Abstract
Background: Theoretical links between seasonal lack of sunlight, hypovitaminosis D and excess cardiovascular disease and death prompted our adding novel to conventional cohort analyses.
Methods: We tested three postulates on 13 224 Scottish Heart Health Extended Cohort participants, assayed for 25-hydroxyvitamin D (25OHD) and followed for 22 years. (i) Endpoints enumerated by month of occurrence mirror annual seasonal oscillation in 25OHD. (ii) Endpoint seasonality is increased in people with below median 25OHD. (iii) Low 25OHD predicts endpoints independently of major risk factors.
Results: Baseline median 25OHD level was 36.4 (other quartiles 26.7, 51.7) nmol/l. The March trough was half the August peak, both well after seasonal solstices. (i) There was no demonstrable monthly variation in First Cardiovascular Event (n = 3307). Peaks and troughs for All Death and Cardiovascular Death (n = 2987, 1350) were near the solstices, earlier than extremes of 25OHD. (ii) Endpoint variability showed no difference between those above and below median 25OHD. (iii) Cox model hazard ratios (HR), by decreasing 25OHD, increased modestly and nonspecifically for all endpoints examined, with no threshold, the gradients diminishing by ∼ 60% following multiple adjustment. For Cardiovascular Disease, HR, by 20 (∼SD) nmol/l decrease, = 1.224 (1.175, 1.275) adjusted for age and sex; additionally adjusted for family history, deprivation index, smoking, systolic blood pressure, total and HDL cholesterol, = 1.093 (1.048, 1.139); All Deaths = 1.238 (1.048, 1.139) and 1.098 (1.050, 1.149). 25OHD made no independent contribution to cardiovascular discrimination and reclassification.
Conclusions: Our analyses challenge vitamin D’s alleged role as major prime mover in cardiovascular disease and mortality.
Keywords: Vitamin D, causality, seasonality, cardiovascular disease, mortality, cohort study
Key Messages
Winter excess of cardiovascular disease and death is attributed to hypovitaminosis D through lack of sunlight.
We added seasonality to a conventional follow-up study of a Scottish cohort.
Despite two-to-one, August-to-March, change in population serum 25-hydroxyvitamin D (25OHD), there was no seasonal change in incident cardiovascular disease.
Peaks in deaths appeared early in winter, therefore out-of-phase.
There was no disproportionate seasonal susceptibility in people with low 25OHD.
25OHD was a modest predictor of endpoints, largely confounded by other risk factors, without an independent predictive effect for cardiovascular disease.
Our results challenge vitamin D’s alleged seasonal and causal role.
Introduction
Debate as to whether low population levels of vitamin D cause mass disease is stimulating large cohort studies, meta-analyses and trials,1–6 and studies of mendelian randomization.7 Earlier research produced inconclusive results8,9 despite rapid increases in clinical testing and prescribing.10,11 Meanwhile an existing natural experiment remains under-investigated. Population vitamin D levels are driven by exposure to sunlight.12,13 In 1981, Scragg hypothesized that excess and winter peaks in cardiovascular disease and death in temperate countries were caused by hypovitaminosis D (analogous to childhood rickets a century earlier, see Figure 1).14 Now embedded in popular medical culture, this seasonality hypothesis warrants critical testing, both in its own right and to pursue this debate along a novel perspective.
Figure 1.
Childhood rickets from hypovitaminosis D, especially prevalent in Scotland in the early 1900s. (Wellcome Library, London).
Midwinter daylight hours in Edinburgh are 39% of midsummer (contrast with 61% in New York and Madrid). The midwinter sun is also low in the sky, and there is a gross seasonal decline in ultraviolet radiation. After deriving three postulates from the seasonality hypothesis, we tested them on a Scottish cohort with long follow-up,15 examining Bradford Hill criteria for causality between vitamin D, cardiovascular disease and mortality–specificity, temporality, strength of association and gradient.16
Methods
Rationale
Participants in the Scottish Heart Health Extended Cohort (SHHEC)15 were recruited over much of 7 years, so archived recruitment serum could be used subsequently to show the annual cycle in vitamin D, averaged for each of the 12 calendar months, measured as 25-hydroxyvitamin D (25OHD). First cardiovascular events and deaths during follow-up were also cumulated to the 12 calendar months of occurrence to record seasonal variation. Because low vitamin D levels occur in winter and higher levels in summer, the bulk of readings swinging up and down en masse, ‘raw’ recruitment readings (25OHDraw) were modified by seasonal adjustment (25OHDadj). This defined the estimated ranking of vitamin D status within the cohort, regardless of season. It was used to divide the population in half for one analysis, and in descending fifths for another.
Postulates
Through the above rationale we developed three ‘Dundee Postulates’ for testing the seasonality hypothesis (see Box 1).
Box 1. Dundee Postulates based on the seasonality hypothesis
Seasonality Postulate 1. Endpoints, (First Cardiovascular Event, All Death and Cardiovascular Death), enumerated by calendar month of occurrence, inversely reflect seasonal fluctuation in recruitment 25-hydroxyvitamin D: 25OHDraw.
Seasonality Postulate 2. This endpoint variability is accentuated in those with low vs high seasonally adjusted 25OHDadj, who experience more hypovitaminosis D in winter.
Postulate 3. Low vs high 25OHDadj is predictive of endpoints, independently of other risk factors.
