Abstract
Previous reports from our lab have shown that Skp2 is necessary for p27 degradation and cell cycle progression during adipocyte differentiation. Data presented here demonstrate that the anti-inflammatory, anti-obesity phytochemical curcumin blocked Skp2 protein accumulation during early adipocyte hyperplasia. In addition, curcumin dose-dependently induced p27 protein accumulation and G1 arrest of synchronously replicating 3T3-L1 preadipocytes. Of note, p27 protein accumulation occurred in the presence of decreased p27 mRNA suggesting a role for post-transcriptional regulation. In support of this hypothesis, curcumin markedly increased p27 protein half-life as well as attenuated ubiquitin proteasome activity suggesting that inhibition of targeted p27 proteolysis occurred through curcumin-mediated attenuation of Skp2 and 26S proteasome activity. While we observed no cytotoxic effects for curcumin at doses less than 20 µM, it is important to note an increase in apoptotic signaling at concentrations greater than 30 µM. Finally, data presented here demonstrate that the anti-proliferative effect of curcumin was critical for the suppression of adipocyte differentiation and the development of the mature adipocyte. Collectively, our data demonstrate that curcumin-mediated post-transcriptional accumulation of p27 accounts in part for the anti-proliferative effect observed in 3T3-L1 preadipocytes.
Keywords: Curcumin, Adipocyte, Proliferation, Cell cycle, Obesity
Highlights
-
•
Curcumin inhibited early stages of adipogenesis during cell cycle progression.
-
•
Curcumin increased p27 protein through post-translational mechanisms.
-
•
Curcumin suppressed Skp2 protein which directs p27 ubiquitylation.
-
•
p27 accumulation may account for late G1 arrest and suppression of adipogenesis.
-
•
Potential mechanism regarding anti-obesity effects of curcumin.
1. Introduction
Obesity-associated co-morbidities such as diabetes and cardiovascular disease contribute to the rising incidence of morbidity and mortality as well as add to the tremendous burden plaguing our health care system [19], [43]. Obesity is defined as excess white adipose tissue accumulation that can occur due to increased adipocyte cell volume (hypertrophy) and cell number (hyperplasia). Though preadipocyte (PA) replication slows during adulthood, periods of positive energy balance at any age can trigger proliferation and subsequent differentiation of dormant PAs known to exist throughout the lifespan [5], [15], [18], [32]. Obesity resulting from adipocyte hyperplasia is associated with the poorest prognosis of treatment [11], [46]. Thus, understanding of molecular and cellular events involved in adipogenesis is critical for elucidating targets in the treatment and prevention of obesity and its co-morbidities.
The hormonal and nutritional mechanisms underlying adipocyte hyperplasia have been extensively studied using the murine 3T3-L1 preadipocyte cell line [24]. In response to a standard adipogenic cocktail, growth-arrested PAs synchronously re-enter the cell cycle for 1–2 rounds of cell division known as mitotic clonal expansion (MCE), a required and necessary step for adipogenesis [26], [40]. MCE is driven by the convergence of mitogen-dependent signaling pathways on sequential expression of regulatory cyclin proteins needed for cyclin-dependent kinase (Cdk) activation. Timely activation of Cdks is critical for orderly cell cycle progression. Transition through the G1/S phase restriction point represents a pivotal and decisive point of cellular autonomy where cell cycle control becomes mitogen-independent [4], [37], [38]. Cell cycle progression through this restriction point is potently suppressed by the cyclin-dependent kinase inhibitor (CKI) p27 [7]. In contrast to the mitogenic activation of cyclins, p27 accumulates in response to anti-mitotic stimuli (e.g. nutrient deprivation, density-arrest, differentiation) to ensure cell cycle arrest and extended quiescence [14], [28], [30], [31]. Indeed, p27 gene ablation has been shown to markedly increase fat mass in mice due to adipocyte hyperplasia [27]. Thus, molecular mechanisms that maintain an optimal balance in p27 during PA replication appear critical in the etiology of obesity.
