Abstract
Background: Despite differences in body shape and adiposity characteristics according to sex and age, a single range of healthy weight [body mass index (BMI, kg/m2) of 18.5–24.9) regardless of sex and age has been recommended. The aim of the study is to examine whether the association between BMI and all-cause mortality varies by sex and age, and, if relevant, to estimate sex-age-specific optimal BMIs associated with a minimal risk of death.
Methods: A total of 12 832 637 Korean adults aged 18–99 years who participated in health examinations during 2001–04 were followed up until 2013. Hazard ratios of death in sex-age groups were calculated using Cox regression models after adjustment for age, smoking status and known pre-existing illness.
Results: During follow-up, 456 175 men and 241 208 women died. Among men, the age-specific optimal BMI was 23.0–25.9 (kg/m2) at 18–34 years, 24.0–27.9 at 45–54 year, and 25.0–28.9 at 65–74 years. Among women, it was 15.5–24.9 at 18–34 years, 21.0–26.9 at 45–54 years and 24.0–28.9 at 65–74 years. Patterns of sex-age-specific association generally did not differ between never-smokers with no known illness and all participants. Progressively increased risks above and below sex-age-specific optimums were observed (reverse J-curve). Smoking had a limited impact on the observed associations.
Conclusions: Women had a lower optimal BMI than men, especially at younger ages. The optimal BMI increased with age. Change in optimal BMI with age, however, was more profound in women than in men. Sex-age-specific optimums were generally higher than the current normal weight (BMI of 18.5–24.9), except in women below 50 years. Sex-age-specific guidelines related to body weight may be needed to guide people for better health.
Keywords: Age factors, Asians, body mass index, Koreans, mortality, sex factors
Key Messages
The optimal BMI with a minimal mortality is lower in women than in men, especially at younger ages.
The optimal BMI increases with age in both women and men, and change in optimal BMI with age is more extreme in women than in men.
Sex-age-specific optimal BMI is higher than the current normal weight (BMI of 18.5–24.9) in Korean adults, except in women below 50 years.
The effect of smoking on the association between BMI and all-cause mortality may be substantially modified by age.
Sex-age-specific guidelines related to body weight may be needed to guide people for better health.
Future research on body weight in relation to health outcomes with careful consideration of participants' sex and age is recommended.
Introduction
Being overweight or obese has been linked with diabetes, heart disease, stroke and some cancers. However, the relationship between body mass index (BMI; weight in kilograms divided by square of height in metres; kg/m2) and all-cause mortality remains controversial.1–7 A recent systematic review2 has indicated that being overweight may modestly decrease the risk of death, and that grade I obesity (BMI of 30–34.9) may not increase the risk of death. However, human body shape and adiposity characteristics differ according to sex and age.8–11 The concept of an age- and sex-independent desirable BMI suggested by the World Health Organization(WHO)12 and the National Heart, Lung, and Blood Institute (NHLBI)13 has been supported by several studies,3,7,14 except perhaps in the older elderly.15
Through a large prospective cohort study that included 12.8 million participants, we set out to elucidate whether the association between BMI and all-cause mortality varies by sex and age and, if relevant, to estimate sex-age-specific optimal BMIs associated with a minimal risk of death. If a sex-age-specific association between BMI and mortality exists, precise sex-age-specific estimates of the relative risk of death associated with BMI can help to inform decision making in the clinical and public health settings. The effect of smoking status on the association between BMI and mortality was also examined.14 In this study, the NHLBI’s terminology for BMI categories of underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9) and obesity [≥30, and grade I obesity (30–34.9)] was used.13
Methods
Ethics approval
All health examination data are collected and maintained by the NHIS in compliance with several Korean laws. Data were anonymized for the analysis and provided to the authors by the NHIS. Data were only available through a specific computer within the NHIS headquarters. Ethics approval was sought for analysis of anonymized data, and this was approved by Institutional Review Board of Kwandong University (Gangneung, Republic of Korea).
