Abstract
Combined spectroscopy measurements and theoretical calculations bring to light a first investigation of a metallic cyanoacetylide, AlC3N, using laser ablation molecular beam Fourier transform microwave spectroscopy. This molecule was synthesized in a supersonic expansion by the reaction of aluminum vapour with C3N, produced from solid aluminum rods and BrCCCN in a newly constructed ablation-heating nozzle device. A set of accurate rotational and 27Al and 14N nuclear quadrupole coupling constants have been determined from the analysis of the rotational spectrum and compared with those predicted in a high-level ab initio study, conducting to the assignment of the observed species to linear AlCCCN. We have searched for this species towards the carbon-rich evolved star IRC + 10216 but only an upper limit to its abundance has been obtained.
I. INTRODUCTION
The nature of the metal-carbon chemical bonding in organometallic species has not been extensively explored, at least from a spectroscopic point of view, and it remains a topic of considerable interest. The experimentally determined molecular parameters of these molecules offer a unique opportunity to examine the competition between ionic and covalent bonding. Since these compounds play a wide range of roles in chemistry, establishing their structures provides a deeper understanding in, among others, catalytic processes and growth mechanisms of nanomaterials. Metal-bearing species are also of interest in astrochemistry. They were detected in space for the first time in the circumstellar envelope of the carbon-rich evolved star IRC + 10216.1 Since then, the number of metal-bearing molecules detected in the interstellar medium has increased significantly. So far, the list of interstellar molecules includes species with Na, K, Mg, Al, and even transition metals such as Fe or Ti. In particular, among refractory species, aluminum-bearing molecules are predicted to be especially abundant according to recent studies.2 Known interstellar aluminum-bearing molecules include halides (AlF,1,3 AlCl1), the oxide and hydroxide species (AlO,4 AlOH5), as well as aluminum isocyanide, AlNC.6 Other metal-containing cyanides and isocyanides have been observed in the interstellar medium. The list includes NaCN,7 KCN,8 MgNC,9 MgCN,10 and FeCN.11 Silicon cyanide and isocyanide have been also detected,12,13 and very recently the observation of a related magnesium molecule, HMgNC, was reported.14
The presence of a relatively wide variety of cyanides and isocyanides in space opens the possibility for the detection of longer carbon chains containing metals similar to the cyanopolyynes family: HC3N, HC5N, HC7N, HC9N, and HC11N. In fact, HC11N is the longest linear molecule observed in space15 so far. Analogue species containing metals could be synthesized through similar reaction schemes as those proposed for the family of cyanopolyynes.16 However, the possible detection of metal chains is hindered by the lack of spectroscopic information on these compounds. To the best of our knowledge none of these potential species have been characterized in laboratory.
In the present work we generated and characterized the first member of the Metal-C3N series, containing aluminum and with formula AlC3N. Laser ablation Fourier transform microwave spectroscopy (LA-MB-FTMW)17 has been used to generate AlC3N and characterize its rotational spectrum across the 2–12 GHz frequency region. To conduct this study, a nozzle which combines a heating-reservoir with laser ablation capabilities has been developed, in order to employ solid precursors to generate the desired species. We have also carried out a complete computational study to guide the identification of the generated species. Finally, a search for the detected species has been performed towards IRC + 10216 but only upper limits have been derived. Experimental and computational strategies are described in Secs. II and III.
II. EXPERIMENTAL
A. LA-MB-FTMW spectroscopy
The AlC3N measurements were carried out using a new LA-MB-FTMW spectrometer18 constructed at the University of Valladolid specially designed to maximize performances at low frequency ranges (from 2 to 12 GHz). It has been recently employed in the studies of large biomolecules.18,19 In this investigation, our conventional ablation nozzle holder17 has been modified by addition of a home-made heatable reservoir extension, placed between the valve and the laser ablation nozzle. The reservoir can hold liquid and/or solid compounds that can be used as precursors of metal bearing species. This new device is accommodated in the backside of the fixed mirror, aligned parallel relative to the optical axis of the resonator.