Scottish Heart Health Extended Cohort
This report comes from the Scottish Heart Health Extended Cohort (SHHEC),15 the MORGAM Biomarker study (MOnica, Risk, Genetics, Archiving and Monograph) and BiomarCaRE (Biomarkers for Cardiovascular Risk Assessment in Europe).17–19 SHHEC combines men and women of the Scottish Heart Health Study (SHHS)l5 aged 40–59 years, recruited across 23 districts of Scotland in 1984–87,with those of Scottish MONICA20 aged 25–64 years from Edinburgh and North Glasgow 1986 and North Glasgow 1989, 1992 (–74 years) and 1995. With ethical committee approval, participants completed questionnaires, attended recruitment survey clinics, gave blood and consented to follow-up of their medical records. Each of the 23 SHHS districts was visited twice in different seasons, whereas the North Glasgow MONICA surveys involved four independent population samples in the same seasons at 3-year intervals. For both studies, general practitioners were recruited randomly first, and their patient lists were then subjected to second-stage random sampling.21
Previously diagnosed cardiovascular disease was identified by self-report and hospital discharge diagnoses: any coronary heart disease; cerebrovascular disease, including transient ischaemic attacks; heart failure; and peripheral arterial disease. Mortality and hospital inpatient episodes were followed by record linkage through 2009.22 Cardiovascular disease morbidity was first diagnosis as above; mortality was any certified cardiovascular cause. Primary endpoints were First Cardiovascular Event, All Death and Cardiovascular Death. Secondary endpoints were Coronary Heart Disease, Cerebrovascular Disease, Non-cardiovascular Death and Cancer Death. The same SHHEC database followed through 2005 was used previously to derive the ASSIGN cardiovascular risk score.23
Laboratory procedures
After venepuncture, blood was left to coagulate up to 4 h. Separated serum was stored at 4°C pending laboratory testing 1–3 days later. Aliquots were biobanked long-term at -40°C24 and assayed for 25OHD in the MORGAM/BiomarCaRE biomarker laboratory as kits became available in 2009 and 2011. The ARCHITECT system (Abbott Laboratories) uses chemiluminescent microparticle immunoassay; the intra-assay coefficient of variation (CoV) was 5.0–9.5% and inter-assay CoV 1.4–8.7%. The assay is highly sensitive to natural 25OHD3, less so to synthetic 25OHD2.11,25
Statistical methods
The main test of the correspondence of seasonality between 25OHDraw and endpoint events (Postulate 1) was an ecological analysis comparing their monthly patterns. To test for increased susceptibility in winter in the ‘LowD people’ vs ‘HighD’ halves of 25OHDadj (Postulate 2), their endpoint events were apportioned separately between ‘Low-D’ and ‘High-D’ seasons and the resulting two-by-two tables tested for heterogeneity using the chi square statistic. Cox proportional hazard analyses for Postulate 3 included endpoints after recruitment, regardless of season. Hazard ratios were adjusted for age and sex, then multiply adjusted for ASSIGN cardiovascular risk factors.23 The cohort was divided into fifths in descending order of 25OHDadj. The baseline hazard ratio of 1·0 was ascribed to the first fifth (100–80th centile).
For First Cardiovascular Event, discrimination, measured using a c-statistic, differences in discrimination and the net reclassification improvement (NRI),(with thresholds of 10% and 20% 10-year risk) on adding 25OHDadj to the prognostic model, over and above ASSIGN variables, were computed allowing for censoring.26–28
Results
Population numbers
Cross-sectional analyses included 13 224 participants assayed for 25OHDraw. Follow-up studies excluded 498 below age 30 and 1098 with previous cardiovascular disease. For Seasonality Postulates 1 and 2, events were followed for whole calendar years so 31 victims afflicted in the calendar year of recruitment were excluded, leaving 11 597, whereas for the Cox proportional hazards analyses (Postulate 3) the 31 were included, making 11 628.
Recruitment 25OHDraw
Seasonal change was as anticipated. The averaged 25OHDraw by calendar month of recruitment was twice as high in August (maximum) as in March (minimum), the extremes occurring weeks after the solstices. Female values were lower than in men, with a smaller summer rise (Table 1). Differences were highly significant. After adjustment for seasonality, there was little variation between months: 25OHDadj. Differing monthly totals reflect cumulative recruitment activity.
Table 1.
Mean monthly 25-hydroxyvitamin D: 25OHDraw (standard deviation) by calendar month of recruitment. Then after adjustment: 25OHDadj. 13 224 participants in the Scottish Heart Health Extended Cohort (SHHEC) with archived serum
| Month (rank) | Number | Men | Women | All | All |
|---|---|---|---|---|---|
| 25OHDraw | 25OHDraw | 25OHDraw | 25OHDadj | ||
| nmol/l | nmol/l | nmol/l | nmol/l | ||
| n = 6539 | n = 6685 | n = 13 224 | n = 13 224 | ||
| January (3)a | 680 | 33.7 (15.6) | 30.9 (17.1) | 32.2 (16.5) | 41.6 (21.2) |
| February (2)a | 689 | 32.3 (13.3) | 29.3 (12.7) | 30.6 (13.1) | 40.6 (17.3) |
| March (1)a | 1266 | 30.1 (12.3) | 29.7 (13.0) | 29.9 (12.6) | 41.2 (17.4) |
| April (4)a | 1448 | 34.3 (17.0) | 31.6 (15.0) | 32.9 (16.1) | 41.6 (20.3) |
| May (5)a | 1585 | 37.9 (15.9) | 33.9 (15.0) | 36.0 (15.6) | 41.3 (17.9) |
| June (9)b | 1543 | 47.0 (22.1) | 40.9 (19.5) | 43.7 (21.0) | 43.2 (20.7) |
| July (11)b | 900 | 60.5 (23.2) | 53.1 (20.0) | 56.8 (22.0) | 40.5 (15.7) |
| August (12)b | 1344 | 64.2 (27.0) | 53.1 (22.1) | 58.6 (25.3) | 41.4 (17.8) |
| September (10)b | 1060 | 58.1 (26.1) | 51.0 (24.6) | 54.6 (25.6) | 41.8 (19.6) |
| October (8)b | 1240 | 45.3 (18.4) | 39.9 (18.2) | 42.4 (18.5) | 41.0 (17.9) |
| November (7)b | 971 | 39.9 (16.8) | 36.6 (16.6) | 38.3 (16.8) | 41.3 (18.1) |
| December (6)a | 498 | 38.8 (18.7) | 33.7 (16.7) | 36.3 (17.9) | 41.5 (20.5) |
| All | 13224 | 44.0 (22.6) | 39.0 (19.9) | 41.5 (21.4) | 41.5 (18.7) |
| Standard error of the mean | 0.28 | 0.24 | 0.19 | 0.16 | |
| March vs August | |||||
| P < 0.0001 |
A general linear model was fitted to estimate the excess log 25OHDraw each month compared with January, adjusting for age, sex and their interaction. The excess was subtracted so that each month had standardized ‘January’ levels; finally all values were reweighted to achieve the observed overall mean, yielding 25OHDadj. The ranking of months (low to high) is based on mean monthly values for sexes combined of 25OHDraw.