Study population
The National Health Insurance Service (NHIS) provides mandatory universal health insurance that covers 97% of the Korean population. A total of 12 845 017 eligible beneficiaries of the NHIS, aged 18–99 years, participated in health examinations during 2001–04; 8505 individuals with missing information about BMI were excluded, as were 964 individuals with body weight below 30 kg, 1870 individuals with short stature (less than 1.30 m among those below 55 years, and less than 1.10 m among those aged 55 years and above), 831 individuals with a BMI of 50 kg/m2 or more and 210 individuals with missing information about the date of the health examination. The final study population included 12 832 637 participants. Of these, 1 548 621 (12.1%) were enrolled in 2001, 5 378 891 (41.9%) in 2002, 3 557 557 (27.7%) in 2003 and 2 347 568 (18.3%) in 2004.
Follow-up and ascertainment of deaths
Deaths of participants until 31 December 2013 were ascertained using the NHIS database of beneficiary status as of 29 May 2014, in which information regarding participants’ deaths was derived from the Resident Register of Korea. Follow-up was complete for all participants.
Data collection
Age at enrolment was calculated using birth year and year at health examination. Weight and height were measured to the nearest 1 kg or 1 cm, respectively, examinees wearing light clothing without shoes. Participants self-reported smoking status and known pre-existing illness either cured or not (including cardiovascular disease, cancer, liver disease, diabetes, a respiratory disease or other disease) through a questionnaire. Information on smoking status (95.0%) and pre-existing illness (99.2%) was available for most participants (Table S1, available as Supplementary data at IJE online). Health examination and data collection were performed using a standard protocol, the Health Examination Practice Guide (Korean; http://www.law.go.kr/admRulLsInfoP.do?chrClsCd=&admRulSeq=2200000012541) publicly released by the Ministry of Health and Welfare.
Statistical analysis
BMI values were categorized into 18 groups [<16.0, 16.0–17.4, 17.5–18.9, 19.0–19.9, 20.0–20.9, 21.0–21.9, 22.0–22.9, 23.0–23.9, 24.0–24.9 (reference for women), 25.0–25.9, 26.0–26.9 (reference for men), 27.0–27.9, 28.0–28.9, 29.0–29.9, 30.0–31.4, 31.5_32.9, 33.0–34.9, ≥ 35.0] and into a further 11 groups [< 17.5, 17.5–18.9, 19.0–20.4, 20.–21.9, 22.0–23.4, 23.5–24.9 (reference for women), 25.0–26.4 (reference for men), 26.5–27.9, 28.0–29.4, 29.5–30.9, ≥ 31.0] or seven groups [< 18.5, 18.5–20.9, 21.0–22.9, 23.0–24.9 (reference for women), 25.0–27.4 (reference for men), 27.5–29.9, ≥ 30.0] for subgroup analyses. Sex-specific reference BMIs were selected based on preliminary analysis that showed that these ranges best represent changes in the curvilinear association with age.
Cox proportional hazards models were used to calculate hazard ratios after adjustment for age at enrolment (continuous variable), sex, smoking status (current smoker, former smoker, never smoker, and those with missing information), and known illness [yes and no (including missing information)]. Metabolic mediators of the effects of BMI such as blood pressure, serum cholesterol and glucose were not included in the analysis,16 to show the full effects of BMI on mortality.2,17 Analyses were performed mainly in sex-age groups. Age at enrolment was categorized for various analyses into 10 groups (years: <35, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, ≥ 75), six groups [<35, 35–44, 45–54, 55–64, (45–49, 50–64 for women), 65–74, ≥ 75], and three groups (<45, 45–64, ≥ 65). Stratified analysis by smoking status was performed both with and without sex-age stratification to examine the effect modification by smoking status. The effect modification between the BMI and age, sex and smoking status (current vs never smokers), one variable at a time,was assessed by introducing interaction terms (linear and quadratic) assuming quadratic association between BMI and mortality. Subgroup analyses among participants (or never smokers) with no known illness (excluding missing information) and/or excluding the first 5 years of deaths were also performed to address possible issues of reverse causality (such that weight loss or gain are a consequence of underlying conditions that lead to death).17,18 BMI was further classified into four (or five) standard categories [<18.5, 18.5–24.9, 25.0–29.9, ≥ 30 (30–34.9, ≥ 35)] for between-study comparisons.2,19 Subgroup analysis and analysis with different BMI categories served as a sensitivity analysis.