Solid samples of BrCCCN were synthesized by bromination of cyanoacetylene as previously reported20 (60 mmol (7.8 g) were obtained in one run starting from 0.1 mol of HC3N and an aqueous Br−-Br2/KOH solution). The precursor was placed in the heatable reservoir to produce a jet expansion with enough concentration of cyanoacetylide. Hence, AlC3N was created by laser ablation of aluminum rods in the throat of a pulsed supersonic expansion of Ne (10 bars stagnation pressure) and BrCCCN. A pulsed picosecond Nd:YAG-laser (λ = 355 nm and 10 mJ pulse−1) at a repetition rate of 2 Hz was used. A motorized micrometer rotates and translates the sample rod each laser pulse, so the laser hits a different point of the sample surface in successive pulses, minimizing the problem of shot-to-shot fluctuation in the amount of the desorbed material.
Briefly, the sequence of an experimental cycle starts with a gas pulse of the mixing carrier gas (typically 450 μs). After an adequate delay, a laser pulse hits the metal rod, vaporizing the solid and producing plasma which trigger the chemical reactions in the precursor mixture.21 Immediately, the resulting products are supersonically expanded between the two mirrors of the Fabry–Pérot resonator and then a microwave pulse (0.3 μs) is applied, producing the macroscopic polarization of the species in the jet. Once the excitation ceases, molecular relaxation gives rise to a transient emission signal (free induction decay) at microwave frequencies, which is captured in the time domain. Its Fourier transformation to the frequency domain yields the rotational transitions that appear as Doppler doublets, because the supersonic jet travels parallel to the resonator axis. The molecular rest frequencies are calculated as the arithmetic mean of the Doppler doublets and are obtained with accuracy better than 3 kHz. Typically 100–200 pulses were accumulated to achieve an adequate signal-to-noise ratio.
B. Computational methods
We have carried out a survey of the AlC3N potential hypersurface in order to characterize possible stable isomers. We are only aware of a previous theoretical study of AlC3N isomers by Petrie,22 where just two isomers were considered. For our purpose we employed the second-order MØller-Plesset (MP2)23 level of theory with Dunning’s correlated consistent triple-zeta basis sets augmented with diffuse and polarization functions, usually denoted as aug-cc-pVTZ.24 Harmonic vibrational calculations were carried out for the stationary points obtained through this exploration in order to confirm that they correspond to true minima. For the more significant species characterized through this procedure we carried out subsequent CCSD(T) calculations25 (coupled cluster with singles and doubles and a perturbative inclusion of triple excitations) with the aug-cc-pVTZ basis set.
In order to aid in the assignment of the rotational spectrum, reliable predictions for the rotational constant, as well as nuclear quadrupole coupling constants, are required. AlC3N possesses two different nuclei with quadrupole moment, 27Al (I = 5/2) and 14N (I = 1), which interact with the electric field gradient created by the rest of the molecule at the nucleus, splitting into a very complex hyperfine pattern each rotational transition. For the geometrical parameters two different procedures have been employed, CCSD(T) optimizations with Dunning’s quadruple-zeta basis set, aug-cc-pVQZ and a composite procedure have been applied. This type of schemes at the coupled-cluster level has been developed by Gauss et al.26,27 A more affordable version of the method, in terms of computational demands, involving geometry optimizations at the MP2 level has been used by Barone et al.28 as an alternative to expensive coupled-cluster calculations, showing also good results. In this context, we have also adopted this MP2-based composite method, to check its performance as an alternative to the expensive CCSD(T)/aug-cc-pVQZ that we have also carried out. For a detailed description of the method we refer to the paper of Barone et al.28
Employing both theoretical procedures, CCSD(T)/augcc-pVQZ and the composite scheme, equilibrium spectroscopic parameters were obtained. In order to achieve an estimate of B0 rotational constant, vibration-rotation interaction constants were estimated using second-order perturbation theory at the MP2/aug-cc-pVTZ level. Aditionally, to aid in a possible experimental detection by IR spectroscopy, anharmonic vibrational frequencies and IR intensities have been predicted at that level of theory (Table S1 of the supplementary material45). Both Gaussian 0929(MP2 geometries) and CFOUR30 (CCSD(T) geometries) program packages were employed in this study.