Curcumin is a bioactive phytochemical found in the rhizome of the perennial herb Curcuma longa Linn (turmeric). Widely used as a culinary agent, curcumin is known to have anti-oxidant, anti-inflammatory, and anti-carcinogenic properties [36], [44]. Of note, high doses of curcumin (12 g/day) have shown no significant cytotoxic effects in humans demonstrating that curcumin is a safe bioactive food component [36], [44]. Recent experimental evidence demonstrated an anti-inflammatory, anti-diabetic, and anti-obesity role for curcumin in mouse models of obesity and diabetes [10], [35], [45]. While these studies postulated a preventive role of curcumin in diet-induced obesity, findings from Ejaz et al. [10] demonstrated an inhibitory role for curcumin during adipocyte differentiation. More recently, it was demonstrated that curcumin blocks adipogenesis during early adipocyte differentiation by inhibiting post-confluent PAs during MCE [17]. Despite these findings that suggest that curcumin is effective in regulating new adipocyte generation, the mechanistic actions of curcumin during adipogenesis remain unclear.
Previous reports from our lab have demonstrated that S-phase kinase-associated protein-2 (Skp2) regulates p27 degradation during S and G2 phase progression of replicating 3T3-L1 PAs during early adipocyte differentiation [2], [3]. Furthermore, we and others have shown that Skp2 promotes cell cycle progression by targeting specific proteins for ubiquitylation and degradation by the 26S proteasome [2], [6], [39]. In this study, we demonstrated that the inhibitory actions of curcumin on adipocyte differentiation occur early during PA proliferation. Moreover, we demonstrated that curcumin arrests proliferating PAs in late-G1 phase and promotes p27 protein accumulation during density arrest by post-transcriptional mechanisms involving Skp2 and 26S proteasome degradation. Together, these data demonstrate an effect of curcumin on p27 protein accumulation and provide a link between cell cycle progression and adipocyte hyperplasia.
2. Materials and methods
2.1. Materials
Dulbecco's Modified Eagle's Medium (DMEM), calf bovine serum (CS), and trypsin-EDTA were purchased from Invitrogen. Fetal bovine serum (FBS) was obtained from HyClone. Propidium Iodide was purchased from Sigma. The following antibodies used for western blot analysis: p27 (Transduction Laboratories); Cleaved caspase-3, cleaved PARP, and ubiquitin (Cell Signaling); and cyclin A, PPARγ, C/EBPα, adipsin, and Skp2 (Santa Cruz Biotechnology). Curcumin was purchased from Alexis Biochemical.
2.2. Cell Culture
Murine 3T3-L1 PAs, purchased from Howard Green, Harvard Medical School, were cultured and differentiated as previously described [2]. Briefly, cells were propagated in DMEM supplemented with 10% CS until density-arrest at 2 days post-confluence then stimulated a hormonal cocktail containing 10% FBS, 0.5 mM 1-methyl-3-isobutylxanthine, 1 µM dexamethasone, and 1.7 µM insulin (MDI). Time post-MDI refers to the time since the administration of MDI to culture medium.
2.3. Immunoblotting
Relative protein abundance was assessed by immunoblotting as previously described [2].
2.4. Oil Red O (ORO)
Cells were fixed in formalin and stained with ORO, washed in deionized water, dried and scanned for analysis.
2.5. Flow cytometry
Cell cycle progression was assessed by DNA histograms generated by propidium iodide staining as previously described [2].
2.6. Real-Time RT-PCR
Relative mRNA abundance was assessed by RT-PCR using the method as previously described [12].
2.7. Proteasome assay
Proteasome activity was assessed using the 20S Proteasome Assay Kit per manufacturer's instructions (BostonBiochem, Cambridge, MA).