Apparent optimal and acceptable ranges of BMI were determined by general inspection of the curvilinear association. In general, the ranges with an excess risk below 5%, relative to the lowest potential risk (the lowest unweighted geometric mean of hazard ratios in three consecutive body mass index categories) were considered the optimal ranges, whereas the ranges with an excess risk below 15%, relative to the potential lowest risk, were deemed the acceptable ranges for each sex-age group.
A uniformly sex-age-standardized death rate per 100 000 person-years (namely, the simple mean of the applicable sex-age-specific rates in 28 sex-age groups at ages 18–24 years, 25–29 years and up to 85 years or older, increasing by 5 years per group, based on age attained during follow-up) was calculated for each BMI category.
All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
During the follow-up period of 9–13 years (135.1 million person-years), 456 175 men and 241 208 women died. At baseline, the average (SD) age was 44.4 (14.2) years and the average BMI was 23.5 (3.2). Of the participants, 56.9% were men and 58.0% were never smokers (Table S1, available as Supplementary data at IJE online). The proportions of underweight and obesity were 4.4% and 2.8%, respectively.
Sex-age specific association between BMI and mortality
In both men and women at all ages combined, the highest and the lowest categories of BMI had the highest mortality risk (reverse J-curve). All-cause mortality was lowest at a BMI of 25.0–27.9 in men and at 24.0–27.9 in women (Figure S1a, available as Supplementary data at IJE online).
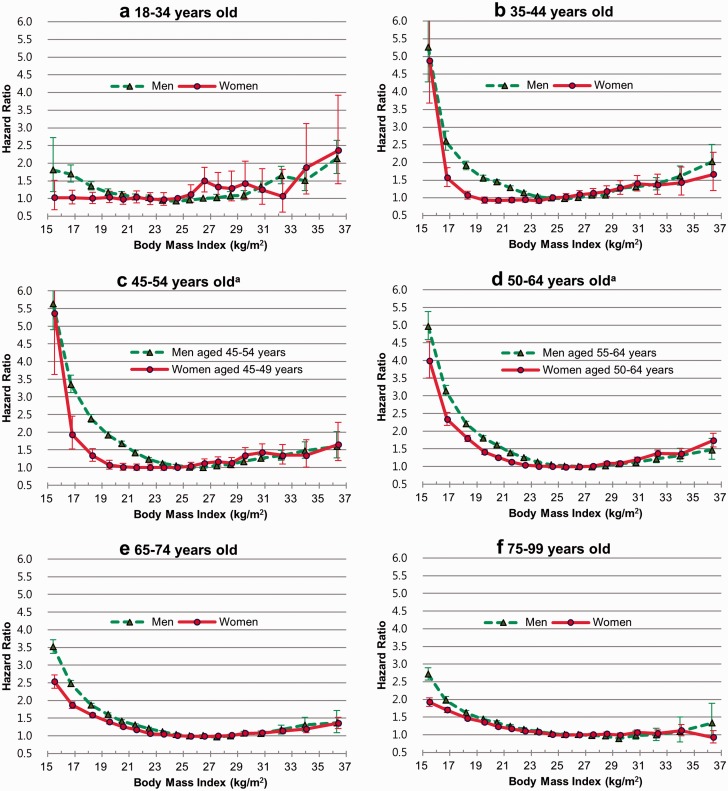
In the sex- and age-stratified analysis, the hazard ratio of the lowest categories of BMI was the lowest at ages below 35 years, the highest at 45–64 years in men and at 50–64 years in women, and decreased with age in those aged 65 years and above (Figure 1; Table S2, Figures S2, S3, available as Supplementary data at IJE online). In general, the younger the age, the higher was the hazard ratio of the highest categories of BMI in both men and women (Figure 1; Figures S2, S3). The P-values for interaction by age were < 0.0001 in both men and women, and those by sex were < 0.0001 in all six age groups.