III. RESULTS AND DISCUSSION
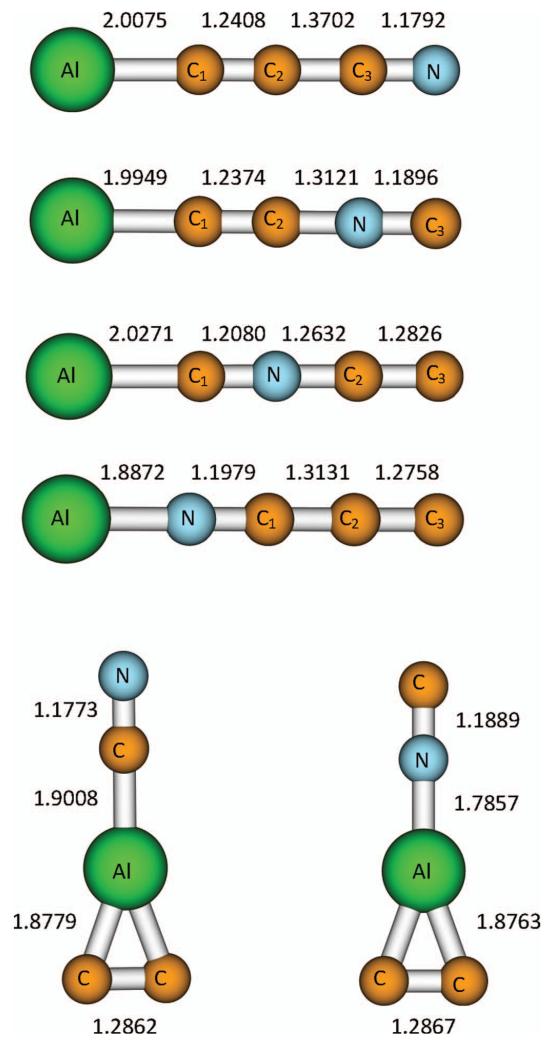
After exploring the AlC3N potential hypersurface four linear isomers were obtained, namely AlCCCN, AlCCNC, AlCNCC, and AlNCCC (Figure 1). We also considered cyclic structures with aluminum in the middle of the chain. Thus, following the imaginary normal modes the optimization ended up with either CN-Al(C2) and NC-Al(C2). These two structures have an AlC2 triangle subunit bonded to CN either through the nitrogen or the carbon atom, respectively. The relative energies of all these species, as well as their rotational constants and dipole moments obtained at the MP2/aug-ccpVTZ level, are summarized in Table I, and the equilibrium bond distances for these isomers obtained at the same level are provided in Figure 1.
FIG. 1.
Equilibrium geometrical parameters for the different AlC3N isomers obtained at the MP2/aug-cc-pVTZ level. Distances are given in Ångstroms.
TABLE I.
Relative energies (kcal/mol) of the different AlC3N species obtained at the MP2 and CCSD(T) levels with the aug-cc-pVTZ basis set. Zero-point vibrational energy (ZPVE) corrections are taken into account at the MP2/aug-cc-pVTZ level. Equilibrium rotational constants (MHz) and dipole moments (Debye) are estimated at the MP2/aug-cc-pVTZ level and CCSD/aug-cc-pVTZ levels, respectively.