aLow-D season.
bHigh-D season.
Follow-up
First Cardiovascular Event, All Death and Cardiovascular Death were enumerated by calendar month of occurrence (Table 2, columns b–d). Resulting endpoint totals were split between the two sub-populations, LowD people / HighD people, either side of the median 25OHDadj of 38.2 nmol/l (Table 2, columns e–j). Results are unadjusted for length of month.
Table 2.
Endpoint numbers by calendar month of follow-up: overall totals, and then by sub-populations of LowD people and HighD people separated at the median (38.2 nmol/l) of 25OHDadj (n = 11 597 for sexes combined, LowD people n = 5799, HighD people n = 5798)
| a | b | c | d | e | f | g | h | i | j |
|---|---|---|---|---|---|---|---|---|---|
| Population | All | All | All | LowD people | HighD people | LowD people | HighD people | LowD people | HighD people |
| endpoint | First CV | All Death | CV Death | First CV | First CV | All Death | All Death | CV Death | CV Death |
| month | Event | Event | Event | ||||||
| January | 281 | 277 | 140 | 148 | 133 | 161 | 116 | 84 | 56 |
| February | 250 | 217 | 93 | 138 | 112 | 121 | 96 | 55 | 38 |
| March | 270 | 236 | 112 | 136 | 134 | 115 | 121 | 59 | 53 |
| April | 295 | 247 | 114 | 174 | 121 | 137 | 110 | 66 | 48 |
| May | 271 | 221 | 100 | 146 | 125 | 119 | 102 | 61 | 39 |
| June | 287 | 216 | 96 | 152 | 135 | 123 | 93 | 54 | 42 |
| July | 285 | 234 | 104 | 165 | 120 | 143 | 91 | 63 | 41 |
| August | 253 | 243 | 115 | 153 | 100 | 139 | 104 | 63 | 52 |
| September | 276 | 224 | 109 | 142 | 134 | 114 | 110 | 55 | 54 |
| October | 296 | 249 | 122 | 143 | 153 | 131 | 118 | 71 | 51 |
| November | 267 | 257 | 122 | 152 | 115 | 140 | 117 | 73 | 49 |
| December | 276 | 276 | 123 | 148 | 128 | 164 | 112 | 75 | 48 |
| Total | 3307 | 2897 | 1350 | 1797 | 1510 | 1607 | 1290 | 779 | 571 |
| Mean | 275·6 | 241·4 | 112·5 | 149·8 | 125·8 | 133·9 | 107·5 | 64·9 | 47·6 |
Numbers are not corrected for length of month, but see Supplementary Table W1 (available as Supplementary data at IJE online). For ranking of months and Low-D/ High-D season see Table 1.
First CV Event, First Cardiovascular Event; All Death, deaths from all causes; CV Death, death from cardiovascular causes.
a–j are for convenience of reference in the main text.
Seasonal pattern of 25OHDraw vs occurrence of endpoints (Postulate 1)
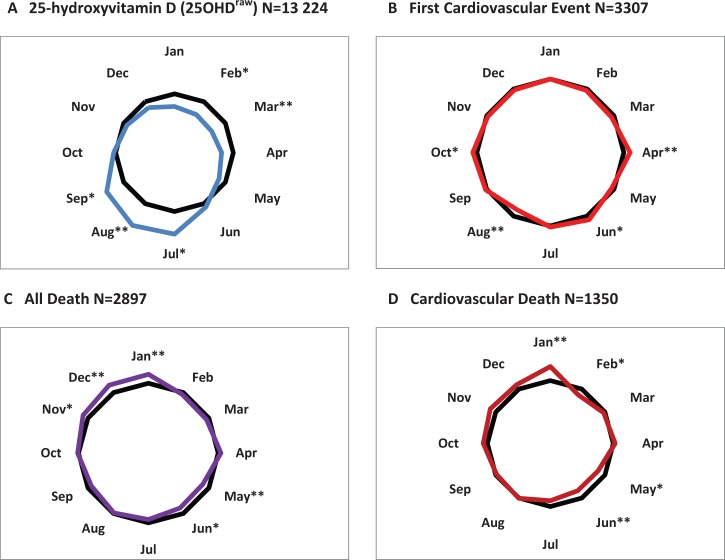
The raw monthly totals of endpoints from Table 2 were adjusted to a monthly duration of 30.44 days. Numbers were then normalized to percentage of the corresponding monthly mean, as was also done for 25OHDraw (see Supplement Table W1, available as Supplementary data at IJE online). Results are displayed as radar plots in Figure 2, contrasting the seasonal pattern in 25OHDraw and the three primary endpoints.