3. Results
3.1. Curcumin inhibits 3T3-L1 adipocyte differentiation during early stages of adipogenesis
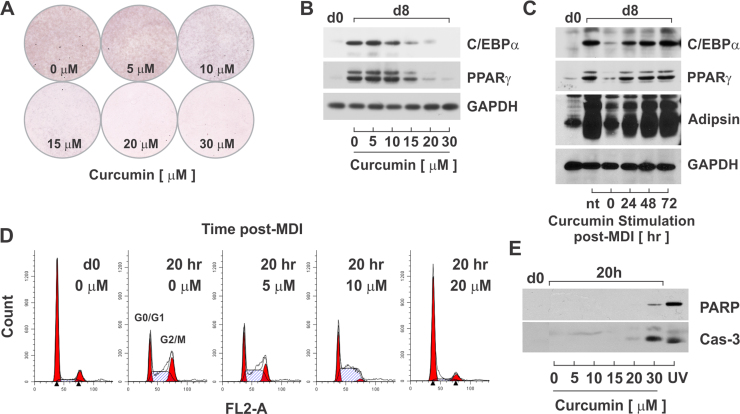
To examine the effects of curcumin on adipocyte differentiation, density-arrested PAs were dose-dependently treated with curcumin and stimulated to differentiate with MDI. On day 8 (d8), cells were fixed and stained with Oil Red O (ORO) to assess lipid accumulation. As shown by ORO, curcumin dose-dependently inhibited adipocyte differentiation (Fig. 1A). Inhibition of differentiation resulted from suppression of PPARγ and C/EBPα, the dominant transcriptional regulators of adipogenesis (Fig. 1B).
Fig. 1.
Curcumin dose-dependently inhibits 3T3-L1 PA proliferation. PAs were stimulated with MDI and increasing concentrations of curcumin and stained with Oil red O at day 8 post-MDI (A) to assess lipid accumulation and cell lysates immunoblotted (B) for key adipogenic transcription factors. (C) Growth-arrested 3T3-L1 PAs were stimulated with MDI in the absence or presence of 20 µM curcumin at the onset of differentiation (0 h), 24 h post-MDI, 48 h post-MDI, or 72 h post-MDI. Cell lysates were harvested 8 days post-MDI and immunoblotted to assess adipocyte differentiation. (D) Density-arrested PAs were stimulated with MDI and increasing concentrations of curcumin. Cells were fixed at d0 and 20 h post-MDI and DNA stained with propidium iodide. DNA histograms were assessed via flow cytometry. (E) Cell lysates were assessed for apoptosis by immunoblotting for cleaved caspase 3 and PARP. Cell lysates treated with 1000 J/m2 UV were used as a positive control for apoptosis.
Mitotic clonal expansion (MCE) is an ‘obligatory’ step of adipocyte differentiation. Inhibition of MCE has been shown necessary for the activation of PPARγ and C/EBPα and acquisition of the mature adipocyte phenotype [24], [25], [26], [33]. To elucidate a role for curcumin during MCE, we treated density-arrested PAs in the absence or presence of 20 µM curcumin at 0 h, 24 h, 48 h, or 72 h post-MDI stimulation. For each treatment, protein expression was assessed on day 8 (d8) for C/EBPα, PPARγ, and adipsin, an adipocyte-specific serine protease. Compared to untreated PAs (d0), MDI-stimulation led to marked increases in protein expression of all three proteins (Fig. 1C). However, treatment with curcumin at the onset of differentiation (0 h) suppressed protein expression of C/EBPα, PPARγ, and adipsin, with only modest suppression observed for curcumin treatment 24 h post-MDI and no effect seen with treatment 48 h or 72 h post-MDI (Fig. 1C).
The findings demonstrated that curcumin blocked early stages (<24 h) of adipocyte differentiation. To determine if curcumin suppressed MCE, density-arrested PAs were stimulated with MDI for 20 h in the absence or presence of increasing doses of curcumin. Cells were fixed and DNA stained with propidium iodide for flow cytometric analysis of cell cycle progression. As shown in Fig. 1D, two DNA peaks in the 2n and 4n range, representing G0/G1 and G2/M cell populations, were present in all histograms. Stimulation with MDI resulted in a marked shift from G0/G1 to S and G2/M phase populations demonstrating synchronous cell cycle progression in the absence of curcumin (Fig. 1D). In contrast, curcumin dose-dependently inhibited MDI-induced cell cycle progression prior to G1/S phase transition (Fig. 1D).
