MucilAir™ tissue following a repeated dose exposure. MucilAir™ tissue was exposed to limonene (500 ppb) vs. limonene (500 ppb)/ozone (100 ppb) for 1 h per day/5 days per week/4 weeks. 72 h following the final weekly exposure, supernatant was evaluated for IL-8 (A), IL-6 (B), MCP-1 (C), and GM-CSF (D) protein production. Cell proliferation was evaluated 72 h following the final experimental exposure (E). Comparisons were made for unexposed vs. limonene (500 ppb) and limonene (500 ppb) vs. limonene (500 ppb)/ozone (100 ppb). Basal cytokine levels for unexposed tissues are as follows: IL-6 (70 ± 8 pg/ml), IL-8 (15 ± 1 pg/ml), MCP-1 (837 ± 263 pg/ml), and GM-CSF (59 ± 9 pg/ml). Bars represent the mean ± SE from three independent biological replicates per exposure group. Significant differences are designated with *(p < 0.05).
4. Discussion
The studies described in this manuscript have utilized an in vitro exposure system to evaluate the toxicity associated with exposure to secondary pollutants generated from the reaction of limonene and ozone using both an isolated epithelial cell line (A549 cells) and highly differentiated epithelial tissue (MucilAir™). The data suggest that exposure to either the parent compound (limonene) or secondary pollutants (reactions of limonene + ozone) can induce alternations in inflammatory responses in A549 cells and MucilAir™ tissue. At higher concentrations exposure to secondary pollutants resulted in greater toxicity as observed in a decrease in cell proliferation in A549 cells. In most cases where alterations in MCP-1, IL-8 or IL-6 cytokine expression occurred in either A549 or MucilAir™ tissue, a greater response was observed following exposure to limonene + ozone as compared to limonene alone. These data are consistent with results from animal studies which have demonstrated increased respiratory distress in animals exposed to reaction products compared to parent compounds. Wolkoff et al. (2012) showed that when mice were exposed to air, limonene (52 ppm/289 mg/m3); ozone (0.1 ppm/0.2 mg/m3); or a reaction mixture of limonene (52 ± 8 ppm) and ozone (0.5, 2.5 and 3.9 ppm) 1 h per day for 10 consecutive days increases in sensory irritation and airflow limitations and a concentration-dependent decrease in respiratory rate developed for the limonene + ozone groups compared to the controls. However, in contrast to the data presented here where there was a trend toward an increase in inflammatory cytokine expression over the 4 week exposure period with MucilAir™ tissue, the severity of the effects observed in the animals did not change with increasing number of exposures. Other studies have demonstrated significant increases in upper airway irritation and airflow limitations in mice exposed for 1 h to reaction products compared to mice exposed to the reactants separately (Rohr et al., 2003; Wilkins et al., 2003). These results support the hypothesis that reaction products or secondary pollutants may yield health effects that are more severe than those resulting from exposure to the parent compounds raising the concern that exposure assessments may be overlooking the most toxic components of indoor air contaminants.
In vitro models play an important role in understanding the biological effects of indoor air pollutants, however standardization of these models will be important in order to interpret data and compare results between laboratories. Culture conditions such as media, number of cells used and growth duration can contribute to variability between studies and may affect result outcomes (Anderson et al., 2010; Feltens et al., 2010; Fischader et al., 2008; Gminski et al., 2010). Heterogeneity in culture conditions can result in different growth characteristics and even phenotypes. For example, in these studies it was determined that the number of cells seeded on the insert and the growth duration prior to exposure can affect cytokine production. Lower cell concentration (250,000) and an increased growth period (48 h following addition to insert) were identified to produce the greatest cytokine production and provide optimal conditions for the detection of cytokine modulation. This is demonstrated in Figs. 2 and 4 where basal levels of cytokine production were much higher in Fig. 4A and B (48 h) compared to Figs. 2A and B (24 h) for clean air and limonene.
