Abstract
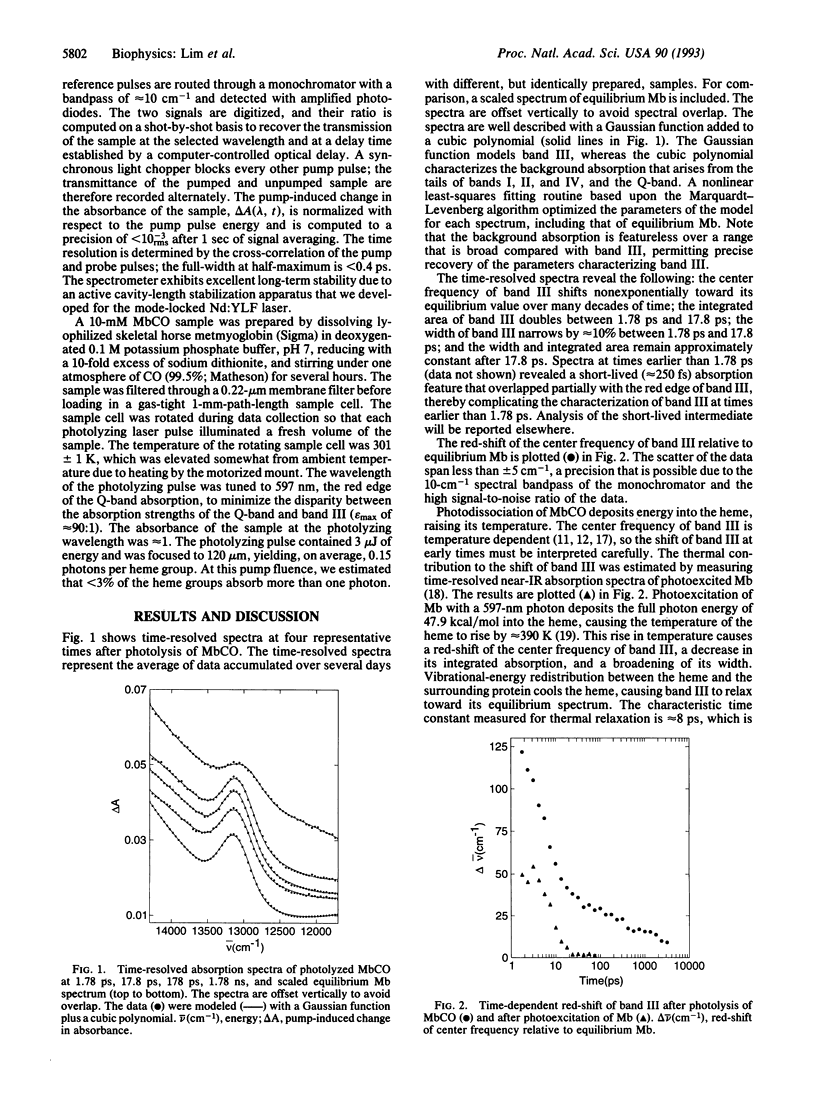
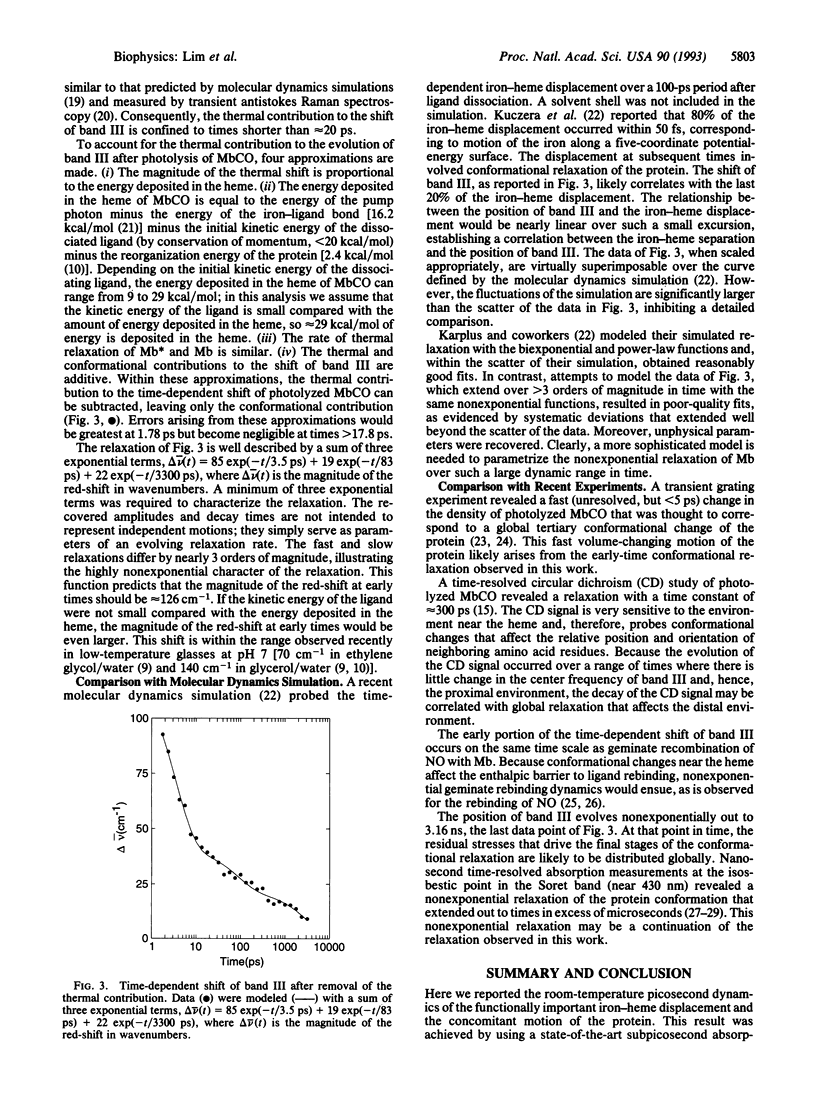
The picosecond evolution of the tertiary conformation of myoglobin (Mb) after photodissociation of MbCO was investigated at room temperature by probing band III, a weak iron-porphyrin charge-transfer transition near 13,110 cm-1 (763 nm) that is sensitive to the out-of-plane displacement of the iron. Upon photolysis, the iron moves out of the plane of the porphyrin, causing a blue-shift of band III and a concomitant change in the protein conformation. The dynamics for this functionally important motion are highly nonexponential, in agreement with recent molecular dynamics simulations [Kuczera, K., Lambry, J.-C., Martin, J.-L. & Karplus, M. (1993) Proc. Natl. Acad. Sci. USA 90, 5805-5807]. The conformational change likely affects the height of the barrier to ligand rebinding and may explain nonexponential NO rebinding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Jones C. M., Henry E. R., Hofrichter J., Eaton W. A. The role of solvent viscosity in the dynamics of protein conformational changes. Science. 1992 Jun 26;256(5065):1796–1798. doi: 10.1126/science.1615323. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Thermal behavior of the 760-nm absorption band in photodissociated sperm whale carbonmonoxymyoglobin at cryogenic temperature: dependence on external medium. Biopolymers. 1990 Feb 15;29(3):639–643. doi: 10.1002/bip.360290316. [DOI] [PubMed] [Google Scholar]
- Cornelius P. A., Hochstrasser R. M., Steele A. W. Ultrafast relaxation in picosecond photolysis of nitrosylhemoglobin. J Mol Biol. 1983 Jan 5;163(1):119–128. doi: 10.1016/0022-2836(83)90032-3. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Structural and dynamic properties of the heme pocket in myoglobin probed by optical spectroscopy. Biopolymers. 1988 Dec;27(12):1977–1997. doi: 10.1002/bip.360271209. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Fiamingo F. G., Alben J. O. Structures of photolyzed carboxymyoglobin. Biochemistry. 1985 Dec 31;24(27):7964–7970. doi: 10.1021/bi00348a019. [DOI] [PubMed] [Google Scholar]
- Genberg L., Richard L., McLendon G., Miller R. J. Direct observation of global protein motion in hemoglobin and myoglobin on picosecond time scales. Science. 1991 Mar 1;251(4997):1051–1054. doi: 10.1126/science.1998121. [DOI] [PubMed] [Google Scholar]