| Species | ΔE(MP2) | ΔE (CCSD(T)) | A | B | C | μ |
|---|---|---|---|---|---|---|
| AlCCCN | 0.0 | 0.0 | 1317.0 | 3.908 | ||
| AlCCNC | 30.66 | 26.04 | 1395.8 | 3.102 | ||
| AlCNCC | 65.18 | 66.23 | 1419.7 | 7.379 | ||
| AlNCCC | 24.71 | 22.07 | 1480.9 | 7.457 | ||
| CN-Al(C2) | 29.1 | 25.58 | 50 875.0 | 2286.9 | 2188.5 | 1.620 |
| NC-Al(C2) | 25.4 | 25.68 | 50 914.0 | 2068.1 | 1987.4 | 1.595 |
The energy results show that linear AlCCCN is the global minimum with the next lowest-lying isomer, namely AlNCCC, located more than 20 kcal/mol higher in energy. Because AlCCCN is the primary target for a possible experimental observation its geometrical parameters have been refined employing higher-level theoretical methods. In order to obtain more accurate predictions which can be useful for rotational spectroscopy, vibration-rotation interactions have been also estimated at the MP2/aug-cc-pVTZ level of theory and a correction of −3.7 MHz to the rotational constant has been found. After taking into account this correction the predicted rotational constant is 1329.2 MHz or 1337.9 MHz, at the CCSD(T)/aug-cc-pVTZ level and composite method, respectively.
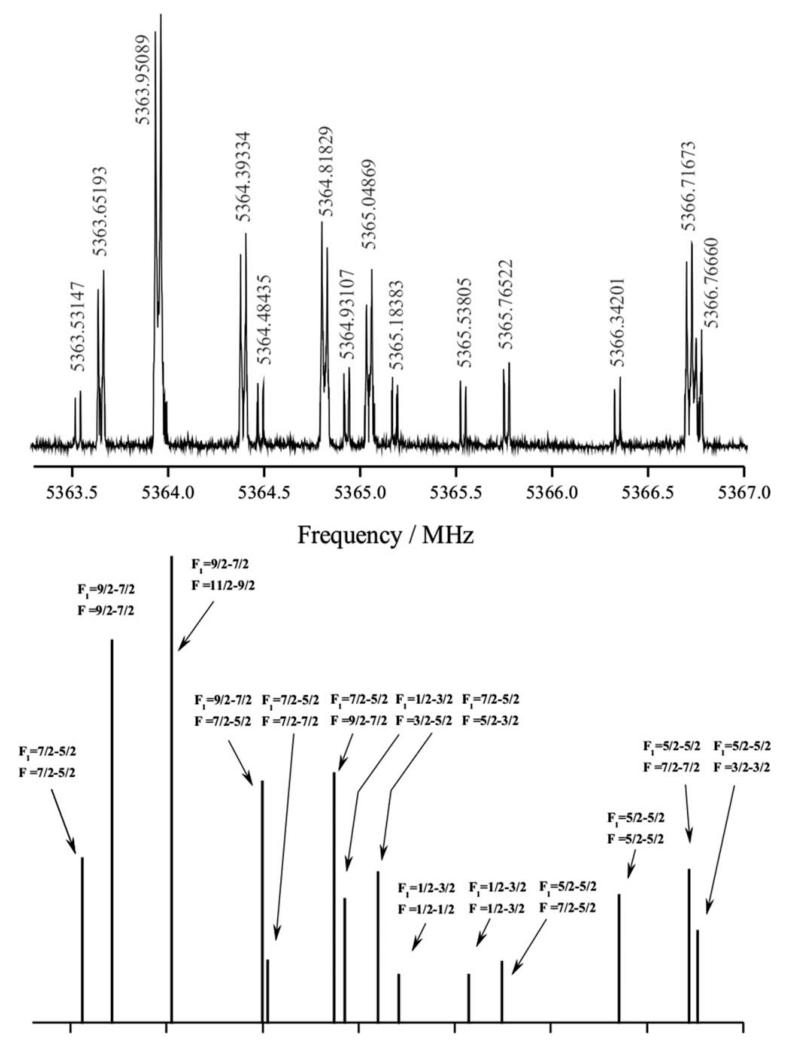
On this basis, wide frequency scans from 5 to 6 GHz were directed to detect plausible signals corresponding to the J = 2–1 rotational transitions of the linear isomers of AlC3N. A rotational transition centered around 5365 MHz, with a very complex hyperfine structure showing many fully resolved components (as shown in the upper panel of Figure 2) was finally found. The experimental value match nicely with that predicted by the composite method for the J = 2–1 transition of the linear AlCCCN isomer at 5351 MHz. However, only a conclusive identification of the observed transition comes from the comparison between the predicted and observed nuclear quadrupole hyperfine structure. As can be seen in Figure 2, an exceptional matching exists between the observed and predicted spectra, when considering the eQq values for the 27Al and 14N nuclei of Table II for the linear AlCCCN isomer.
