Abstract
Human metapneumovirus (hMPV) is a common cause of respiratory tract infection in the paediatrics population. Recently, we and others have shown that retinoic acid-inducible gene 1 (RIG-I)-like receptors (RLRs) are essential for hMPV-induced cellular antiviral signalling. However, the contribution of those receptors to host immunity against pulmonary hMPV infection is largely unexplored. In this study, mice deficient in mitochondrial antiviral-signalling protein (MAVS), an adaptor of RLRs, were used to investigate the role(s) of these receptors in pulmonary immune responses to hMPV infection. MAVS deletion significantly impaired the induction of antiviral and pro-inflammatory cytokines and the recruitment of immune cells to the bronchoalveolar lavage fluid by hMPV. Compared with WT mice, mice lacking MAVS demonstrated decreased abilities to activate pulmonary dendritic cells (DCs) and abnormal primary T-cell responses to hMPV infection. In addition, mice deficient in MAVS had a higher peak of viral load at day 5 post-infection (p.i.) than WT mice, but were able to clear hMPV by day 7 p.i. similarly to WT mice. Taken together, our data indicate a role of MAVS-mediated pathways in the pulmonary immune responses to hMPV infection and the early control of hMPV replication.
Introduction
Human metapneumovirus (hMPV), a negative ssRNA virus belonging to the family Paramyxovirus, was identified in 2001 (van den Hoogen et al., 2001). Soon after its discovery, hMPV was quickly recognized as an important cause of lower respiratory infection in infants and children worldwide (Crowe, 2004; Kahn, 2006; Principi et al., 2006; Williams et al., 2004). Virtually all children older than 5 years show serological evidence of hMPV infection (van den Hoogen et al., 2001). Reinfection with hMPV occurs symptomatically throughout life without significant antigenic changes, suggesting incomplete or short-lived immunity. In addition, hMPV is also associated with higher rates of hospitalization and enhanced disease severity among elderly and immunocompromised patients with respiratory tract infections (Englund et al., 2006; Falsey et al., 2003). Given the impact of hMPV on public health, it is important to determine host factor(s) that control host immunity against hMPV infection, to facilitate the development of antiviral strategies, such as adjuvants in future hMPV vaccines and therapeutics.
We and others have shown that pattern recognition receptors belonging to the retinoic-acid-inducible protein I (RIG-I)-like receptors (RLRs) and toll-like receptors (TLRs) play a significant role in launching cell type-specific immune responses to hMPV infection (Baños-Lara et al., 2013; Goutagny et al., 2010; Kolli et al., 2011; Liao et al., 2008). We also confirmed the contribution of TLR-mediated signalling to hMPV-induced host immunity in an experimental mouse model (Ren et al., 2013). However, the in vivo role of RLRs in hMPV-induced host immunity, especially their role in mediating immune cell functions, has not been elucidated.
Mitochondrial antiviral-signalling protein (MAVS) is an essential common adaptor for RLRs, such as RIG-I and melanoma differentiation-associated protein 5 (MDA5) (Sun et al., 2006). In response to viral infection, the polyubiquitination and phosphorylation of MAVS are important in recruiting and activating IKK and TBK1 for the activation of NF-kB and IRF-3 and, subsequently, in the transcription of antiviral and pro-inflammatory genes (Liu et al., 2013, 2015). In this study, we used MAVS knockout mice to determine the contribution of RLR-mediated signalling to hMPV-induced host immunity in vivo. We found that MAVS is vital in inducing pulmonary innate immune responses, which include the secretion of immune mediators and the recruitment and maturation of immune cells. In addition, MAVS is critical for the initiation of T-cell responses, thereby confirming its role in immune responses to hMPV infection. To our knowledge, this report demonstrates, for the first time, a role of MAVS in controlling pulmonary immune responses against hMPV, confirming the relevance of RLR-mediated pathways in hMPV-induced host immunity.