Curcumin has been shown to induce apoptosis in various cancer cell lines [21], [29], To assess cytotoxicity, density-arrested PAs were stimulated with MDI for 20 h in the absence or presence of increasing concentrations of curcumin or 1000 J/m2 UV, a known activator of apoptosis. Protein expression was examined for caspase 3 and PARP cleavage, mid- and late-stage markers of apoptosis.
3.2. Curcumin inhibits p27 protein degradation in proliferating Pas
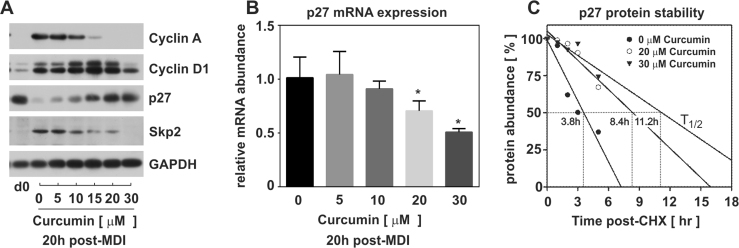
To further elucidate the effect on cell cycle progression, density-arrested PAs were stimulated with MDI for 20 h in the absence or presence of increasing doses of curcumin. Cell lysates were harvested and analyzed for inducible cyclin protein expression corresponding to specific phases of the cell cycle. Curcumin dose-dependently suppressed cyclin A which is known to accumulate and function during S phase progression [22], [25]. To elucidate if cell cycle arrest occurred in G0 or G1, lysates were also immunoblotted for cyclin D1 which is known to accumulate and function during G1 phase progression [24], [25]. As illustrated, cyclin D1 increased with increasing concentrations of curcumin up through 20 µM, yet was suppressed with 30 µM curcumin (Fig. 2A). Collectively, these data suggest that doses <30 µM curcumin block cells in G1 phase of the cell cycle.
Fig. 2.
Curcumin inhibits p27 protein degradation during PA proliferation. (A) Density-arrested PAs were stimulated with MDI and increasing concentrations of curcumin. Cell lysates were harvested 20 h post-MDI and immunoblotted for cell cycle protein expression. (B) RNA was also isolated from cells at 20 h post-MDI and curcumin and p27 mRNA examined using real time qRT-PCR. Data were set relative to MDI-stimulated PAs in the absence of curcumin (0 µM) and normalized to 18S rRNA. Statistical significance was set at p<0.05 as measured via ANOVA with Tukey's post-hoc. (C) PAs were stimulated with MDI and increasing concentrations of curcumin. After 18 h, all cells were exposed to cycloheximide (CHX; 30 µM) to block protein synthesis. Lysates were harvested over time post-CHX and immunoblotted for p27 decay. Immunoblots were digitized and liner regression of remaining protein abundance over time was assessed for protein half-life (t1/2).
Previous reports from our lab and others have shown that transition across the G1/S restriction point involves p27 regulation [2], [3], [4]. Therefore, we examined the effect of curcumin treatment on p27 protein expression. Density-arrested PAs were stimulated as indicated above and cell lysate harvested at d0 and 20 h to examine p27 protein expression. Consistent with a G1 phase arrest, p27 protein accumulation occurred with curcumin in a concentration dependent manner (Fig. 2A). To elucidate a mechanism of curcumin on p27 protein accumulation, we examined the effect of curcumin on p27 mRNA expression. In contrast to p27 protein accumulation, curcumin treatment suppressed p27 mRNA abundance (Fig. 2B) suggesting a post-transcriptional role for curcumin on p27 protein accumulation. Consistent with this hypothesis, we observed a dose-dependent decrease in Skp2 protein accumulation (Fig. 2A). Previous reports from our lab have shown that increased Skp2 targets p27 for ubiquitylation and proteasome degradation in adipocytes [2], [3]. To determine a role for curcumin on p27 protein stability, growth-arrested PAs were stimulated with MDI for 18 h in the absence or presence of curcumin prior to exposure to 30 µM cycloheximide (CHX) to block de novo protein synthesis. Cell lysates were subsequently harvested over time post-CHX and immunoblotted for p27. Linear regression of protein remaining over time post-CHX was used for protein half-life (T1/2) estimation. As shown (Fig. 2C), p27 protein half-life markedly increased in response to curcumin (20 µM=8.4 h; 30 µM=11.2 h) compared to MDI alone (0 µM=3.8 h).