It is critical to identify the conditions that will be sensitive enough to predict alterations, yet robust enough to be applied to various systems and across chemicals because the sample requirements often limit analysis to a single endpoint. Based on their relationship to human disease there are several endpoints which have been chosen for analysis. The airway epithelium is a complex physicochemical barrier that plays a pivotal role in host defense and is a rich source of modulatory compounds including cytokines which have been shown to play an import role in the etiology of airway disease (Mills et al., 1999). The development of specific epithelial cell culture techniques has enabled investigators to examine differences that exist in the airway between health and disease states. Soluble inflammatory cytokines such as, IL-6, IL-8 and MCP-1 are often described in the literature as markers for the analysis of adverse outcomes induced by chemical exposure in cell lines such as A549; these can be collected directly from the supernatant and analyzed easily using methods such as ELISA or flow cytometry (Fischader et al., 2008; Persoz et al., 2010). The choice of these markers is supported by studies using primary cultures of human nasal epithelial cells from atopic individual with and without rhinitis (Calderon et al., 1997). In general, nasal epithelial cells from atopic individuals release significantly greater amounts of MCP-1, IL-8, TNF-α, and GM-CSF compared to cells collected from non-atopic, non-rhinitic individuals. Additionally, IL-8 levels have been shown to be increased in asthmatics and MCP-1 has been implicated in the pathogenesis of diseases characterized by monocytic infiltrates (Wood et al., 2012; Bafadhel et al., 2012). However, there is not a consensus in the literature with regards to the need or impact of cell stimulation prior to chemical exposure in the evaluation of cytokine modulation. For example, Persoz et al. (2010) found that pre-stimulation was required to detect changes in IL-8 production following formaldehyde exposure. In the studies presented here contradictory results were obtained when TNF-α was used for pre-stimulation in the A549 studies. Although following exposure to limonene reaction products cytokine levels in TNF-α stimulated cells were increased for both IL-8 and MCP-1 compared to unstimulated cultures, there was no longer a statistically significant increase in cytokine production between exposure groups and controls as was seen with unstimulated cells (Figs. 4 and 5). This points out the need for optimization such that there is room on the dose response curve to observe both up and down regulatory events.
One important shortcoming of more primitive in vitro methods is the lack of complexity. Cells in culture represent very simplified living systems; they do not possess the complexity of integrated functioning tissues. The use of differentiated tissue such as MucilAir™ helps to overcome some of these issues. MucilAir™ tissues are made of primary human cells isolated from the nasal cavity, the trachea and the bronchus to mimics the human respiratory epithelium. They contain basal, goblet, ciliated cells, and mucus and have features such as cilia beating, tight junctions, active ion transport, metabolic activity/detoxification (CYP450), and cytokine/chemokine/metalloproteinase release. Due to growth requirements, cell lines such as A549 are often limited to a single acute exposure and high doses representing cumulative exposure are frequently tested. The MucilAir™ model allows for repeated exposures and the studies presented here demonstrate the use of this more complex in vitro model to evaluate repeated exposures testing chemical concentrations closely related to indoor environments for up to 4 weeks. Due to growth requirements, cell lines are often limited to a single exposure. In these A549 cells studies, a high dose single exposure induced a similar pattern of cytokine modulation as seen in the lower dose MucilAir™ studies.
In summary, these studies suggest that secondary reaction products may be a significant contributor to adverse health effects associated with contaminated indoor air exposure. A combined approach using representative cell cultures as a screening tool, followed when appropriate, with more complex tissues including engineered tissues or lung slices may provide a valuable tool in investigating the role of indoor contaminants in respiratory disease. Further development, standardization, and validation of these in vitro test methods could play a significant role in understanding the cellular, biochemical, and molecular mechanisms underlying the pulmonary toxicity resulting from exposure to indoor environment.
Acknowledgments
This work was supported in part by the Inter-Agency Agreement NIEHS Y1-ES0001-06.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Abbreviations
- A549
alveolar epithelia cells