Figure 2.
Radar plots from the SHHEC population of monthly variation in: A, recruitment 25-hydroxyvitamin D (25OHDraw); B, First Cardiovascular Event; C, All Death; and D, Cardiovascular Death, testing Seasonality Postulate 1. Sexes combined, n = 11 597, 22-year follow-up. Mean for all months (100%) shown in black. (Plotted from Supplementary Table W1, available as Supplementary data at IJE online).*Outside coefficient of variation, **Highest and lowest monthly values (see text).
Circular plots in colour are shown against 100% in black. Calendar months in which deviation exceeds the coefficient of variation (CoV) are asterisked; highest and lowest months have double asterisks.
Monthly values of 25OHDraw (Figure 2A) show a cyclical annual doubling and halving, peak and trough each lasting several months, centred on late summer (August) and late winter (March), respectively. The seasonality hypothesis, Postulate 1, predicts the converse pattern for endpoints.
For First Cardiovascular Event (Figure 2B), the plot shows no deviation from circularity (P = 0·84). Between-months CoV is small at 5.0%; no deviation persists. Huge seasonal changes in 25OHDraw appear to leave the monthly incidence of First Cardiovascular Events unperturbed.
Patterns for All Death (Figure 2C) and Cardiovascular Death (Figure 2D) are ovoid and consistent with seasonal variation in deaths in national statistics. CoVs are larger than for First Cardiovascular Events at 7.8% and 10.7%. For both, the peak is in early winter; the trough in early summer. With smaller numbers, Cardiovascular Death appears more subject to random deviation. For neither fatal endpoint do February or March plots, the nadir in 25OHDraw, show the peak predicted by the seasonality hypothesis.14 The reciprocals of the monthly vitamin D values (Supplement Table W1, available as Supplementary data at IJE online) are 127% in February and 130% in March, but no endpoint value exceeds 100%.
In contrast to 25OHDraw there is no seasonal pattern for First Cardiovascular Event. What there is for All Death and Cardiovascular Death precedes rather than follows the late seasonal swings in 25OHDraw, failing Bradford Hill’s criterion of temporality.16
Increased Low-D season susceptibility to endpoints in those with low 25OHDadj (Postulate 2)
The null hypothesis for Postulate 2 involved testing for heterogeneity in endpoint occurrences. The cohort is split in half at the median 25OHDadj value of 38.2 nmol/l into LowD people and HighD people and the year into two 6-month periods, Low-D season and High-D season, at the 39.3 nmol/l monthly median for 25OHDraw. After the lowest 6 months against the highest, this was repeated for the lowest vs highest four, and then lowest three by highest, identifiable in Table 1. The seasons were not conventional summer and winter but reflected the delayed response of blood levels of 25OHDraw to seasonal sunlight. Aggregate endpoint totals were sums of appropriate monthly numbers, see Table 2, columns e–j.
The results in Table 3 show no evidence of heterogeneity by season in endpoints between LowD and HighD sub-populations. There is no increased susceptibility by season in the former when tested on a six-by-six, four-by-four or three-by-three basis. The second seasonality postulate is thus not supported in the SHHEC data.
Table 3.
Testing the null hypothesis for Dundee Seasonality Postulate 2 (greater seasonal susceptibility in LowD people) using 2 × 2 tables. Endpoints are partitioned by LowD people/HighD people at risk, and by Low-D season/High-D season of occurrence. Comparisons are of lowest and highest months for 25OHDraw, 6 months vs 6, 4 vs 4 and 3 vs 3 (see Table 1)
| High-D vs Low-D months compared | Endpoint numbers in LowD people, Low-D season | Endpoint numbers in LowD people, High-D season | Endpoint numbers in HighD people, Low-D season | Endpoint numbers in HighD people, High-D season | chi square for two-by-two table | P-value (Yates) | |
|---|---|---|---|---|---|---|---|
| n = 5799 | n = 5799 | n = 5798 | n = 5798 | ||||
| First CV Event | 6 vs 6 | 890 | 907 | 753 | 757 | 0·03 | 0·86 |
| 4 vs 4 | 596 | 612 | 500 | 489 | 0.28 | 0.60 | |
| 3 vs 3 | 422 | 460 | 379 | 354 | 2.23 | 0.14 | |
| All Death | 6 vs 6 | 817 | 790 | 657 | 633 | 0·00 | 1·00 |
| 4 vs 4 | 534 | 519 | 443 | 398 | 0.65 | 0.42 | |
| 3 vs 3 | 397 | 396 | 333 | 305 | 0.56 | 0.45 | |
| CV Death | 6 vs 6 | 400 | 379 | 282 | 289 | 0·43 | 0·51 |
| 4 vs 4 | 264 | 235 | 195 | 189 | 0.31 | 0.58 | |
| 3 vs 3 | 198 | 181 | 147 | 147 | 0.25 | 0.62 |
Half-populations and half-years are almost identical in size. Endpoints from each population are being compared in two different time periods, so there is no need to adjust for other factors. Correction for trivial differences both in numbers at risk and in cumulated numbers of days in comparison months had no effect on chi square or P-values. Event numbers from Table 2. Further ratios explored in Supplement Table W2 (available as Supplementary data at IJE online). Numbers are not corrected for length of month–but see Supplementary Table W1 (available as Supplementary data at IJE online). For ranking of months and Low-D/ High-D season see Table 1.