Figure 1.
Sex-age-specific hazard ratios of risk of death associated with body mass index. Hazard ratios were calculated using Cox proportional hazards models; 18 categories of body mass index (reference: 26.0–26.9 for men and 24.0–24.9 for women) were used. The midpoint was used as a representative value for each body mass index category, except for both ends (15.5 and 36.4) for which the median of all participants was used. The analyses were adjusted for age, smoking status and known pre-existing illness. aMen and women have different age groups. Panel c. Men aged 45–54 years, women aged 45–49 years. Panel d. Men aged 55–64 years, women aged 50–64 years. Results of women aged 45–54 years and 55–64 years are presented in Table S2. The P-values for interaction between BMI and sex were < 0.0001 in all age groups.
As people aged, the optimal BMI tended to increase a bit. In men, the optimal BMI range with a minimal risk of death was 23.0–25.9 at 18–34 years and 25.0–28.9 at 65–74 years (Table 1, Figure 1; Figure S2). In women, the optimal BMI was 15.5–24.9 at 18–34 years and 24.0–28.9 at 65–74 years (Table 1, Figure 1; Figure S3). Women below 16 kg/m2 did not have a higher risk of death at 18–34 years (Figure 1a). The change in the optimum BMI with age from 45–49 to 50–54 years was more extreme than from 50–54 years to 60–64 years in women (Figure S3b, c). Women had a lower optimal BMI than men, especially at younger ages (Figure 1).
Table 1.
| Men |
Women |
||||
|---|---|---|---|---|---|
| Age (years) | Optimal BMIb | Acceptable BMIb | Age (years) | Optimal BMIb | Acceptable BMIb |
| 18–34 | 23.0–25.9 | 21.0–28.9 | 18–34 | 15.5–24.9c | 15.5–25.9c |
| 35–44 | 23.0–26.9 | 22.0–28.9 | 35–44 | 19.0–23.9 | 17.5–25.9 |
| 45–54 | 24.0–27.9 | 23.0–28.9 | 45–49 | 20.0–25.9 | 19.0–26.9 |
| 50–54 | 22.0–26.9 | 21.0–27.9 | |||
| 55–64 | 24.0–28.9 | 23.0–31.4 | 55–64 | 23.0–27.9 | 22.0–29.9 |
| 65–74 | 25.0–28.9 | 23.0–31.4 | 65–74 | 24.0–28.9 | 22.0–31.4 |
| 75–99 | 25.0–32.9 | 24.0–34.9 | 75–99 | 24.0–29.9 | 22.0–36.4d |
aGenerally, the ranges with an excess risk below 5%, relative to the lowest potential risk (the lowest unweighted geometric mean of hazard ratios in three consecutive body mass index categories), were considered the optimal ranges, whereas the ranges with an excess risk below 15%, relative to the lowest potential risk, were deemed the acceptable ranges for each sex-age group. For example, in men aged 18–34 years, the lowest potential risk was 0.94 [the geometric mean of hazard ratios at BMI of 23–23.9 (hazard ratio = 0.94), 24–24.9 (0.93), and 25–25.9 (0.96); Table S2]. The relative hazard at 26–26.9 kg/m2 (hazard ratio = 1.0) was 1.06 (1.0/0.94), and the excess risk was 6%. Thus, in men aged 18–34 years, a BMI of 26–26.9 kg/m2 was considered the acceptable range.
bBMI: body mass index (weight in kilograms divided by the square of height in metres; kg/m2). Weight and height were measured while examinees wore light clothing without shoes.
cThe lower end (15.5) of the range is the median of the body mass index in the lowest body mass index category (< 16) among all participants.
dThe higher end (36.4) of the range is the median of the body mass index in the highest body mass index category (35.0–50.0) among all participants.