Results
MAVS is critical for innate pulmonary cytokine/chemokine induction
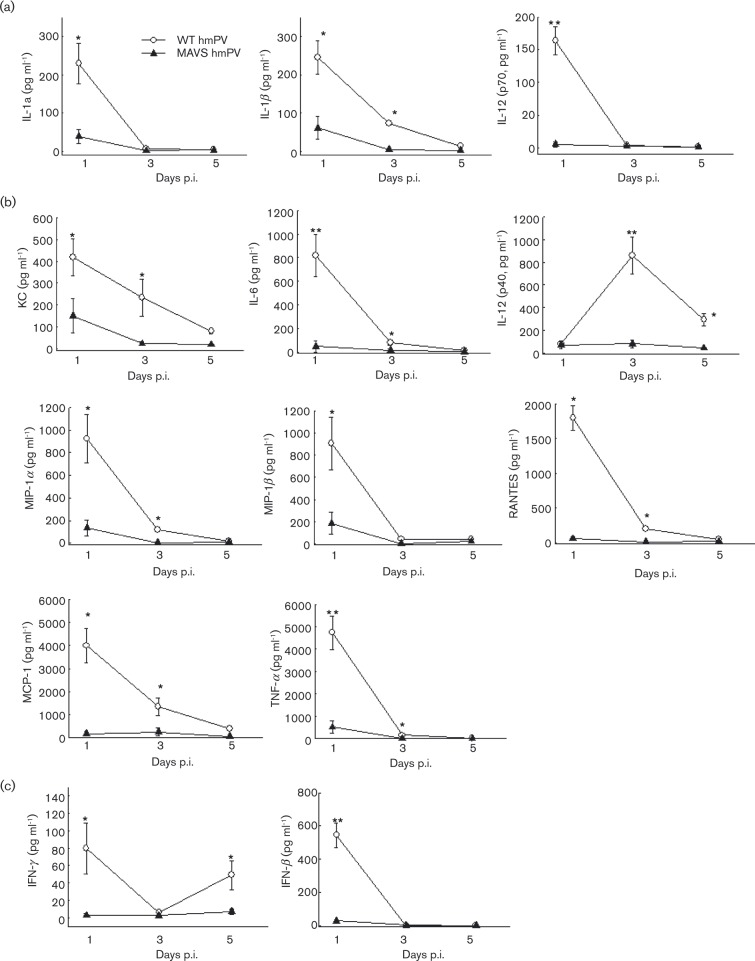
Although the importance of MAVS in inducing innate cytokines and chemokines has been demonstrated in hMPV-infected airway epithelial cells, the overall role of MAVS in pulmonary innate immunity against hMPV has not been explored except for its role in producing IFNs (Ren et al., 2012; Spann et al., 2014). In this study, we investigated the contribution of MAVS to pulmonary innate immunity by comparing the secretion of immune mediators in infected lungs of WT and MAVS− / − mice. In brief, WT and MAVS− / − mice were infected intranasally (i.n.) with hMPV, and bronchoalveolar lavage (BAL) fluid was analysed at days 1, 3 and 5 p.i. Mock infection was used as a negative control. We observed that the overall cytokine/chemokine basal level of WT and MAVS− / − mice was comparable, and no more than that of hMPV-infected MAVS− / − mice (data not shown). The induced cytokines/chemokines in WT mice ≤ 300 pg ml− 1 or ≥ 400 pg ml− 1 induction at day 1 p.i. are grouped in Fig. 1(a, b), respectively. IFNs are shown in Fig. 1(c). As shown in Fig. 1, many pro-inflammatory and antiviral cytokines and chemokines were induced as early as day 1 after hMPV infection. The production of these immune mediators was significantly less in MAVS− / − mice. In WT mice, at day 3 p.i., the induction of IL-1α, IL-12-p70, MIP-1β and IFN-β became undetectable, and the production of other mediators declined but was still detectable. However, all these immune mediators were absent in MAVS− / − mice. At day 5 p.i., most cytokines and chemokines were undetectable in WT and MAVS− / − mice, while IL-12-p40 and IFN-γ were sustained in infected WT mice, but not in MAVS− / − mice. Overall, the data from Fig. 1 demonstrates that MAVS contributes to hMPV-induced lung innate immune responses.
Fig. 1. Effect of MAVS on pulmonary cytokine and chemokine production in hMPV-infected lung. BAL samples collected from mock- and hMPV-infected mice, WT and MAVS− / − , at indicated time points p.i., were assessed for IFN-β by ELISA. Other cytokine and chemokine production was measured by a multi-Plex Cytokine detection system. Data are mean ± SEM from n = 10–12 mice in each group from three independent experiments. *P < 0.05 and **P < 0.01 for comparison with hMPV-infected samples from WT mice. Induced cytokines and chemokines in WT mice with levels of ≤ 300 pg ml− 1 or ≥ 400 pg ml− 1 induction at day 1 p.i. were grouped in (a) and (b), respectively. IFNs are shown in (c).
MAVS affects immune cell infiltration
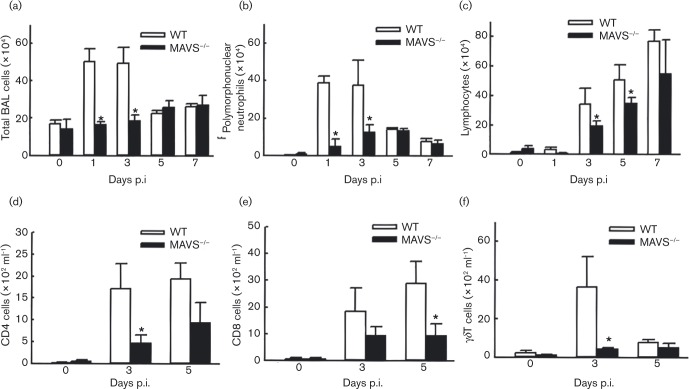
Immune cell migration in response to pathogen-induced chemotactic cytokines is important for immunological defence (Banchereau et al., 2000; Gill et al., 2005). Compared with WT-infected mice, the total BAL cells in response to hMPV were significantly less in MAVS− / − infected mice, at days 1 and 3 p.i. (Fig. 2a), suggesting that the pulmonary innate response to hMPV is significantly impaired by MAVS deletion. Changes in specific BAL cell populations were also investigated by cytospin or FACS. Consistent with our previous finding (Kolli et al., 2008), we observed a remarkable neutrophilia in hMPV-infected WT mice within the first 3 days of infection, and this hMPV-enhanced neutrophilia was significantly alleviated by MAVS knockout (Fig. 2b). It is well known that KC is a neutrophil chemoattractant. Therefore, the result regarding the impaired neutrophil infiltration was in alignment with decreased induction of KC in MAVS deficient mice by hMPV. We also found that MAVS affected lymphocyte migration at relatively late time points p.i. The numbers of lymphocytes increased by day 3 in WT mice. At days 3 and 5 p.i., MAVS− / − mice had significantly fewer infiltrated lymphocytes, but the deficiency was fully abrogated at day 7 p.i. (Fig. 2c). To specify the lymphocyte population particularly affected by MAVS, we also used FACS to analyse cells in BAL. We found that the migration of both CD4+T-cells and gamma delta T-cells (γδ T-cells) was significantly impaired by MAVS at day 3 p.i., while CD8+T-cell infiltration was impaired at day 5 p.i (Fig. 2d–f). Overall, the BAL cell analysis demonstrates the importance of MAVS in mediating pulmonary innate response to hMPV infection.