3.3. Curcumin inhibits proteasome activity
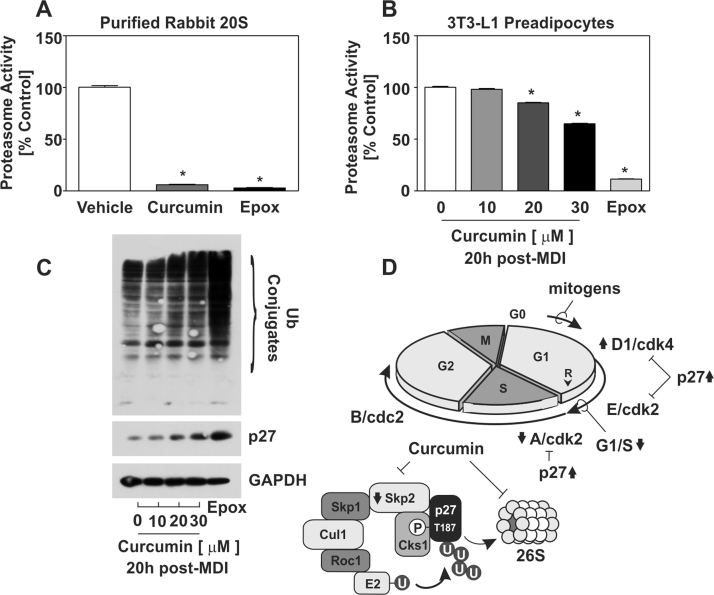
Data presented above suggested a role for curcumin on p27 regulated proteolysis via mechanisms involving SCFSkp2 E3 ligase and 26S proteasome during PA proliferation. However, recent studies have reported a role for curcumin in the direct regulation of 26S proteasome activity [9], [16], [21]. To examine this, we utilized a proteasome activity assay to measure chymotrypsin-like activity in vitro of purified 20S proteasome. Activated purified rabbit 20S proteasome was incubated in the absence or presence of curcumin or epoxomicin (Epox), a potent proteasome inhibitor, and proteasome activity measured over time. Direct interaction with purified 20S proteasome revealed a potent role for curcumin (~94%, p<0.05) on proteasome inhibition equivalent to Epox (97%, p<0.05) (Fig. 3A). We also determined that curcumin dose-dependently attenuated proteasome activity in PAs. While 20 µM (~15%, p<0.05) and 30 µM (~35%, p<0.05) curcumin presented with a statistically significant decrease in proteasome activity, its effects were minimal compared to Epox (~89%, p<0.05) (Fig. 3B). As 26S proteasome inhibition would be expected to yield increased protein ubiquitylation, we next examined a role for curcumin on ubiquitin protein expression. Density-arrested PAs were stimulated with MDI in the absence or presence of increasing doses of curcumin or 1 µM Epox and harvested 8 h post-MDI to assess ubiquitin conjugates. As shown in Fig. 3C, curcumin increased ubiquitin conjugate protein expression, consistent with its inhibition of the proteasome.
Fig. 3.