Confounding by smoking and reverse causality
In analyses with all ages combined excluding those participants with known illness or death within 5 years after health examination, the results were generally unchanged, except for a small decrease in the hazard ratios in the lowest BMI categories (Figure S1c-f). In men, the hazard ratio associated with low BMI was similar between current smokers and never smokers (Figure S4c, available as Supplementary data at IJE online), whereas in women it was lowest in current smokers (Figure S4e). Hazard ratios related to lower than optimal BMI were higher in the analyses in never smokers with no known pre-existing illness than in never smokers among all participants (including those with known illness; Figure S4b, d, f,vs S4a, c, e).
Sex-age-specific optimal BMIs estimated in never smokers were generally similar to those in all participants (Table S3 and Figure S5, available as Supplementary data at IJE online). Compared with those of all participants, patterns of sex-age-specific association generally did not differ among never smokers with no known illness or never smoker-survivors after 5 years of follow-up with no known illness, except for a small decrease in the hazard ratios in the lower BMI categories. Sex-age-specific optimal BMIs in these groups seemed to be slightly lower than those in all participants (Figures S6, S7, available as Supplementary data at IJE online).
Effect modification by smoking
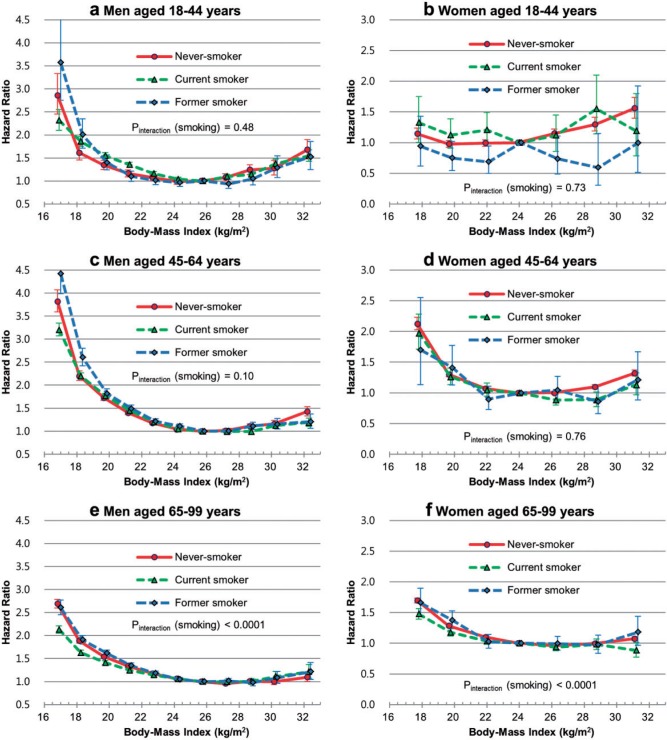
The association between BMI and mortality was modified by smoking status; however, hazard ratios associated with BMI according to smoking status in themselves were substantially modified by age and sex (Figure 2; Figures S4 and S8, available as Supplementary data at IJE online). In general, hazard ratios associated with lower than optimal BMI in never smokers were low at ages below 45 years, were similar at 45–64 years and were high at 65 years and above, compared with the results in current smokers, among both men and women (Figure 2; Tables S4, S5, available as Supplementary data at IJE online). However, effect modification by former smoking was inconsistent between sex-age groups.
Figure 2.