Fig. 2. Effect of MAVS on immune cell infiltration. Mice were either infected with 1 × 107 p.f.u. of hMPV or mock infected, and at days as indicated p.i., cells were isolated from BAL, and subjected to cytospin (a–c), or stained with antibodies for lineage-specific markers and analysed by FACS (d–f). Data are means ± sem from 12 mice in each group at each time point, and are representative of three independent experiments. *P < 0.05 for comparison with WT mice.
MAVS deficiency impairs the maturation of pulmonary dendritic cells
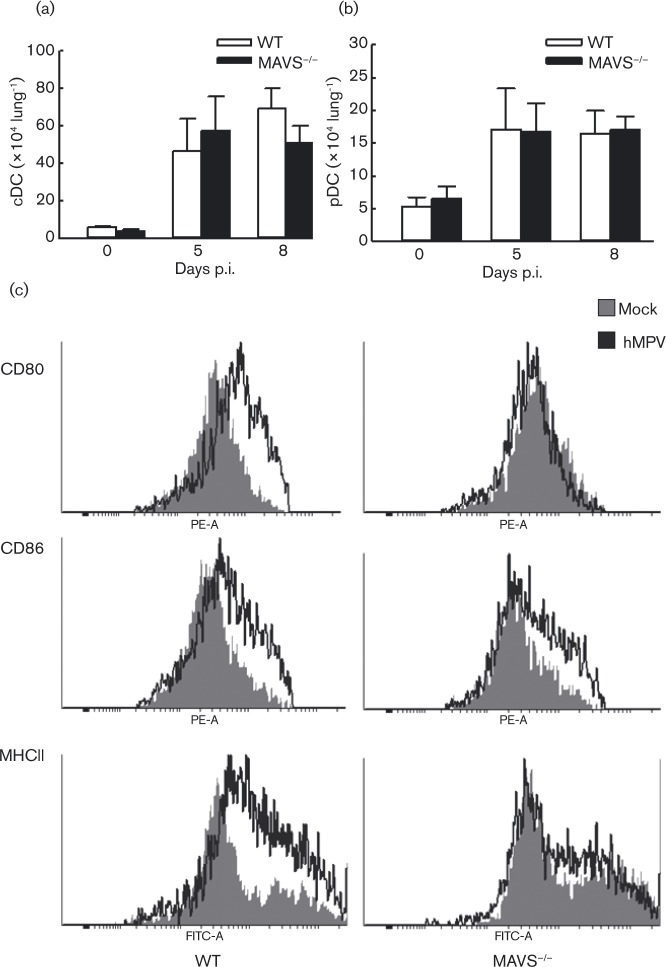
Dendritic cells (DC), including conventional DC (cDC) and plasmacytoid DC (pDC), generate not only cellular antiviral responses but also migrate through the afferent lymph to the lymph nodes where they prime naive T-cells, playing a significant role in bridging innate and adaptive immune responses (Guerrero-Plata et al., 2006, 2009). Herein, we determined the recruitment of pDC and cDC to the lung after hMPV infection in WT and MAVS− / − mice. We have previously demonstrated that pulmonary DC migration in response to hMPV starts at day 5 and peaks at day 8 p.i. (Guerrero-Plata et al., 2009). Therefore, we chose these two time points to investigate the impact of MAVS on DC migration, and found comparable infiltration in WT and MAVS− / − mice (Fig. 3a, b). Since DC maturation, after detection, uptake and degradative processing of invading pathogens, is critical to presenting processed viral antigen to lymphocytes (Pulendran et al., 2001; Steinman & Hemmi, 2006), we investigated whether MAVS affects DC maturation. As evident in the histogram of DC maturation markers (Fig. 3c), the expression of CD80, CD86 and MHC class II on CD11c+ lung cells of WT mice was significantly increased at day 5 p.i, compared with mock-infected mice. However, in hMPV-infected MAVS− / − mice, the induction of CD80 and MHC II was significantly lower (Fig. 3c). The inhibitory effect of MAVS on hMPV-induced CD80 and MHCII maturation was minimized at day 7 p.i. (data not shown). Overall, our data demonstrates that MAVS is essential for hMPV-induced DC maturation.
Fig. 3. Effect of MAVS on DC migration and maturation. Mice were infected with hMPV or mock infected as described above. At days 5 and 8 p.i., pulmonary cDC (a) and pDC (b) were quantified by FACS. The total cell numbers are presented as means ± SEM, n = 4 in each group from two independent experiments. (c) The maturation of DCs were also investigated by comparing the expression of MHCII, CD80 or CD89 on the cell surface of lung CD11C+ cells from WT and MAVS-/- mice. Representative histograms to elucidate the expression of DC maturation are shown.