Curcumin inhibits 20S proteasome activity and leads to increased accumulation of ubiquitylated proteins. (A) Purified 20S proteasome (0.2 µg/200 µl) was incubated with curcumin (20 µM) or epoxomicin (Epox; 1 µM) and spectral analysis used to examine chymotrypsin-like protease activity. (B) Density-arrested PAs were stimulated with MDI in the absence or presence of curcumin or Epox (1 µM). Cell lysates were collected 20 h post-MDI and processed for proteasome activity. Epox was used as a positive control for proteasome inhibition. One-way ANOVA with Dunnet's post-hoc was used to determine significance (n=4, p<0.05) compared to control and all data expressed as SEM. (C) Immunblot analysis of cells collected 8 h post-MDI with increasing concentrations curcumin were assessed for ubiquitylated protein conjugates. Epox (1 µM) was used as a positive control. (D) Proposed model for curcumin-mediated inhibition of PA proliferation.
4. Discussion
This study defines curcumin as an anti-proliferative agent in cultured 3T3-L1 PAs undergoing adipocyte differentiation. Further, data presented here elucidate an early anti-adipogenic role for curcumin via post-transcriptional regulation of p27. We propose a model in which curcumin increases p27 protein half-life through mechanisms involving both inhibition of Skp2 protein accumulation and 26S proteasome activity thus promoting G1 arrest (Fig. 3D).
Excess energy intake stimulates PA proliferation and differentiation into mature adipocytes during the development of hyperplastic obesity [13], [32]. The cyclin dependent kinase inhibitor (CKI) p27 plays a critical role in the development of adipocyte hyperplasia as a potent inhibitor of G1/S transition [30], [31], [42]. Other studies have shown that p27 null mice have excessive adipose tissue mass due to adipocyte hyperplasia and not hypertrophy [27]. Using 3T3-L1 PAs, we had previously shown that targeted p27 proteolysis was regulated by the SCFSkp2 E3 ligase and 26S proteasome during PA proliferation [2], [3]. Intriguingly, Skp2 null mice are protected from genetic and diet-induced obesity due to a decrease in adipocyte cell number [8], [34]. Importantly, decreased adipocyte number and fat pad mass of Skp2 null mice was completely lost in Skp2 and p27 double knockout animals [8]. Collectively, these data suggest that optimal balance of p27 is tightly controlled by the SCFSkp2 E3 ligase. Data presented here demonstrated a dose-dependent increase in p27 protein accumulation that tightly correlated with Skp2 suppression during PA proliferation. Moreover, changes in p27 protein accumulation were not the result of changes in mRNA expression. In support of Skp2-mediated p27 degradation, we further showed that curcumin markedly increased p27 protein stability, as p27 half-life more than doubled in the presence of 20 µM curcumin.
Recent evidence has shown that curcumin can inhibit 26S proteasome activity in cancer cells [9], [16], [21], suggesting another route to increase p27 protein stability. Consistent with these reports, we demonstrated a potent suppression of purified 20S proteasome activity in vitro. However, curcumin only modestly attenuated 20S activity in 3T3-L1 PAs. One postulate for the differences in curcumin-mediated proteasome activity rely on the well-known observation that curcumin is poorly soluble and bioavailable [36], [44]. However, it should be noted that 20 µM curcumin was sufficient in increasing protein ubiquitin conjugates in 3T3-L1 PAs, further supporting proteasome inhibition. Taken together, our data highlight two anti-proliferative mechanisms for curcumin in 3T3-L1 PAs, where curcumin increased p27 protein stability by (1) inhibiting Skp2-mediated p27 proteolysis and (2) inhibiting proteasome-mediated p27 degradation.
In summary, early reports have demonstrated that curcumin blocked adipocyte differentiation, but the underlying mechanisms were not fully elucidated [20]. Since then, it has been proposed that curcumin blocks adipocyte differentiation during an early phase of PA replication known as MCE [17] and that this inhibition potentially resulted from inhibition of Wnt signaling [1]. Our results demonstrated that curcumin potently blocked Skp2 protein expression that was tightly correlated to the increase in p27 protein accumulation. Intriguingly, genetic loss of Wnt signaling has been shown to increase p27 accumulation and cell cycle arrest [23], [41]. Moreover, inhibition of the Wnt pathway has been shown to inhibit Skp2 expression and increase p27 accumulation [41]. Thus, future investigation into the role of curcumin on Wnt-regulated p27 protein expression during early adipogenesis will be of interest.