Age-specific hazard ratios of risk of death associated with body mass index according to sex and smoking status. Hazard ratios were calculated using Cox proportional hazard models; 11 categories of body mass index for men (reference: 25.0–26.4) and 7 categories for women (reference: 23.0–24.9) were used. The midpoint was used as a representative value of each body mass index category, except for both ends [16.9 and 32.3 for men (due to 11 groups); 17.8 and 31.2 for women (7 groups)], for which the median of all participants was used. The analyses were adjusted for age and known pre-existing illness. Pinteraction (smoking) = P-value for interaction between BMI and smoking (current vs never smoker).
Discussion
In our large cohort study of more than 12.8 million participants with information on measured BMI, we found that the optimal body weight relative to height for a minimal risk of death varied by sex and increased with age.5 Observed sex-age-specific optimums were generally higher than the current normal weight, except in women below 50 years. Women had lower age-specific optimums than men, especially at younger ages. In general, the highest and the lowest categories of BMI had the highest mortality risk (reverse J-curve).
Relationship to previous studies
The estimated optimal ranges at all ages combined were similar between men and women, in accordance with previous research.3,7,14 Results at all ages combined, however, did not fully reflect sex-age-specific optimums in the current study. Large cohort studies that examined a relatively narrow age range,4,20 showed similar results to the current study, when considering the ages and BMI ranges covered. For example, the relative risks among all women in the Nurses’ Health Study20 were similar to the results among women aged 35–49 years in the current study.
Although there have been several attempts to highlight the sex-age-specific impact on the association between BMI and the risk of death,5,14,15 previous evidence for a sex-age-specific association has been less than convincing. We suggest that previous studies have not been able to reveal clear sex-age-specific associations due, mainly, to small numbers of participants, especially at younger ages,21 and due to examining a mixture of populations that may have varying risk associated with BMI according to follow-up times and chronological periods,1,3,5,22 and due to excessively long periods of follow-up that may not reflect weight change with age and period.5,17
The findings of Flegal and colleagues2 (that being overweight may modestly decrease mortality and that grade I obesity may not increase mortality) seem to result from using the current normal weight as the reference, and a lack of detailed consideration of age (Tables S6, S7, available as Supplementary data at IJE online). Our results clearly showed that grade I obesity (BMI of 30–34.9) is related to higher mortality among people below 75 years compared with age-sex-specific optimums (Figure 1). However, compared with the current normal weight, grade I obesity was related to higher mortality among men below 45 years and women below 65 years. Our results also showed that being overweight is associated with higher mortality in women below 45 years compared with the current normal weight. The risk associated with overweight and obesity compared with normal weight may be overestimated in the study by Flegal and colleagues,2 due to performing meta-analysis on studies mainly in US and European populations that include few individuals with low-normal weight.
Causality and possible mechanisms underlying sex-age-specific associations
The progressive increases in the risk associated with BMI below or above the optimum and the clear associations even at ages below 50 years, when there is a smaller possibility of comorbidity, support causal relationships. Additionally, as previously shown, common approaches to address the reverse causality and confounding by smoking did not sufficiently explain the risk in the current study.2,18 However, the observational design of the study limiting the drawing of definite causal inferences, and some of the observed excess mortality associated with BMI, may still be non-causal.
Deaths from respiratory diseases and some cancers, including lung and upper aero-digestive cancers, have been linked with excess death in the underweight, and vascular mortality has been considered to be mainly responsible for excess deaths in the overweight and obese.3,6,23 The increase in the risk associated with BMI above 26 kg/m2 in women aged below 35 years could be partly accounted for by breast cancer,21 the incidence of which increases sharply in women at around 25 years of age in Korea.24 A sharp increase in the lower end of the optimum BMI in women aged from 45–49 to 50–54 years may be explained by physiological changes related to menopause.25,26 Further cause-specific analysis in this population may address the detailed nature of the excess mortality. Those smokers most vulnerable to smoking-induced weight loss and subsequent death seem to quit smoking at younger ages in men.