MAVS controls T-cell responses
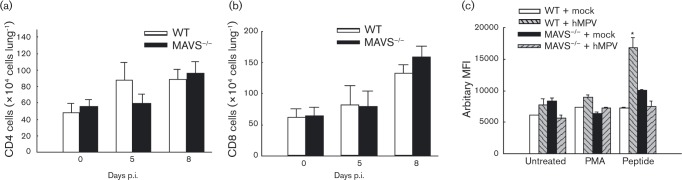
In addition to investigating the effect of MAVS on the infiltration of T-cells to BAL (Fig. 2d–f), we also studied whether MAVS potentially regulates lung T-cell responses. We found that although the number of pulmonary CD4+ and CD8+T-cells was comparable in WT- and MAVS− / − -infected mice (Fig. 4a, b), MAVS seemed to affect T-cell responses during primary hMPV infection. Total lung cells after infection were cultured with PMA plus ionomycin, or an hMPV-derived peptide, as we previously described (Ren et al., 2013). The percentage, total number and mean fluorescence intensity (MFI) of IFN-γ-producing CD8+ pulmonary T-cells was quantified by FACS. We found that the percentage and total number of IFN-γ+ CD8 cells in response to hMPV-peptide stimulation seemed unaffected by MAVS deficiency (data not shown). In term of MFI, we found that mock-infected mice, WT or MAVS− / − had few IFN-γ-producing CD8+ pulmonary T-cells (about 0.1 %) and low background. Upon stimulation of hMPV-peptide, lung cells isolated from hMPV-infected WT mice showed a significant increase in the MFI of IFN-γ-producing CD8+ pulmonary T-cells by hMPV-peptide simulation, but not by PMA stimulation. On the other hand, the MFI of IFN-γ+ CD8 cells was not significantly increased in infected MAVS− / − mice, demonstrating the importance of MAVS in T-cell function (Fig. 4c).
Fig. 4. Effect of MAVS on T-cell function. Lung cells from infected mice were harvested at days 5 and 8 p.i., and the total CD3+CD4+ population (a) and CD3+CD8+ population (b) were qualified by FACS. For the intracellular staining, isolated lung cells were untreated or treated with an hMPV-derived peptide. Stimulation with PMA and ionomycin was used as a control. Five hours later, intracellular IFN-γ levels in CD3+CD8+ cells were measured by FACS. (c) MFI of the IFN-γ+CD3+CD8+ population from infected cells was normalized to the MFI of the IFN-γ+CD3+CD8+ population from uninfected and non-stimulated cells, and data were plotted for each genotype. Data presented are representative of three similar experiments with n = 6 mice in each group. *P < 0.05 compared with peptide-untreated and mock-infected WT mice.
MAVS deficiency affects pulmonary viral replication and function
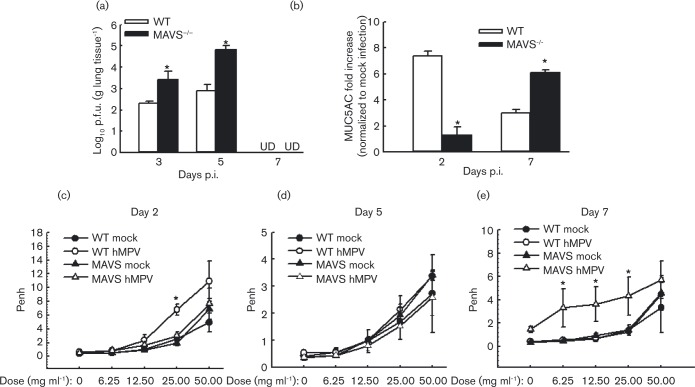
MAVS was essential not only for the induction of immune mediators, but also for immune cell migration and function. Since primary hMPV replication is controlled by multiple factors, including innate antiviral chemokines and pulmonary T-cells (Guerrero-Plata et al., 2005a; Kolli et al., 2008; Spann et al., 2014), our data suggest a possible role of MAVS in affecting hMPV replication. Therefore, we evaluated the overall effect of MAVS on viral replication by comparing viral loads in WT and mutant lungs. We found that MAVS− / − mice permitted more hMPV replication than WT mice at days 3 and 5 p.i., following the primary infection (Fig. 5a). By day 7 p.i., both WT and mutant mice completely eliminated infectious particles (data not shown), suggesting that lack of MAVS does not prevent clearance. To evaluate the effect of hMPV on lung function, we first quantified the gene transcription of MUC5AC, a primary mucin gene upregulated during severe airway diseases associated with mucus overproduction (Morcillo & Cortijo, 2006; Voynow et al., 2006). We found that WT mice produced more MUC5AC than MAVS− / − mice at day 2 p.i. However, hMPV induced more MUC5AC expression in MAVS− / − mice than WT mice at day 7 p.i. (Fig. 5b), demonstrating that MAVS regulates lung inflammation in response to hMPV infection.
Fig. 5. Effect of MAVS on lung viral replication and function. WT and MAVS− / − mice were infected i.n. with 1 × 107 p.f.u. of hMPV or mock-infected, total lung was harvested at indicated time points p.i. and lung infectious viral particles were titrated on LLC-MK2 cell monolayers by immune staining assays (a) and MUC5AC was quantified by real-time PCR (b). (c–e) Lung function in hMPV-infected mice, either WT or MAVS, at days 2, 5 and 7 p.i. was assayed by post-methacholine challenge. Penh values were determined by unrestrained plethysmography. n = 3–5 mice in each group. The results are from two independent experiments. *P < 0.05 for comparison with hMPV-infected WT or MAVS− / − mice. UD, undetectable.