Acknowledgments
The authors are grateful for technical assistance from Dr. Robin G. Hopkins (UNC Greensboro). Study was supported by NIH funding to BSF (F31-DK847702) and RFM (R15-DK082799).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.11.014.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Reference
- 1.Ahn J., Lee H., Kim S., Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol. Cell Physiol. 2010;298:C1510–C1516. doi: 10.1152/ajpcell.00369.2009. [DOI] [PubMed] [Google Scholar]
- 2.Auld C.A., Fernandes K.M., Morrison R.F. Skp2-mediated p27(Kip1) degradation during S/G2 phase progression of adipocyte hyperplasia. J .Cell. Physiol. 2007;211:101–111. doi: 10.1002/jcp.20915. [DOI] [PubMed] [Google Scholar]
- 3.Auld C.A., Morrison R.F. Evidence for cytosolic p27(Kip1) ubiquitylation and degradation during adipocyte hyperplasia. Obesity (Silver Spring) 2006;14:2136–2144. doi: 10.1038/oby.2006.250. [DOI] [PubMed] [Google Scholar]
- 4.Blagosklonny M.V., Pardee A.B. The restriction point of the cell cycle. Cell Cycle. 2002;1:103–110. [PubMed] [Google Scholar]
- 5.Brook C.G., Lloyd J.K., Wolf O.H. Relation between age of onset of obesity and size and number of adipose cells. Br. Med. J. 1972;2:25–27. doi: 10.1136/bmj.2.5804.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrano A.C., Eytan E., Hershko A., Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell. Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 7.Cheng M., Olivier P., Diehl J.A., Fero M., Roussel M.F., Roberts J.M., Sherr C.J. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin d-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooke P.S., Holsberger D.R., Cimafranca M.A., Meling D.D., Beals C.M., Nakayama K., Nakayama K.I., Kiyokawa H. The F box protein S phase kinase-associated protein 2 regulates adipose mass and adipocyte number in vivo. Obesity (Silver Spring) 2007;15:1400–1408. doi: 10.1038/oby.2007.168. [DOI] [PubMed] [Google Scholar]
- 9.Dutta K., Ghosh D., Basu A. Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J. Neuroimmune Pharmacol. 2009;4:328–337. doi: 10.1007/s11481-009-9158-2. [DOI] [PubMed] [Google Scholar]
- 10.Ejaz A., Wu D., Kwan P., Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009;139:919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 11.Faust I.M., Johnson P.R., Stern J.S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am. J. Physiol. 1978;235:E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson B.S., Nam H., Hopkins R.G., Morrison R.F. Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes. PLoS One. 2010;5:e15208. doi: 10.1371/journal.pone.0015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausman D.B., DiGirolamo M., Bartness T.J., Hausman G.J., Martin R.J. The biology of white adipocyte proliferation. Obes. Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 14.Hengst L., Dulic V., Slingerland J.M., Lees E., Reed S.I. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch J., Batchelor B. Adipose tissue cellularity in human obesity. Clin. Endocrinol. Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 16.Jana N.R., Dikshit P., Goswami A., Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J. Biol. Chem. 2004;279:11680–11685. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 17.Kim C.Y., Le T.T., Chen C., Cheng J.X., Kim K.H. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J. Nutr. Biochem. 2011;22:910–920. doi: 10.1016/j.jnutbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Knittle J.L., Timmers K., Ginsberg-Fellner F., Brown R.E., Katz D.P. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J. Clin. Invest. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.K., Lee W.S., Hwang J.T., Kwon D.Y., Surh Y.J., Park O.J. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J. Agric. Food Chem. 2009;57:305–310. doi: 10.1021/jf802737z. [DOI] [PubMed] [Google Scholar]