Clinical implications
Being even mildly obese (BMI of 30–34.9) is associated with higher mortality for those below 75 years. However, our study shows that a little excess weight does not necessarily increase the risk of death, except for women below 50 years, in whom the current normal weight is generally associated with a minimal mortality. Although being underweight is generally deleterious, being a low-normal weight may also increase mortality in women aged 50 years and above, and in men at all ages. Gaining weight gradually and becoming overweight with age may not be particularly harmful for longevity in individuals with normal weight,27,28 especially at older ages (particularly in women).
Implications for research on adiposity
Age and sex could be the two most important effect modifiers on the association between BMI and mortality. The effect of smoking on the association was also substantially modified by age and, to a lesser degree, by sex in the current study. Common approaches to address reverse causality may introduce a bias in risk estimation due to a changing age distribution in the study population (e.g. an average of 54.2 and 42.7 years old for those with or without known illness, respectively, in the current study). Stratification by sex and age is strongly recommended for studies examining the optimal BMI in a population, in order to avoid a bias of shifting optimal weights downward (when younger people are included), or the higher end of optimal weights upwards (when older people are included). Furthermore, since various measures of adiposity are correlated for all sex-age groups,29 future research on measures of adiposity in relation to health outcomes with careful consideration of effect modification by sex and age is recommended.
Strengths and limitations of the study
The very large number of participants enabled us to calculate more precise estimates of sex-age-specific risks associated with measured BMI. Additional strengths are that our results can be applied to the current population because risks associated with BMI were calculated from a recently recruited population,5,22 and that the follow-up of deaths was complete.
This study also has several limitations. This study only examined all-cause mortality. Whereas adjustment for age, sex and smoking could be considered adequate,2,3 unadjusted confounding may affect the study results. Due to a low prevalence of obesity, estimated risks associated with obesity were focused on around the level of grade I obesity. Although self-reported smoking status collected at NHIS health examination has been used in various studies,6,30 data on self-reported smoking status and pre-existing illness might vary in quality, which could lead to residual confounding. The analysis based on a single measurement (BMI) might underestimating the true association.31 This study did not examine other measures of adiposity such as fat and lean mass and central obesity. These measures may provide information in addition to BMI on the association between body adiposity and mortality.32,33 Finally, our study participants being Korean may affect the generalizability to the global population.4,7 Sex-age-specific associations may need to be assessed in other populations. Koreans generally have a leaner body shape than do Whites. However, Korean populations have a similar longevity to residents of the UK and a higher longevity than residents of the USA as of 2012, according to 2014 Organization for Economic Co-operation and Development (OECD) health statistics.34
It is also worth noting that the study population is considered to be healthier and to have higher socioeconomic status than the general population of Korea because the study participants were volunteers for health examination and did not include the poorest people (around 3% of the population), who are covered by the Medical Aid rather than the National Health Insurance.
Conclusions
Among Korean adults, the association between BMI and mortality varied by sex and age, especially at younger ages. The optimal BMI with a minimal mortality was lower in women than in men. The optimal BMI increased with age in both women and men. Change in optimal BMI with age, however, was more extreme in women than in men. Observed sex-age-specific optimal BMI was generally higher than the current normal weight, except in women below 50 years. Grade I obesity was related to higher mortality among people below 75 years compared with sex-age-specific optimums, as generally was being overweight in women below 50 years. Many of those with a BMI below sex-age-specific optimums were at a greater risk of mortality than those with grade I obesity. These sex-age-specific associations may need to be confirmed in other populations. Sex-age-specific guidelines related to body weight may be needed to guide people for better health. Future research on body weight in relation to health outcomes with careful consideration of participants' sex and age is recommended.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Health examinations were funded and managed by the NHIS.There was no funding specific to the current work.This report is independent research using the data collected and maintained by the NHIS. The data were provided under the condition that the NHIS would be given opportunity to comment on the manuscript before submission for publication. The findings and views expressed in this publication are those of the authors and not necessarily those of the NHIS.