It has been previously shown that, in response to hMPV infection, BALB/c mice have modest body weight loss and rapid recovery following an initial phase (days 1 to 3) and then significant body weight loss (20 % of initial weight) starting around day 5, with a peak at days 7 to 9 p.i. (Kolli et al., 2008). Unlike BALB/c mice, mice with a B6129SF2/J background did not have a second wave of body weight loss (data not shown). Therefore, body weight was not used as a clinical parameter to determine the importance of MAVS in hMPV-induced disease pathogenesis. Since hMPV is a mucosal pathogen and airway hyperresponsiveness (AHR) is commonly used as a clinical parameter to determine the pulmonary function, AHR was investigated to evaluate the importance of MAVS in pulmonary disease following hMPV infection, as we and others previously described (Abu-Harb et al., 1999; Chakraborty et al., 2012; Fullmer et al., 2005; Kolli et al., 2008). We found that uninfected WT and MAVS− / − mice have comparable and low Penh values. At day 2 p.i, immediately after the peak of most chemokine/cytokine induction, the Penh values were slightly higher in WT-infected mice than in MAVS− / − , mice when mice were challenged by 25 mg ml− 1 nebulized methacholine (Fig. 5c). The difference in AHR of infected WT and MAVS− / − mice was diminished after day 2 p.i. (data at day 5 p.i. is shown as an example in Fig. 5d), except at day 7 p.i., when a moderate increase in Penh value in MAVS− / − mice was observed, compared with WT mice (Fig. 5e). The increased Penh in MAVS− / − mice at day 7 p.i. was consistent with the enhanced MUC5AC expression in MAVS deficient mice. Overall, our data demonstrate that MAVS is important for controlling hMPV-induced pulmonary responses.
Discussion
We have provided in vitro evidence that MAVS is essential for the induction of antiviral and pro-inflammatory cytokines in response to hMPV infection (Ren et al., 2012). Herein, we provide in vivo data to support our in vitro observation. The importance of MAVS in controlling immune mediators is virus-dependent. In coxsackie B virus infection, MAVS is critical for type I IFN secretion; however, inflammatory cytokine production is independent of MAVS (Wang et al., 2010). MAVS is not critical in inducing type I IFN and inflammatory cytokines in response to lymphocytoid choriomeningitis virus (Jung et al., 2008), and only partially responsible for the induction of type I IFN by influenza virus (Koyama et al., 2007). The role of MAVS in controlling chemokine/cytokine induction in respiratory syncytial virus infection is similar to what we found in hMPV infection (Bhoj et al., 2008). Therefore, these data suggest the need to investigate the role of MAVS in host immunity in a pathogen-specific manner.
We have recently demonstrated the requirement of MyD88 for hMPV-induced innate cytokine responses (Ren et al., 2013). We found that MyD88 deficiency leads to about 30 % reduction in IFN-β, while MAVS knockout abolished more than 95 % of IFN-β. We have previously shown that the RIG-I–MAVS pathway dominantly regulates IFN-β secretion in airway epithelial cells (Ren et al., 2012). Besides airway epithelial cells, pulmonary macrophages and pDCs are also major sources of type I IFN secretion, which seem to be mediated by RLR–MAVS and TLR–MyD88 pathways, respectively (Hemmi et al., 2003; Honda et al., 2005; Sun et al., 2006). These published data, combined with current results of MAVS-dependent IFN-β secretion, suggests epithelial cells and macrophages to be key contributors to pulmonary IFN-β secretion, and that MAVS plays a significant role in IFN induction in these cells.
In addition to the role of MAVS in hMPV-induced immune mediators, we characterized the function of MAVS in immune cell infiltration and T-cell responses to hMPV infection. Although the defective cytokine response in MAVS− / − mice led to impaired immune cell migration to BAL, DC maturation and T-cell responses, the overall pulmonary migration of DCs and T-cells was comparable in WT and mutant mice. Although the mechanism is not clear, it is possible that hMPV-induced cytokines/chemokines in MAVS− / − mice met the basal requirements for DC and T-cell pulmonary migration, but was not enough for DC maturation and T-cell function. We also found that mice lacking MAVS had higher viral loads than WT mice, suggesting that MAVS contributes to immunity. However, MAVS− / − mice were able to clear the virus effectively. A possible answer to effective virus clearance might be the involvement of RLR–MAVS-independent antiviral pathways, which helped the MAVS− / − mice clear the viruses. Further studies are needed to characterize this phenomenon. Overall, our results demonstrate that MAVS is required for pulmonary immune responses. Therefore, agents that activate the RLR–MAVS pathway may serve as important adjuvants in future hMPV vaccines and therapeutics.
Methods
hMPV preparation and titre determination
The Canadian isolate hMPV 97-83 were propagated in LLC-MK2 cells at 35 °C in the absence of serum and in the presence of 1 μg trypsin ml− 1 (Worthington), and were sucrose purified, as described previously (Bao et al., 2008a, 2008b; Ren et al., 2012). Viral titres of purified hMPV or infected lung were determined by immunostaining in LLC-MK2 cells, as described previously (Bao et al., 2008a, 2008b; Ren et al., 2012).
Mice
Female MAVS− / − mice (B6;129-Mavs tm1Zjc/J) were purchased from the Jackson Laboratory and grown under specific-pathogen-free conditions at the University of Texas Medical Branch (UTMB). Age- and sex-matched control WT mice were purchased from the Jackson Laboratory as well. Mice were anaesthetized and infected intranasally (i.n.) with 107 p.f.u. hMPV diluted in Dulbecco's PBS (D-PBS) (Invitrogen) as described (Guerrero-Plata et al., 2005b). As mock treatment, mice were inoculated with an equivalent volume of sucrose diluted in D-PBS. All of the studies have been approved by the UTMB Institutional Animal Care and Use Committee Full Board.