Further analysis by rearrangement of two-by-two results is shown in Supplement Table W2 (available as Supplementary data at IJE online). The Low-D season overall does not show excess of First Cardiovascular Events compared with High-D, but LowD people do have more endpoint events than HighD people. The latter finding is further explored in population fifths, using the Cox proportional hazards model.
Cox proportional hazards by declining fifths of 25OHDadj (testing Postulate 3)
Table 4 shows the Cox proportional hazards model for primary endpoints, sexes combined and separately. Results are by declining fifths of 25OHDadj, corrected for age and sex, and then multiply adjusted (ma) for factors in the ASSIGN score: age, sex, family history of coronary heart disease, Scottish Index of Multiple Deprivation, tobacco smoking dose, total cholesterol and high-density lipoprotein (HDL) cholesterol. Note that BMI (body mass index) did not contribute significantly to the ASSIGN score, nor did adding it in here.22 Ranges and mean values by fifths for the sexes together and separately for 25OHDadj are shown in Supplement Table W3 (available as Supplementary data at IJE online).
Table 4.
Hazard ratios for primary endpoints, Cox proportional hazards model by fifths of descending 25OHDadj values, and by 20-nmol/l change (HRD20), age and sex adjusted; and then after multiple adjustment (ma) for ASSIGN variables, sexes combined and separately.a All n = 11 628, men n = 5658, women n = 5970
| Fifths | 2 | 3 | 4 | 5 | 95% CL for 5 | HRD20 | 95% CL for HRD20 | |
|---|---|---|---|---|---|---|---|---|
| Allb | Events / At risk | 2327 | 2323 | 2325 | 2326 | |||
| First CV Event | 3332 | 1.26 | 1.48 | 1.59 | 1.70 | 1.52,1.90 | 1.224 | 1.175,1.275 |
| ma | 1.13 | 1.27 | 1.24 | 1.25 | 1.11,1.40 | 1.093 | 1.048,1.139 | |
| All death | 2928 | 1.25 | 1.40 | 1.65 | 1.67 | 1.48,1.89 | 1.238 | 1.185,1.294 |
| ma | 1.13 | 1.17 | 1.28 | 1.22 | 1.08,1.39 | 1.098 | 1.050,1.149 | |
| CV Death | 1368 | 1.48 | 1.86 | 2.11 | 2.21 | 1.83,2.66 | 1.363 | 1.275,1.458 |
| ma | 1.27 | 1.51 | 1.55 | 1.48 | 1.21,1.79 | 1.168 | 1.090,1.252 | |
| Menb | Events / At risk | 1131 | 1132 | 1132 | 1131 | |||
| First CV Event | 1936 | 1.33 | 1.62 | 1.60 | 1.84 | 1.58,2.13 | 1.238 | 1.176,1.304 |
| ma | 1.24 | 1.44 | 1.32 | 1.42 | 1.21,1.65 | 1.123 | 1.065,1.184 | |
| All Death | 1715 | 1.36 | 1.55 | 1.62 | 1.89 | 1.62,2.22 | 1.255 | 1.188,1.327 |
| ma | 1.25 | 1.35 | 1.29 | 1.39 | 1.18,1.64 | 1.116 | 1.055,1.180 | |
| CV Death | 823 | 1.66 | 2.11 | 2.09 | 2.57 | 2.01,3.27 | 1.394 | 1.282,1.516 |
| ma | 1.49 | 1.82 | 1.63 | 1.80 | 1.40, 2.33 | 1.212 | 1.112,1.323 | |
| Womenb | Events / At risk | 1195 | 1192 | 1197 | 1193 | |||
| First CV Event | 1396 | 1.05 | 1.21 | 1.54 | 1.43 | 1.21,1.70 | 1.187 | 1.111,1.269 |
| ma | 0.99 | 1.01 | 1.17 | 1.08 | 0.90,1.30 | 1.046 | 0.976,1.121 | |
| All Death | 1213 | 1.12 | 1.14 | 1.59 | 1.45 | 1.21,1.75 | 1.207 | 1.123,1.298 |
| ma | 1.05 | 0.98 | 1.20 | 1.13 | 0.93,1.37 | 1.067 | 0.991,1.149 | |
| CV Death | 545 | 1.23 | 1.37 | 1.95 | 1.74 | 1.30,2.33 | 1.295 | 1.157,1.450 |
| ma | 1.10 | 1.15 | 1.33 | 1.25 | 0.92,1.69 | 1.094 | 0.974,1.228 |
Where 95% confidence limits >1.0, hazard ratios are printed bold. HRD20 is per 20-nmol/l decrease in 25OHDadj (∼ one standard deviation).
HRD, hazard ratio decrease; CL, confidence limits.
aAge, sex, family history of coronary heart disease, Scottish Index of Multiple Deprivation, tobacco smoking dose, total cholesterol and HDL cholesterol. BMI did not contribute significantly to the ASSIGN score, nor did adding it in here make any difference.
bFirst fifth(1) omitted as HR = 1.0. For sexes combined, n = 2327, men n = 1132, women n = 1193. Cutpoints for centiles 80, 60, 40, 20 sexes combined are: 53.84, 42.25, 34.54, 27.12 nmol/l, respectively; men: 56.53, 45.16, 36.66, 28.92 nmol/l, respectively; women: 50.64, 39.66, 32.73, 25.79 nmol/l, respectively. For full ranges and means see Supplement Table W3, available as Supplementary data at IJE online.