FIG. 2.
(Upper panel) Central section of the J = 2–1 rotational transition of AlC3N near 5.4 GHz. The nuclear quadrupole coupling hyperfine structure arising from both 27Al (I = 5/2) and 14N (I = 1) nuclei is clearly resolved. The coaxial arrangement of the adiabatic expansion and the resonator axis produces an instrumental Doppler doubling. The resonances frequencies are calculated as the average of the two Doppler components. (Lower panel) Theoretical simulation of the nuclear quadrupole hyperfine structure for the J = 2–1 rotational transition of AlCCCN isomer.
TABLE II.
Theoretical and experimental spectroscopic constants for AlCCCN isomer. Theoretical rotational constants have been corrected with vibration-rotation interaction estimated at MP2/aug-cc-pVTZ level.
| Parameter | Theoretical | Experimental |
|---|---|---|
| B (MHz) | 1329.2a / 1337.9b | 1340.757500 (32) |
| D (kHz) | 0.0600c | 0.0673 (14) |
| eQq (Al) (MHz) | −38.224d | −38.5993 (8) |
| eQq (N) (MHz) | −4.458d | −4.2475 (6) |
| CI (Al) (kHz) | … | 1.257 (31) |
| rms (kHz) | … | 0.6 |
| N | … | 73 |
CCSD(T)/aug-cc-pVQZ level.
Composite method.
MP2/aug-cc-pVTZ.
CCSD/aug-cc-pVTZ.
An initial fit,31 including the 27 hyperfine components measured for the J = 2–1 rotational transition gives rise a first set of constants which were used to predict and measure the J = 1–0, J = 3–2, and J = 4–3 centered at 2683, 8044, and 10 725 MHz, respectively. A total of 73 hyperfine components (collected in Table III) were analyzed31 using a 1Σ Hamiltonian of the following form:32 H = HR + HQ + Hnsr where HR contains rotational centrifugal distortion parameters, HQ the quadrupole coupling interactions while Hnsr describes the nuclear spin-rotation terms. The energy levels involved in each transition are labeled with the quantum numbers J, F1, and F, where F1 = J + IAl and F = F1 + IN. The experimental values for the ratational parameters B0 and D, the electric quadrupole coupling constant eQq for the 27Al and 14N nuclei along with the nuclear spin-rotation parameter CI (27Al) for the aluminum nucleus were derived from the analysis. Attempting to fit this last constant for the 14N nucleus resulted in values that were undefined to within their 3σ uncertainties. The standard deviation obtained for the fit is 0.6 kHz. Confirmation of the observed species as the linear AlCCCN isomer resides in the excellent agreement between the experimental and theoretical values of the rotational and 27Al and 14N nuclear quadrupole coupling constants collected in Table II.
TABLE III.