- FBS
fetal bovine serum
- ROS
reactive oxygen species
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GSH
glutathione
- TNF-α
tumor necrosis factor alpha
- VOC
volatile organic compounds
- IL-8
interleukin 8
- IL-6
interleukin 6
- MCP-1
monocyte chemoattractant protein 1
References
- Anderson SE, Jackson LG, Franko J, Wells JR. Evaluation of dicarbonyls generated in a simulated indoor air environment using an in vitro exposure system. Toxicol Sci. 2010;115:453–461. doi: 10.1093/toxsci/kfq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health. 2007;80:711–719. doi: 10.1007/s00420-007-0183-2. [DOI] [PubMed] [Google Scholar]
- Bafadhel M, McCormick M, Saha S, McKenna S, Shelley M, Hargadon B, Mistry V, Reid C, Parker D, Dodson P, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83:36–44. doi: 10.1159/000330667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakand S, Winder C, Khalil C, Hayes A. Toxicity assessment of industrial chemicals and airborne contaminants: transition from in vivo to in vitro test methods: a review. Inhalation Toxicol. 2005;17:775–787. doi: 10.1080/08958370500225240. [DOI] [PubMed] [Google Scholar]
- Bonisch U, Bohme A, Kohajda T, Mogel I, Schutze N, von Bergen M, Simon JC, Lehmann I, Polte T. Volatile organic compounds enhance allergic airway inflammation in an experimental mouse model. PLoS ONE. 2012;7:e39817. doi: 10.1371/journal.pone.0039817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon MA, Devalia JL, Prior AJ, Sapsford RJ, Davies RJ. A comparison of cytokine release from epithelial cells cultured from nasal biopsy specimens of atopic patients with and without rhinitis and nonatopic subjects without rhinitis. J Allergy Clin Immunol. 1997;99:65–76. doi: 10.1016/s0091-6749(97)70302-6. [DOI] [PubMed] [Google Scholar]
- Elms J, Beckett PN, Griffin P, Curran AD. Mechanisms of isocyanate sensitisation. An in vitro approach. Toxicol In Vitro: An Int J (Published in Association with BIBRA) 2001;15:631–634. doi: 10.1016/s0887-2333(01)00078-9. [DOI] [PubMed] [Google Scholar]
- Feltens R, Mogel I, Roder-Stolinski C, Simon JC, Herberth G, Lehmann I. Chlorobenzene induces oxidative stress in human lung epithelial cells in vitro. Toxicol Appl Pharmacol. 2010;242:100–108. doi: 10.1016/j.taap.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Fischader G, Roder-Stolinski C, Wichmann G, Nieber K, Lehmann I. Release of MCP-1 and IL-8 from lung epithelial cells exposed to volatile organic compounds. Toxicol In Vitro: An Int J (Published in Association with BIBRA) 2008;22:359–366. doi: 10.1016/j.tiv.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Forester CD, Ham JE, Wells JR. Geraniol (2,6-dimethyl-2,6-octadien-8-ol) reactions with ozone and OH radical: rate constants and gas-phase products. Atmos Environ. 2007;41:1188–1199. [Google Scholar]
- Forester CD, Wells JR. Yields of carbonyl products from gas-phase reactions of fragrance compounds with OH radical and ozone. Environ Sci Technol. 2009;43:3561–3568. doi: 10.1021/es803465v. [DOI] [PubMed] [Google Scholar]
- Gminski R, Tang T, Mersch-Sundermann V. Cytotoxicity and genotoxicity in human lung epithelial A549 cells caused by airborne volatile organic compounds emitted from pine wood and oriented strand boards. Toxicol Lett. 2010;196:33–41. doi: 10.1016/j.toxlet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Ham JE, Proper SP, Wells JR. The gas-phase chemistry of citronellol with ozone and OH radical: rate constants and products. Atmos Environ. 2006;40:726–735. [Google Scholar]
- Harrison JC, Ham JE, Wells JR. Citronellal reactions with ozone and OH radical: rate constants and gas-phase products detected using PFBHA derivatization. Atmos Environ. 2007;41:4482–4491. [Google Scholar]
- Jang AS, Choi IS, Koh YI, Park CS. Volatile organic compounds contribute to airway hyperresponsiveness. Korean J Intern Med. 2007;22:8–12. doi: 10.3904/kjim.2007.22.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer T. Pentoxifylline inhibits ICAM-1 expression and chemokine production induced by proinflammatory cytokines in human pulmonary epithelial cells. Immunopharmacology. 2000;46:253–261. doi: 10.1016/s0162-3109(99)00186-1. [DOI] [PubMed] [Google Scholar]
- Lambre CR, Auftherheide M, Bolton RE, Rubini B, Haagsman HP, Hext PM, Jorissen M, Landry Y, Morin JP, Nemery B, Nettesheim P, Pauluhn J, Richards RJ, Vickers AEM, We R. In vitro test for respiratory toxicity, The report and recommemdations of ECVAM working 18. Altern Lab Anim J. 1996;24:671–681. [Google Scholar]