Supplementary Material
Acknowledgements
The authors thank the staff at the Big Data Steering Department at the NHIS for providing the data and support.
Specific author contributions: S.Y. conceived the study concept and design, analysed and interpreted data and wrote the first draft. S.Y., S.S. and J.Y. reviewed the literature. S.S., J.Y. and H.O. contributed to interpretation of results and critical revision of the manuscript. All authors have read and approved of the final submitted version of the manuscript. S.Y. is the study guarantor.
Conflict of interest: S.Y. had been paid a consulting fee and S.S. has been paid a salary by the NHIS during the conduct of the study. The authors have no other competing interests to declare.
References
- 1.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million White adults. N Engl J Med 2010;363:2211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763–78. [DOI] [PubMed] [Google Scholar]
- 5.Engeland A, Bjorge T, Selmer RM, Tverdal A. Height and body mass index in relation to total mortality. Epidemiology 2003;14:293–99. [PubMed] [Google Scholar]
- 6.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med 2006;355:779–87. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 2011;364:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003;46:459–69. [DOI] [PubMed] [Google Scholar]
- 9.Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr 1999;70:405–11. [DOI] [PubMed] [Google Scholar]
- 10.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009;8:339–48. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–97. [DOI] [PubMed] [Google Scholar]
- 12.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:1–253. [PubMed] [Google Scholar]
- 13.Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr 1998;68:899–917. [DOI] [PubMed] [Google Scholar]
- 14.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341:1097–105. [DOI] [PubMed] [Google Scholar]
- 15.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med 1998;338:1–7. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014;383:970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med 1999;341:427–34. [DOI] [PubMed] [Google Scholar]
- 18.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol 2011;173:1–9. [DOI] [PubMed] [Google Scholar]
- 19.Flegal KM, Kit BK, Graubard BI. Body mass index categories in observational studies of weight and risk of death. Am J Epidemiol 2014;180:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med 1995;333:677–85. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Flanders WD, Ward EM, Jemal A. Body mass index in young adulthood and premature death: analyses of the US National Health Interview Survey linked mortality files. Am J Epidemiol 2011;174:934–44. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005;293:1861–67. [DOI] [PubMed] [Google Scholar]
- 23.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 2014;384:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013;45:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res 2010;1350:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haffner SM. Sex hormones, obesity, fat distribution, type 2 diabetes and insulin resistance: epidemiological and clinical correlation. Int J Obes Relat Metab Disord 2000;24(Suppl 2):S56–58. [DOI] [PubMed] [Google Scholar]
- 27.Shimazu T, Kuriyama S, Ohmori-Matsuda K, Kikuchi N, Nakaya N, Tsuji I. Increase in body mass index category since age 20 years and all-cause mortality: a prospective cohort study (the Ohsaki Study). Int J Obes (Lond) 2009;33:490–96. [DOI] [PubMed] [Google Scholar]
- 28.Yun KE, Park HS, Song YM, Cho SI. Increases in body mass index over a 7-year period and risk of cause-specific mortality in Korean men. Int J Epidemiol 2010;39:520–28. [DOI] [PubMed] [Google Scholar]
- 29.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 2009;89:500–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jee SH, Golub JE, Jo J, Park IS, Ohrr H, Samet JM. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol 2009;170:1478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–53. [DOI] [PubMed] [Google Scholar]
- 32.Heitmann BL, Erikson H, Ellsinger BM, Mikkelsen KL, Larsson B. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old swedish men-a 22-year follow-up. The study of men born in 1913. Int J Obes Relat Metab Disord 2000;24:33–37. [DOI] [PubMed] [Google Scholar]
- 33.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–20. [DOI] [PubMed] [Google Scholar]
- 34.OECD. OECD Health Statistics 2014 – Frequently Requested Data. 2014. http://www.oecd.org/els/health-systems/oecd-health-statistics-2014-frequently-requested-data.htm (18 May 2015, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.