- 21.Milacic V., Banerjee S., Landis-Piwowar K.R., Sarkar F.H., Majumdar A.P., Dou Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minshull J., Golsteyn R., Hill C.S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990;9:2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miranda-Carboni G.A., Krum S.A., Yee K., Nava M., Deng Q.E., Pervin S., Collado-Hidalgo A., Galic Z., Zack J.A., Nakayama K., Nakayama K.I., Lane T.F. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev. 2008;22:3121–3134. doi: 10.1101/gad.1692808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison R.F., Farmer S.R. Insights into the transcriptional control of adipocyte differentiation. J. Cell Biochem. 1999;Suppl. 32–33:S59–S67. doi: 10.1002/(sici)1097-4644(1999)75:32+<59::aid-jcb8>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Morrison R.F., Farmer S.R. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J. Biol. Chem. 1999;274:17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 26.Morrison R.F., Farmer S.R. Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 2000;130:3116S–3121S. doi: 10.1093/jn/130.12.3116S. [DOI] [PubMed] [Google Scholar]
- 27.Naaz A., Holsberger D.R., Iwamoto G.A., Nelson A., Kiyokawa H., Cooke P.S. Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J. 2004;18:1925–1927. doi: 10.1096/fj.04-2631fje. [DOI] [PubMed] [Google Scholar]
- 28.Nourse J., Firpo E., Flanagan W.M., Coats S., Polyak K., Lee M.H., Massague J., Crabtree G.R., Roberts J.M. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 29.Pae H.O., Jeong S.O., Jeong G.S., Kim K.M., Kim H.S., Kim S.A., Kim Y.C., Kang S.D., Kim B.N., Chung H.T. Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem. Biophys. Res. Commun. 2007;353:1040–1045. doi: 10.1016/j.bbrc.2006.12.133. [DOI] [PubMed] [Google Scholar]
- 30.Polyak K., Kato J.Y., Solomon M.J., Sherr C.J., Massague J., Roberts J.M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Polyak K., Lee M.H., Erdjument-Bromage H., Koff A., Roberts J.M., Tempst P., Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 32.Prins J.B., O’Rahilly S. Regulation of adipose cell number in man. Clin. Sci. 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 33.Rosen E.D., Spiegelman B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 34.Sakai T., Sakaue H., Nakamura T., Okada M., Matsuki Y., Watanabe E., Hiramatsu R., Nakayama K., Nakayama K.I., Kasuga M. Skp2 controls adipocyte proliferation during the development of obesity. J. Biol. Chem. 2007;282:2038–2046. doi: 10.1074/jbc.M608144200. [DOI] [PubMed] [Google Scholar]
- 35.Seo K.I., Choi M.S., Jung U.J., Kim H.J., Yeo J., Jeon S.M., Lee M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 36.Shehzad A., Ha T., Subhan F., Lee Y.S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 2011;50:151–161. doi: 10.1007/s00394-011-0188-1. [DOI] [PubMed] [Google Scholar]
- 37.Sherr C.J., Roberts J.M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 38.Sherr C.J., Roberts J.M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 39.Sutterluty H., Chatelain E., Marti A., Wirbelauer C., Senften M., Muller U., Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 40.Tang Q.Q., Otto T.C., Lane M.D. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y., Simoneau A.R., Liao W.X., Yi G., Hope C., Liu F., Li S., Xie J., Holcombe R.F., Jurnak F.A., Mercola D., Hoang B.H., Zi X. WIF1, a Wnt pathway inhibitor, regulates SKP2 and c-myc expression leading to G1 arrest and growth inhibition of human invasive urinary bladder cancer cells. Mol. Cancer Ther. 2009;8:458–468. doi: 10.1158/1535-7163.MCT-08-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 43.Tsai A.G., Williamson D.F., Glick H.A. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes. Rev. 2011;12:50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R., Bapat P., Kwun I., Shen C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisberg S.P., Leibel R., Tortoriello D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang M.U., Presta E., Bjorntorp P. Refeeding after fasting in rats: effects of duration of starvation and refeeding on food efficiency in diet-induced obesity. Am. J. Clin. Nutr. 1990;51:970–978. doi: 10.1093/ajcn/51.6.970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material