- Henry E. R., Eaton W. A., Hochstrasser R. M. Molecular dynamics simulations of cooling in laser-excited heme proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8982–8986. doi: 10.1073/pnas.83.23.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Sommer J. H., Hofrichter J., Eaton W. A. Geminate recombination of carbon monoxide to myoglobin. J Mol Biol. 1983 May 25;166(3):443–451. doi: 10.1016/s0022-2836(83)80094-1. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Yamamoto H., Kotani M., Yonetani T. Low temperature photodissociation of hemoproteins: carbon monoxide complex of myoglobin and hemoglobin. Biochim Biophys Acta. 1974 Nov 5;371(1):126–139. doi: 10.1016/0005-2795(74)90161-5. [DOI] [PubMed] [Google Scholar]
- Kuczera K., Lambry J. C., Martin J. L., Karplus M. Nonexponential relaxation after ligand dissociation from myoglobin: a molecular dynamics simulation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5805–5807. doi: 10.1073/pnas.90.12.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Frauenfelder H. Spectroscopic evidence for conformational relaxation in myoglobin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2902–2906. doi: 10.1073/pnas.89.7.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich J. W., Lambry J. C., Kuczera K., Karplus M., Poyart C., Martin J. L. Ligand binding and protein relaxation in heme proteins: a room temperature analysis of NO geminate recombination. Biochemistry. 1991 Apr 23;30(16):3975–3987. doi: 10.1021/bi00230a025. [DOI] [PubMed] [Google Scholar]
- Petrich J. W., Poyart C., Martin J. L. Photophysics and reactivity of heme proteins: a femtosecond absorption study of hemoglobin, myoglobin, and protoheme. Biochemistry. 1988 May 31;27(11):4049–4060. doi: 10.1021/bi00411a022. [DOI] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Rudolph S. A., Boyle S. O., Dresden C. F., Gill S. J. A calorimetric study of the binding of carbon monoxide to myoglobin. Biochemistry. 1972 Mar 14;11(6):1098–1101. doi: 10.1021/bi00756a024. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Rousseau D. L. Time dependence of near-infrared spectra of photodissociated hemoglobin and myoglobin. Biochemistry. 1987 Jun 2;26(11):3092–3098. doi: 10.1021/bi00385a022. [DOI] [PubMed] [Google Scholar]
- Srajer V., Champion P. M. Investigations of optical line shapes and kinetic hole burning in myoglobin. Biochemistry. 1991 Jul 30;30(30):7390–7402. doi: 10.1021/bi00244a005. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Tian WD, Sage JT, Srajer V, V, Champion PM. Relaxation dynamics of myoglobin in solution. Phys Rev Lett. 1992 Jan 20;68(3):408–411. doi: 10.1103/PhysRevLett.68.408. [DOI] [PubMed] [Google Scholar]
- Xie X. L., Simon J. D. Protein conformational relaxation following photodissociation of CO from carbonmonoxymyoglobin: picosecond circular dichroism and absorption studies. Biochemistry. 1991 Apr 16;30(15):3682–3692. doi: 10.1021/bi00229a013. [DOI] [PubMed] [Google Scholar]