BAL analysis
At different days p.i., BAL fluid was collected by washing the lungs two times with 1 ml D-PBS. An aliquot of BAL samples was used to determine total cell counts and counts of different types of cells, as previously described (Guerrero-Plata et al., 2005a; Kolli et al., 2008; Ren et al., 2013). IFN-β and other cytokines/chemokines from another aliquot of BAL samples were quantified by ELISA (PBL) and Luminex-based Bio-Plex system (Bio-Rad Laboratories) according to the manufacturer's instructions. The lower limit of detection for all cytokines measured by this assay is 3 pg ml− 1.
Real-time PCR
Lung RNA samples were prepared using Trizol (Invitrogen), quantified using a Nanodrop Spectrophotometer (Thermo Fisher Scientific) and qualified by analysis on an RNA Nano chip using the Agilent 2100 Bioanalyser (Agilent Technologies). Synthesis of cDNA was performed with 0.5 μg total RNA in a 20ul reaction using the reagents in a Taqman Reverse Transcription Reagents kit from Thermo Fisher Scientific. The reaction conditions were as follows: 25 °C, 10 min; 48 °C, 30 min; and 95 °C, 5 min. RT-PCR amplifications (performed in triplicate) were done using 1 μl cDNA in a total volume of 20 μl using a Faststart Universal SYBR Green Master Mix (Roche Applied Science). The final concentration of the primers was 300 nM. Relative RT-PCR assays were performed with either 18S RNA as a normalizer. All PCR assays were run in an ABI Prism 7500 Sequence Detection System and the conditions were as follows: 50 °C, 2 min; 95 °C, 10 min; and 40 cycles of 95 °C, 15 s and 60 °C, 1 min.
FACS analysis of leukocytes
BAL cells or total lung cells were harvested at different time points up to 8 days after mock or hMPV infection as previously described (Guerrero-Plata et al., 2009; Ren et al., 2013). Isolated cells were incubated with anti-FcγRII/FcγRIII mAb (24G2; BD Biosciences). For cell-surface marker staining, an aliquot of cells was stained with the following anti-mouse antibodies: anti-CD11c, anti-I-A/I-E (MHC II), anti-CD11b, (all from BD-Pharmingen) and anti-mPDCA1 (Miltenyi Biotec). In a separate aliquot of samples, cells were stained with anti-CD11c and anti-MHC II in combination with anti-CD80 and anti-CD86, or stained with a combination of antibodies: anti-CD3, anti-CD4, anti-CD8 and anti-TCR γ/δ. Samples were stained at 4 °C in PBS with 1 % FBS and analysed with a FACS Canto flow cytometer equipped with BD FACSDiva software (both from Becton Dickinson Immunocytometry Systems). Analysis was performed using WinMDI2.8 (Scripps).
CD8+ T-cell responses
Intracellular cytokine staining was performed for CD8+ cells during the primary infection of hMPV as we previously described (Ren et al., 2013). In brief, lung cells were isolated on day 8 after mock or hMPV infection and then stimulated at 2.5 × 106 cells ml− 1 with 50 ng phorbol 12-myristate 13-acetate (PMA) ml− 1 and 500 ng ionomycin ml− 1 as a control (Sigma-Aldrich) or an hMPV-derived peptide (VGALIFTKL, 10 μg ml− 1) for 5 h at 37 °C (Herd et al., 2006; Rutigliano et al., 2005). Golgi Stop (BD Biosciences) was added during the final 3.5 h. The cells were harvested, stained with anti-CD3 and -CD8 antibodies, and fixed in 2 % paraformaldehyde. The cells were then stained for IFN-γ using Becton Dickinson fix/perm reagent according to the manufacturer's instructions. Samples were run on a Becton Dickinson FACS Canto flow cytometer equipped with BD FACSDiva software. Analysis was performed using WinMDI2.8 (Scripps).
Airway hyperresponsiveness (AHR)
AHR was assessed in unrestrained mice using whole-body barometric plethysmography (Buxco) to record enhanced pause (Penh), as previously described (Kolli et al., 2008; Ren et al., 2013). Penh, a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration, has previously been validated in animal models of AHR (Hamelin et al., 2006; Jacoby et al., 1988; Jafri et al., 2004). Respiratory activity was recorded for 4 min in order to establish baseline Penh values. Mice were subsequently exposed to increasing doses of nebulized methacholine (6.25, 12.5, 25 and 50 mg ml− 1) for 1.5 min, and data were recorded for another 3 min.
Statistical analysis
Statistical significance was determined by ANOVA with a Student–Neuman–Kuhl post-test, Values of P < 0.05 were considered significant. Values for phenotype analysis, viral replication and cytokine production experiments are presented as means ± sem.
Acknowledgements
All authors concur that there are no conflicts of interest in this work. This work was supported by grants from the National Institutes of Health-National Institute of Allergy and Infectious Diseases (R56 AI107033-01A1), Research Pilot Grant from John Sealy Memorial Foundation, UTMB, the American Lung Association (RG232529N), and American Heart Association (12BGIA12060008) to X. B. Y. C. is partially supported by an Overseas Training Grant of TongJi Hospital, TongJi Medical College, Huazhong University of Science and Technology, China. The work was also partially supported by the National Institute of Environmental Health Sciences (5P30ES006676) and a NIH Clinical and Translational Science Award (CTSA) (UL1TR000071).