- Lehmann AD, Daum N, Bur M, Lehr CM, Gehr P, Rothen-Rutishauser BM. An in vitro triple cell co-culture model with primary cells mimicking the human alveolar epithelial barrier. Eur J Pharm Biopharm. 2011;77:398–406. doi: 10.1016/j.ejpb.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lehmann I, Roder-Stolinski C, Nieber K, Fischader G. In vitro models for the assessment of inflammatory and immuno-modulatory effects of the volatile organic compound chlorobenzene. Exp Toxicol Pathol: Official J Gesellschaft fur Toxikol Pathol. 2008;60:185–193. doi: 10.1016/j.etp.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Mendell MJ, Fisk WJ, Kreiss K, Levin H, Alexander D, Cain WS, Girman JR, Hines CJ, Jensen PA, Milton DK, et al. Improving the health of workers in indoor environments: priority research needs for a national occupational research agenda. Amer J Public Health. 2002;92:1430–1440. doi: 10.2105/ajph.92.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PR, Davies RJ, Devalia JL. Airway epithelial cells, cytokines, and pollutants. Am J Respir Crit Care Med. 1999;160:S38–S43. doi: 10.1164/ajrccm.160.supplement_1.11. [DOI] [PubMed] [Google Scholar]
- Pariselli F, Sacco MG, Rembges D. An optimized method for in vitro exposure of human derived lung cells to volatile chemicals. Exp Toxicol Pathol: Official J Gesellschaft fur Toxikol Pathol. 2009;61:33–39. doi: 10.1016/j.etp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Persoz C, Achard S, Leleu C, Momas I, Seta N. An in vitro model to evaluate the inflammatory response after gaseous formaldehyde exposure of lung epithelial cells. Toxicol Lett. 2010;195:99–105. doi: 10.1016/j.toxlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Persoz C, Achard S, Momas I, Seta N. Inflammatory response modulation of airway epithelial cells exposed to formaldehyde. Toxicol Lett. 2012 doi: 10.1016/j.toxlet.2012.03.799. [DOI] [PubMed] [Google Scholar]
- Pichavant M, Charbonnier AS, Taront S, Brichet A, Wallaert B, Pestel J, Tonnel AB, Gosset P. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J Allergy Clin Immunol. 2005;115:771–778. doi: 10.1016/j.jaci.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Ritter D, Knebel JW, Aufderheide M. In vitro exposure of isolated cells to native gaseous compounds–development and validation of an optimized system for human lung cells. Exp Toxicol Pathol: Official J Gesellschaft fur Toxikol Pathol. 2001;53:373–386. doi: 10.1078/0940-2993-00204. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Shore SA, Spengler JD. Repeated exposure to isoprene oxidation products causes enhanced respiratory tract effects in multiple murine strains. Inhal Toxicol. 2003;15:1191–1207. doi: 10.1080/08958370390229870. [DOI] [PubMed] [Google Scholar]
- Schmalz C, Wunderlich HG, Heinze R, Frimmel FH, Zwiener C, Grummt T. Application of an optimized system for the well-defined exposure of human lung cells to trichloramine and indoor pool air. J Water Health. 2011;9:586–596. doi: 10.2166/wh.2011.144. [DOI] [PubMed] [Google Scholar]
- Singer BC, Destaillats H, Hodgson AT, Nazaroff WW. Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air. 2006;16:179–191. doi: 10.1111/j.1600-0668.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- Verstraelen S, Bloemen K, Nelissen I, Witters H, Schoeters G, Van Den Heuvel R. Cell types involved in allergic asthma and their use in in vitro models to assess respiratory sensitization. Toxicol In Vitro: An Int J (Published in Association with BIBRA) 2008a;22:1419–1431. doi: 10.1016/j.tiv.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Verstraelen S, Wens B, Hooyberghs J, Nelissen I, Witters H, Schoeters G, Cauwenberge PV, Heuvel RV. Gene expression profiling of in vitro cultured macrophages after exposure to the respiratory sensitizer hexamethylene diisocyanate. Toxicol In Vitro: An Int J (Published in Association with BIBRA) 2008b;22:1107–1114. doi: 10.1016/j.tiv.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Wells JR. Gas-phase chemistry of alpha-terpineol with ozone and OH radical: rate constants and products. Environ Sci Technol. 2005;39:6937–6943. doi: 10.1021/es0481676. [DOI] [PubMed] [Google Scholar]
- Weschler CJ. Chemical reactions among indoor pollutants: what we’ve learned in the new millennium. Indoor Air. 2004;14:184–194. doi: 10.1111/j.1600-0668.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- Wilkins CK, Wolkoff P, Clausen PA, Hammer M, Nielsen GD. Upper airway irritation of terpene/ozone oxidation products (TOPS). Dependence on reaction time, relative humidity and initial ozone concentration. Toxicol Lett. 2003;143:109–114. doi: 10.1016/s0378-4274(03)00115-2. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Clausen PA, Larsen ST, Hammer M, Nielsen GD. Airway effects of repeated exposures to ozone-initiated limonene oxidation products as model of indoor air mixtures. Toxicol Lett. 2012;209:166–172. doi: 10.1016/j.toxlet.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012 doi: 10.1378/chest.11-1838. [DOI] [PubMed] [Google Scholar]
- Yoon HI, Hong YC, Cho SH, Kim H, Kim YH, Sohn JR, Kwon M, Park SH, Cho MH, Cheong HK. Exposure to volatile organic compounds and loss of pulmonary function in the elderly. Eur Respir J: Official J Eur Soc Clin Respi Physiol. 2010;36:1270–1276. doi: 10.1183/09031936.00153509. [DOI] [PubMed] [Google Scholar]