There was some interaction by sex, significant for cardiovascular deaths. The gradient of risk for women is shallower than for men despite lower 25OHDadj values. The modest increase in risk, in results adjusted for age and sex alone, is attenuated by ∼ 60% after multiple adjustment, losing statistical significance in women despite good numbers. There was no evidence of a threshold, confirmed by spline analyses (not shown). There was no increase in risk associated with the highest 25OHDadj values (see Supplement Table W4, available as Supplementary data at IJE online). Linearity was thus modelled by analyses of hazard ratios for a 20-nmol/l decrease (HRD20) in 25OHDadj (the standard deviation was 18.7 nmol/l). Very similar hazard ratios for secondary endpoints (see Supplement Table W5, available as Supplementary data at IJE online) suggest lack of specificity in the apparent vitamin D effect. These effects were, again, strongly attenuated following multiple adjustment.
Most ASSIGN risk factors showed a significant linear relationship with 25OHDadj. The ASSIGN score for cardiovascular risk was 25% higher in the final fifth of 25OHDadj than in the first (see Supplement Table W6, available as Supplementary data at IJE online), consistent with the impact of ASSIGN factors in multiple adjustment.
In analyses of discrimination and net reclassification improvement for First Cardiovascular Event, sexes combined and separately, changes in the c statistic and the NRI were small, with confidence intervals straddling zero (Supplement Table W7, available as Supplementary data at IJE online).
Vitamin D, measured as 25OHDadj, appears to be a modest, non-specific predictor of many endpoints. Its effect is largely attenuated by competing risk factors. It has no independent effect in predicting First Cardiovascular Events beyond that of established ASSIGN risk factors, and would not therefore contribute significantly to cardiovascular risk assessment in a multifactorial risk score.
Discussion
Neglect of the hypothesized link of seasonal sunlight to cardiovascular disease and death is overdue for correction. It opens a new window on the vitamin D debate. Mendelian randomization studies7 examine fixed differences in levels. Clinical trials raise levels, often by medication, in compliant volunteers.3–6 Cohort studies accumulate follow-up data (perhaps to be examined differently after this).1,2 Meanwhile a huge natural experiment, whose ‘protocol’ is dictated by Earth’s 23.4° axis deviation and speed of angular rotation, annually raises blood levels but then lowers them to levels research ethicists might veto. Our novel analyses and results raise questions for answering, as follows.
Baseline recruitment 25OHDraw
Serial single assays across the 13 224 participants were used to describe the presumed monthly cycle of change over both the years of recruitment and following decades. Results are consistent with others, including the delays from the solstices to peak and trough values; but, with Scotland’s high latitude, the two-to-one oscillation may be unusually large.12,13 Similarity of those recruited at different times is shown by consolidated results of the two contrasting seasons in the Scottish districts. These show trivial differences in mean risk factor levels, excepting an obvious separation in mean 25OHDraw which predictably closes with seasonal adjustment to 25OHDadj.
Seasonally adjusted 25OHDadj
As have others, we adjusted seasonally unstable 25OHDraw readings to an estimated longer-term ranking within the SHHEC cohort: 25OHDadj. Without adjustment, the seasonal variation is extreme (see Supplement Figure 1 showing winter and summer values, available as Supplementary data at IJE online). The 50th centile reading for men in January–March of 28.8 nmol/l is equal to the 6th centile in July–September. Contrast the ‘LowD’ half of the population of 25OHDadj coming very evenly from different recruitment calendar months, with 25OHDraw at the same cutpoint: 52% of participants from January–March were LowD for 25OHDadj but 75% for 25OHDraw; whereas for July—September, the figures were 50% and 20%, respectively. Unadjusted readings are confounded by date of sampling (also true for uncorrected clinical samples). Because the seasonal swing in values is unlikely to be uniform and will vary with the amount of sunshine in a given year and with personal exposure, 25OHDadj can only be an estimate of ranking. Increased precision would necessitate multiple measurements, and even a long-term tendency would be affected by changing lifestyle, for example from inability to venture outdoors.
Single measurements as a long-term predictor: tracking
That 25OHD appears so unstable but nonetheless appears predictive suggests long-term stability. Both 25OHDraw and 25OHDadj are predictive in Cox proportional hazards analyses. The latter is a better predictor, but the difference is not great. External literature confirms ‘tracking’ over time.4,29 Fortuitously, independent random sampling in North Glasgow for MONICA surveys at 3-year intervals sampled 35 people twice. Both 25OHDraw and 25OHDadj values were more repeatable than other risk factors, excluding height (see the scatter plot, Supplement Figure 2, available as Supplementary data at IJE online). The value of 25OHDadj as a predictor is further demonstrated in Supplement Tables (available as Supplementary data at IJE online). Table W8 shows that the LowD vs HighD event ratio during follow-up appears similarly predictive whether originally derived from Low-D season or High-D season measurements, and Table W9 that the LowD vs HighD event ratio persists during successive 5-year periods of follow-up.
Long-term prediction from single measurements is a challenge to many cohort studies, not unique to 25OHD in SHHEC. What is unusual is that the measurement is unstable over a period of months, but must go back on track again despite these seasonal oscillations. Our current analyses exploit both phenomena. No other risk factor shows such extraordinary two-to-one seasonal variation.