Footnotes
† These authors contributed equally to this paper.
References
- Abu-Harb M., Bell F., Finn A., Rao W.H., Nixon L., Shale D., Everard M.L. (1999). IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis Eur Respir J 14 139–143 10.1034/j.1399-3003.1999.14a23.x . [DOI] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. (2000). Immunobiology of dendritic cells Annu Rev Immunol 18 767–811 10.1146/annurev.immunol.18.1.767 . [DOI] [PubMed] [Google Scholar]
- Baños-Lara M.R., Ghosh A., Guerrero-Plata A. (2013). Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo J Virol 87 1242–1251 10.1128/JVI.01213-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X., Liu T., Shan Y., Li K., Garofalo R.P., Casola A. (2008a). Human metapneumovirus glycoprotein G inhibits innate immune responses PLoS Pathog 4 e1000077 10.1371/journal.ppat.1000077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X., Sinha M., Liu T., Hong C., Luxon B.A., Garofalo R.P., Casola A. (2008b). Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarray analysis Virology 374 114–127 10.1016/j.virol.2007.12.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoj V.G., Sun Q., Bhoj E.J., Somers C., Chen X., Torres J.P., Mejias A., Gomez A.M., Jafri H., otherauthors (2008). MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus Proc Natl Acad Sci U S A 105 14046–14051 10.1073/pnas.0804717105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K., Zhou Z., Wakamatsu N., Guerrero-Plata A. (2012). Interleukin-12p40 modulates human metapneumovirus-induced pulmonary disease in an acute mouse model of infection PLoS One 7 e37173 10.1371/journal.pone.0037173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J.E., Jr (2004). Human metapneumovirus as a major cause of human respiratory tract disease Pediatr Infect Dis J 23 S215–S221 10.1097/01.inf.0000144668.81573.6d . [DOI] [PubMed] [Google Scholar]
- Englund J.A., Boeckh M., Kuypers J., Nichols W.G., Hackman R.C., Morrow R.A., Fredricks D.N., Corey L. (2006). Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients Ann Intern Med 144 344–349 10.7326/0003-4819-144-5-200603070-00010 . [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Erdman D., Anderson L.J., Walsh E.E. (2003). Human metapneumovirus infections in young and elderly adults J Infect Dis 187 785–790 10.1086/367901 . [DOI] [PubMed] [Google Scholar]
- Fullmer J.J., Khan A.M., Elidemir O., Chiappetta C., Stark J.M., Colasurdo G.N. (2005). Role of cysteinyl leukotrienes in airway inflammation and responsiveness following RSV infection in BALB/c mice Pediatr Allergy Immunol 16 593–601 10.1111/j.1399-3038.2005.00248.x . [DOI] [PubMed] [Google Scholar]
- Gill M.A., Palucka A.K., Barton T., Ghaffar F., Jafri H., Banchereau J., Ramilo O. (2005). Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections J Infect Dis 191 1105–1115 10.1086/428589 . [DOI] [PubMed] [Google Scholar]
- Goutagny N., Jiang Z., Tian J., Parroche P., Schickli J., Monks B.G., Ulbrandt N., Ji H., Kiener P.A., otherauthors (2010). Cell type-specific recognition of human metapneumoviruses (HMPVs) by retinoic acid-inducible gene I (RIG-I) and TLR7 and viral interference of RIG-I ligand recognition by HMPV-B1 phosphoprotein J Immunol 184 1168–1179 10.4049/jimmunol.0902750 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A., Baron S., Poast J.S., Adegboyega P.A., Casola A., Garofalo R.P. (2005a). Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections J Virol 79 10190–10199 10.1128/JVI.79.16.10190-10199.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A., Casola A., Garofalo R.P. (2005b). Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus J Virol 79 14992–14997 10.1128/JVI.79.23.14992-14997.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A., Casola A., Suarez G., Yu X., Spetch L., Peeples M.E., Garofalo R.P. (2006). Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus Am J Respir Cell Mol Biol 34 320–329 10.1165/rcmb.2005-0287OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A., Kolli D., Hong C., Casola A., Garofalo R.P. (2009). Subversion of pulmonary dendritic cell function by paramyxovirus infections J Immunol 182 3072–3083 10.4049/jimmunol.0802262 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin M.E., Prince G.A., Gomez A.M., Kinkead R., Boivin G. (2006). Human metapneumovirus infection induces long-term pulmonary inflammation associated with airway obstruction and hyperresponsiveness in mice J Infect Dis 193 1634–1642 10.1086/504262 . [DOI] [PubMed] [Google Scholar]
- Hemmi H., Kaisho T., Takeda K., Akira S. (2003). The roles of Toll-like receptor 9. MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets J Immunol 170 3059–3064 10.4049/jimmunol.170.6.3059 . [DOI] [PubMed] [Google Scholar]
- Herd K.A., Mahalingam S., Mackay I.M., Nissen M., Sloots T.P., Tindle R.W. (2006). Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice J Virol 80 2034–2044 10.1128/JVI.80.4.2034-2044.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Ohba Y., Yanai H., Negishi H., Mizutani T., Takaoka A., Taya C., Taniguchi T. (2005). Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction Nature 434 1035–1040 10.1038/nature03547 . [DOI] [PubMed] [Google Scholar]