Measured frequencies and residuals (in MHz) for the nuclear quadrupole coupling hyperfine components of AlCCCN.
| J′ | J″ | F′1 | F″1 | F′ | F″ | ν obs. | νobs. – ν calc. |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 2.5 | 2.5 | 1.5 | 1.5 | 2674.63471 | −0.00049 |
| 2.5 | 2.5 | 3.5 | 3.5 | 2675.05122 | −0.00046 | ||
| 2.5 | 2.5 | 2.5 | 2.5 | 2676.00137 | −0.00024 | ||
| 3.5 | 2.5 | 3.5 | 2.5 | 2682.89096 | −0.00029 | ||
| 3.5 | 2.5 | 2.5 | 1.5 | 2683.57333 | −0.00196 | ||
| 3.5 | 2.5 | 4.5 | 3.5 | 2683.66385 | −0.00011 | ||
| 1.5 | 2.5 | 1.5 | 1.5 | 2686.77891 | −0.00138 | ||
| 1.5 | 2.5 | 0.5 | 1.5 | 2687.13311 | −0.00050 | ||
| 1.5 | 2.5 | 2.5 | 3.5 | 2687.41089 | −0.00059 | ||
| 2 | 1 | 2.5 | 1.5 | 1.5 | 0.5 | 5354.26762 | −0.00106 |
| 2.5 | 1.5 | 3.5 | 2.5 | 5354.35676 | 0.00020 | ||
| 2.5 | 1.5 | 2.5 | 2.5 | 5354.93291 | −0.00076 | ||
| 2.5 | 1.5 | 2.5 | 1.5 | 5355.56552 | −0.00025 | ||
| 3.5 | 3.5 | 2.5 | 2.5 | 5356.10914 | 0.00042 | ||
| 3.5 | 3.5 | 4.5 | 4.5 | 5356.20544 | 0.00001 | ||
| 3.5 | 3.5 | 3.5 | 3.5 | 5356.64182 | −0.00018 | ||
| 3.5 | 2.5 | 3.5 | 2.5 | 5363.53147 | −0.00017 | ||
| 4.5 | 1.5 | 3.5 | 2.5 | 5360.55638 | −0.00009 | ||
| 4.5 | 3.5 | 4.5 | 3.5 | 5363.65193 | 0.00049 | ||
| 1.5 | 1.5 | 2.5 | 1.5 | 5360.44958 | −0.00053 | ||
| 1.5 | 1.5 | 1.5 | 2.5 | 5360.50497 | −0.00007 | ||
| 1.5 | 1.5 | 1.5 | 0.5 | 5360.78391 | 0.00008 | ||
| 4.5 | 3.5 | 5.5 | 4.5 | 5363.95089 | 0.00037 | ||
| 1.5 | 1.5 | 1.5 | 1.5 | 5361.13692 | −0.00022 | ||
| 4.5 | 3.5 | 3.5 | 2.5 | 5364.39334 | −0.00023 | ||
| 3.5 | 2.5 | 3.5 | 3.5 | 5364.48435 | 0.00064 | ||
| 3.5 | 2.5 | 4.5 | 3.5 | 5364.81829 | 0.00058 | ||
| 0.5 | 1.5 | 1.5 | 2.5 | 5364.93107 | 0.00021 | ||
| 3.5 | 2.5 | 2.5 | 1.5 | 5365.04869 | −0.00012 | ||
| 0.5 | 1.5 | 0.5 | 0.5 | 5365.18383 | −0.00009 | ||
| 0.5 | 1.5 | 0.5 | 1.5 | 5365.53805 | 0.00081 | ||
| 2.5 | 2.5 | 3.5 | 2.5 | 5365.76522 | 0.00093 | ||
| 2.5 | 2.5 | 2.5 | 2.5 | 5366.34201 | 0.00061 | ||
| 2.5 | 2.5 | 3.5 | 3.5 | 5366.71673 | 0.00037 | ||
| 2.5 | 2.5 | 1.5 | 1.5 | 5366.76660 | −0.00049 | ||
| 2.5 | 2.5 | 2.5 | 3.5 | 5367.29364 | 0.00017 | ||
| 3 | 2 | 4.5 | 4.5 | 5.5 | 5.5 | 8037.77458 | 0.00109 |
| 1.5 | 0.5 | 2.5 | 1.5 | 8041.00381 | 0.00079 | ||