Pre-analytical and laboratory processing
Serum specimens analysed for 25OHD appear robust to handling.24 Our archived frozen specimens, kept for decades for analysis, were run off in two batches between August 2009 and February 2012 with a 17-month gap. The two (3511 and 9713) had similar descriptors and no evidence of laboratory drift. By defining population halves and fifths both separately and together, we found negligible effects on risk calculations. Use of multivitamin tablets by SHHEC recruits was uncommon (see Supplement Addendum Table A4, available as Supplementary data at IJE online), so the ARCHITECT assay’s comparative lack of sensitivity to 25OHD2 was immaterial. Official laboratory lower limits did however cut across the SHHEC cohort, with 9.9% below the European standard of 20 nmol/l and 40.7% below the more stringent US Food and Drug Administration (FDA) limit of 32.5 nmol/l, higher percentages of 25OHDraw occurring in winter.11,25 Our Supplement Figure 2, the analyses themselves and listing of the centiles for the assays in Supplement Table W10 (available as Supplementary data at IJE online), raise no questions about the validity of these low readings for our epidemiological purposes. The 9.9% would involve part only of the bottom population fifth (numbered 5 in the Cox model tables), the reference fifth, number 1, HR 1·0, being the highest 20%. Readings below laboratory limits do not necessarily imply disease.
Missing data
The 13 224 SHHEC participants were those with serum available in 2009 when the new assay kits arrived at the Mainz laboratory. Recruitment in 1984–95 had numbered 18 107, a 64% response rate.21 Virtually complete data on 16 000 were sent to Helsinki for the MORGAM cohort collaboration in 2000. In 2007, 14 751 serum specimens were sent to Mainz for the biomarker substudy.17–19 Sequential losses were predominantly from non-availability of serum—first refusal or failure of venepuncture at recruitment (during an HIV needle scare) and later depletion in laboratory studies. Completeness of data in the 13 224 exceeds 99%, the exception being HDL-cholesterol at 97.3%. Record linkage depends on a death or hospital record and cumulated during follow-up to 91.2%; no link means either loss to follow-up or continuing good health.22 The 13 224 with 25OHD measurements were comparable to the 4883 without in age, sex, social deprivation, risk factors and endpoint risk. (STROBE statement is in Supplement Table W11, available as Supplementary data at IJE online.)
Winter morbidity and mortality, other explanations
Excess winter mortality has declined since 1981. Related to factors other than vitamin D—home heating, influenza, respiratory disease and social conditions—they do not necessarily exclude it.30 Short-term meteorological and air pollution precipitants of cardiovascular disease contribute.31,32 With excess mortality in northern countries beginning in December, Healy33 found the least excess among 14 European countries was in Finland; winter excess correlated negatively with latitude—contrary to the seasonality hypothesis (r = -0.68, P < 0.01).14,33 Data for deaths (all ages and causes) registered by calendar month and year in Scotland over the years 1989–2009 confirm that the winter peak each year is in December or January, therefore preceding the SHHEC trough of 25OHD in March. The summer low trough in deaths varied from May to September34 (Supplement Table W12, available as Supplementary data at IJE online). SHHEC data on monthly incidence of cardiovascular events and deaths need replication from studies elsewhere.
Conclusion
Our conventional Cox proportional hazard model analyses show 25OHDadj to be a modest non-specific predictor of many primary and secondary endpoints, the effect being strongly attenuated by adjustment for risk factors in the ASSIGN cardiovascular risk score.23 Strength and gradient are not marked: findings could result from residual confounding, or alternatively from over-adjustment. It does not improve prediction of First Cardiovascular Event over and above ASSIGN variables, an obligatory question for the MORGAM Biomarker and BiomarCaRE studies.17–19
Seasonality analyses show that a two-to-one annual oscillation in population 25OHD has no apparent impact on incidence of First Cardiovascular Events, and follows rather than precedes the peak in All Deaths and Cardiovascular Deaths. Winter appears to influence fatality rather than incidence. There was no disproportionate susceptibility to endpoint events in the low-vitamin-D months in those ranked as having low vitamin D, as predicted by the concept of hypovitaminosis D. These results were consistent with the absence of a demonstrable 25OHD threshold both in spline analyses and in the quasi-linear gradient of increasing risk with declining levels in the Cox model fifths. Our 25OHD readings were low, many below laboratory limits; but with no visible threshold for disease in SHHEC, we cannot estimate the prevalence of ‘hypovitaminosis D’ in the Scottish population, a concept originating with rickets and osteomalacia. Others may do so.13,14
In this study, 25OHD failed two out of three of our postulate testings and was borderline for the third. Bradford Hill criteria for causality were found wanting for specificity, temporality, strength and gradient.16 Although not excluding causation completely—there could be a modest delayed effect integrated over years—our results question the seasonal hypothesis of vitamin D, and therefore challenge its alleged causal role. They need replication in other studies.
Supplementary data
Further tables and figures are available as specified in the text. The Supplement also includes an addendum of descriptive epidemiology of 25OHD in SHHEC covering means by age and sex (Table A1), by year of recruitment (Table A2), in relation to lifestyle factors (Table A3) and diet habits from a food frequency questionnaire (Table A4).
Funding
Scottish Heart Health Extended Cohort (SHHEC): Scottish Health Department Chief Scientist Organization; British Heart Foundation; FP Fleming Trust.
MORGAM: European Commission Seventh Framework Programme FP7/2007-2013 [HEALTH-F4-2007-2014113, ENGAGE; HEALTH-F3-2010-242244, CHANCES].
MORGAM Biomarker Study (Serum biomarkers in the MORGAM Populations): Medical Research Council London [G0601463, No 80983].
BiomarCaRE (Biomarkers for Cardiovascular Risk Assessment in Europe): European Commission Seventh Framework Programme FP7/2007-2013 [HEALTH-F2-2011-278913].