- Jacoby D.B., Tamaoki J., Borson D.B., Nadel J.A. (1988). Influenza infection causes airway hyperresponsiveness by decreasing enkephalinase J Appl Physiol 64 (1985), 2653–2658 . [DOI] [PubMed] [Google Scholar]
- Jafri H.S., Chavez-Bueno S., Mejias A., Gomez A.M., Rios A.M., Nassi S.S., Yusuf M., Kapur P., Hardy R.D., otherauthors (2004). Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice J Infect Dis 189 1856–1865 10.1086/386372 . [DOI] [PubMed] [Google Scholar]
- Jung A., Kato H., Kumagai Y., Kumar H., Kawai T., Takeuchi O., Akira S. (2008). Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88 J Virol 82 196–206 10.1128/JVI.01640-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J.S. (2006). Epidemiology of human metapneumovirus Clin Microbiol Rev 19 546–557 10.1128/CMR.00014-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolli D., Bataki E.L., Spetch L., Guerrero-Plata A., Jewell A.M., Piedra P.A., Milligan G.N., Garofalo R.P., Casola A. (2008). T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection J Virol 82 8560–8569 10.1128/JVI.00699-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolli D., Bao X., Liu T., Hong C., Wang T., Garofalo R.P., Casola A. (2011). Human metapneumovirus glycoprotein G inhibits TLR4-dependent signaling in monocyte-derived dendritic cells J Immunol 187 47–54 10.4049/jimmunol.1002589 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S., Ishii K.J., Kumar H., Tanimoto T., Coban C., Uematsu S., Kawai T., Akira S. (2007). Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination J Immunol 179 4711–4720 10.4049/jimmunol.179.7.4711 . [DOI] [PubMed] [Google Scholar]
- Liao S., Bao X., Liu T., Lai S., Li K., Garofalo R.P., Casola A. (2008). Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling J Gen Virol 89 1978–1986 10.1099/vir.0.2008/000778-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Chen J., Cai X., Wu J., Chen X., Wu Y.T., Sun L., Chen Z.J. (2013). MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades eLife 2 e00785 10.7554/eLife.00785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., Du F., Ren J., Wu Y.T., otherauthors (2015). Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation Science 347 aaa2630 10.1126/science.aaa2630 . [DOI] [PubMed] [Google Scholar]
- Morcillo E.J., Cortijo J. (2006). Mucus and MUC in asthma Curr Opin Pulm Med 12 1–6 10.1097/01.mcp.0000198064.27586.37 . [DOI] [PubMed] [Google Scholar]
- Principi N., Bosis S., Esposito S. (2006). Human metapneumovirus in paediatric patients Clin Microbiol Infect 12 301–308 10.1111/j.1469-0691.2005.01325.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Palucka K., Banchereau J. (2001). Sensing pathogens and tuning immune responses Science 293 253–256 10.1126/science.1062060 . [DOI] [PubMed] [Google Scholar]
- Ren J., Wang Q., Kolli D., Prusak D.J., Tseng C.T., Chen Z.J., Li K., Wood T.G., Bao X. (2012). Human metapneumovirus M2-2 protein inhibits innate cellular signaling by targeting MAVS J Virol 86 13049–13061 10.1128/JVI.01248-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Kolli D., Deng J., Fang R., Gong B., Xue M., Casola A., Garofalo R.P., Wang T., Bao X. (2013). MyD88 controls human metapneumovirus-induced pulmonary immune responses and disease pathogenesis Virus Res 176 241–250 10.1016/j.virusres.2013.06.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutigliano J.A., Rock M.T., Johnson A.K., Crowe J.E., Jr, Graham B.S. (2005). Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus Virology 337 335–343 10.1016/j.virol.2005.04.032 . [DOI] [PubMed] [Google Scholar]
- Spann K.M., Loh Z., Lynch J.P., Ullah A., Zhang V., Baturcam E., Werder R.B., Khajornjiraphan N., Rudd P., otherauthors (2014). IRF-3, IRF-7, and IPS-1 promote host defense against acute human metapneumovirus infection in neonatal mice Am J Pathol 184 1795–1806 10.1016/j.ajpath.2014.02.026 . [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Hemmi H. (2006). Dendritic cells: translating innate to adaptive immunity Curr Top Microbiol Immunol 311 17–58 . [DOI] [PubMed] [Google Scholar]
- Sun Q., Sun L., Liu H.H., Chen X., Seth R.B., Forman J., Chen Z.J. (2006). The specific and essential role of MAVS in antiviral innate immune responses Immunity 24 633–642 10.1016/j.immuni.2006.04.004 . [DOI] [PubMed] [Google Scholar]
- van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A., Osterhaus A.D. (2001). A newly discovered human pneumovirus isolated from young children with respiratory tract disease Nat Med 7 719–724 10.1038/89098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow J.A., Gendler S.J., Rose M.C. (2006). Regulation of mucin genes in chronic inflammatory airway diseases Am J Respir Cell Mol Biol 34 661–665 10.1165/rcmb.2006-0035SF . [DOI] [PubMed] [Google Scholar]
- Wang J.P., Cerny A., Asher D.R., Kurt-Jones E.A., Bronson R.T., Finberg R.W. (2010). MDA5 and MAVS mediate type I interferon responses to coxsackie B virus J Virol 84 254–260 10.1128/JVI.00631-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.V., Harris P.A., Tollefson S.J., Halburnt-Rush L.L., Pingsterhaus J.M., Edwards K.M., Wright P.F., Crowe J.E., Jr (2004). Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children N Engl J Med 350 443–450 10.1056/NEJMoa025472 . [DOI] [PMC free article] [PubMed] [Google Scholar]