Funding bodies had no role in the planning of the study, analyses, interpretation, writing, or publication of the manuscript, or recruitment of participants.
Supplementary Material
Acknowledgements
The MORGAM/ BiomarCaRE Biomarker laboratory in Hamburg (formerly Mainz) is directed by Stefan Blankenberg and headed by Tanja Zeller, whose team carried out the 25-hydroxyvitamin D measurements. The Scottish Heart Health Extended Cohort (SHHEC) is a national and international resource made possible by thousands of volunteer participants, medical practitioners and others in public health medicine.
Conflict of interest: Abbott Diagnostics provided test reagents for 25-hydroxyvitamin D determinations within the frame of the MORGAM Biomarker Study and the BiomarCaRE project. The authors do not report any conflict of interest. M.W. is a consultant for Novartis and AMGEN.
References
- 1.Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-Hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol 2012;32:2794–802. [DOI] [PubMed] [Google Scholar]
- 2.Tomson JT, Emberson J, Hill M, et al. Vitamin D and risk of death from vascular and non-vascular causes in the Whitehall study and meta-analyses of 12,000 deaths. Eur Heart J 2013;34:1365–74. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury R, Kunutsor S, Vitezova A, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014:348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 Trial (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 2012;33:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnish Vitamin D Trial (FIND). https://www.uef.fi/fi/nutritionepidemiologists/find2 (5 February 2015, date last accessed).
- 7.Afzal S, Brøndum-Jacobsen P, Benn M, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: mendelian randomisation analysis in three large cohorts . BMJ 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scragg R. Vitamin D and public health: an overview of recent research on common diseases and mortality in adulthood. Public Health Nutr 2011;14:1515–32. [DOI] [PubMed] [Google Scholar]
- 9.Annuzzi G, Della Pepa G, Vetrani C. Vitamin D and cardiovascular disease: is there evidence to support the bandwagon? Curr Atheroscler Rep 2012;14:525–34. [DOI] [PubMed] [Google Scholar]
- 10.Sattar N, Welsh P, Panarelli M, Forouhi NG. Increasing requests for vitamin D measurements: costly, confusing and without credibility. Lancet 2012;379:95–96. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann M. The measurement of 25-hydroxy vitamin D–an analytical challenge. Clin Lab Med 2012;50:1873–75. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) serum seasonality in the United States . PLoS One 2013;8:e65785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007;85:860–68. [DOI] [PubMed] [Google Scholar]
- 14.Scragg R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int J Epidemiol 1981;10:337–41. [DOI] [PubMed] [Google Scholar]
- 15.Tunstall-Pedoe H, Woodward M, Tavendale R, A’Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ 1997;315:722–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford Hill A. The environment and disease: association or causation. Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankenberg S, Zeller T, Saarela O, et al. ; for the MORGAM Investigators. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation 2010;121:2388–97. [DOI] [PubMed] [Google Scholar]
- 18.Zeller T, Tunstall-Pedoe H, Saarela O, et al. ; for the MORGAM Investigators. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker project Scottish cohort. Eur Heart J 2014;35:271–81. [DOI] [PubMed] [Google Scholar]
- 19.BiomarCaRE. Biomarkers for Cardiovascular Risk Assessment in Europe. http://www.biomarcare.eu/ (5 February 2015, date last accessed). [Google Scholar]
- 20.Tunstall-Pedoe H, ed; for the WHO MONICA Project. MONICA Monograph and Multimedia Sourcebook. Geneva: World Health Organization; 2003. [Google Scholar]
- 21.Smith WCS, Crombie IK, Tavendale R, Irving JM, Kenicer MB, Tunstall-Pedoe H. The Scottish Heart Health Study: objectives and development of methods. Health Bull (Edinb) 1987;45:211–17. [PubMed] [Google Scholar]
- 22.Kendrick S, Clarke J. The Scottish Record Linkage System. Health Bull (Edinb) 1993;51:72–79. [PubMed] [Google Scholar]
- 23.Woodward M, Brindle P, Tunstall-Pedoe H; for the SIGN group on risk estimation. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2007;93:172–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wielders JP, Wijnberg FA. Preanalytic stability of 25(OH)-vitamin D3 in human blood or serum at room temperature: solid as a rock. Clin Chem 2009;55:1584–85. [DOI] [PubMed] [Google Scholar]
- 25.Cavalier E, Carlisi A, Bekaert AC, Rousselle O, Chapelle JP, Souberbielle JC. Analytical evaluation of the new Abbott Architect 25-OHvitamin D assay. Clin Biochem 2012;45:505–08. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–46. [PubMed] [Google Scholar]
- 27.Newsom RB. Comparing the predictive power of survival models using Harrell’s C or Somers’ D. Stata J 2010;10:339–58. [Google Scholar]
- 28.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 2010;171:903–08. [DOI] [PubMed] [Google Scholar]
- 30.The Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet 1997;349:1341–46. [PubMed] [Google Scholar]
- 31.Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Strachan DP. Air pollution and daily mortality in London 1987–92. BMJ 1996;312:665–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnett AG, Dobson AJ, McElduff P, Salomaa V, Kuulasmaa K, Sans S; for the WHO MONICA Project. Cold periods and coronary events: an analysis of populations worldwide. J Epidemiol Community Health 2005;59:551–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Healy JD. Excess winter mortality in Europe: a cross-country analysis identifying key risk factors. J Epidemiol Community Health 2003;57:784–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.http://www.gro-scotland.gov.uk/statistics/theme/vital-events/general/weekly-monthly-births-death-data/monthly-deaths.html (5 February 2015, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.