| 2.5 | 1.5 | 2.5 | 1.5 | 8041.06585 | −0.00021 | ||
| 2.5 | 1.5 | 3.5 | 2.5 | 8041.68165 | 0.00057 | ||
| 3.5 | 2.5 | 3.5 | 2.5 | 8042.11209 | 0.00081 | ||
| 2.5 | 1.5 | 1.5 | 0.5 | 8042.15330 | 0.00041 | ||
| 4.5 | 2.5 | 3.5 | 2.5 | 8043.70899 | −0.00040 | ||
| 3.5 | 2.5 | 2.5 | 1.5 | 8043.78790 | 0.00070 | ||
| 3.5 | 2.5 | 4.5 | 3.5 | 8043.81635 | −0.00030 | ||
| 5.5 | 4.5 | 5.5 | 5.5 | 8043.82945 | −0.00101 | ||
| 4.5 | 3.5 | 4.5 | 4.5 | 8044.50608 | −0.00075 | ||
| 3.5 | 3.5 | 3.5 | 2.5 | 8044.77355 | 0.00022 | ||
| 4.5 | 3.5 | 4.5 | 3.5 | 8044.84035 | −0.00048 | ||
| 5.5 | 4.5 | 5.5 | 4.5 | 8044.90024 | 0.00014 | ||
| 5.5 | 4.5 | 4.5 | 3.5 | 8044.98067 | 0.00003 | ||
| 4.5 | 3.5 | 5.5 | 4.5 | 8045.51866 | 0.00008 | ||
| 1.5 | 1.5 | 1.5 | 1.5 | 8045.67326 | 0.00124 | ||
| 1.5 | 1.5 | 2.5 | 2.5 | 8046.11592 | 0.00005 | ||
| 4.5 | 3.5 | 3.5 | 2.5 | 8046.37197 | 0.00053 | ||
| 4.5 | 3.5 | 3.5 | 3.5 | 8046.51845 | −0.00070 | ||
| 2.5 | 2.5 | 2.5 | 2.5 | 8046.63682 | −0.00061 | ||
| 2.5 | 2.5 | 3.5 | 3.5 | 8047.14293 | 0.00040 | ||
| 2.5 | 2.5 | 1.5 | 1.5 | 8047.23619 | −0.00079 | ||
| 2.5 | 3.5 | 2.5 | 3.5 | 8049.44645 | −0.00074 | ||
| 4 | 3 | 2.5 | 1.5 | 2.5 | 1.5 | 10 724.05291 | −0.00040 |
| 3.5 | 2.5 | 3.5 | 2.5 | 10 724.49903 | 0.00018 | ||
| 2.5 | 1.5 | 3.5 | 2.5 | 10 724.69515 | −0.00129 | ||
| 3.5 | 2.5 | 4.5 | 3.5 | 10 724.98315 | 0.00030 | ||
| 1.5 | 0.5 | 2.5 | 1.5 | 10 725.34913 | 0.00028 | ||
| 4.5 | 3.5 | 4.5 | 3.5 | 10 725.73774 | −0.00044 | ||
| 1.5 | 0.5 | 0.5 | 0.5 | 10 725.74059 | −0.00014 | ||
| 4.5 | 3.5 | 3.5 | 2.5 | 10 725.88506 | −0.00013 | ||
| 4.5 | 3.5 | 5.5 | 4.5 | 10 726.00496 | 0.00018 | ||
| 4.5 | 4.5 | 5.5 | 5.5 | 10 726.20217 | 0.00066 | ||
| 5.5 | 4.5 | 5.5 | 4.5 | 10 726.25886 | −0.00028 | ||
| 6.5 | 5.5 | 5.5 | 4.5 | 10 726.32945 | 0.00095 | ||
| 5.5 | 4.5 | 4.5 | 3.5 | 10 726.37781 | −0.00059 |
Additional searches to find rotational signatures of other species of AlC3N failed. At this point, it is interesting to note that both aluminum cyanide and isocyanide have been experimentally observed by rotational spectroscopy,33,34 being AlNC predicted to be about 5 kcal/mol more stable than AlCN.35,36 BrCCCN can be considered as a good generator of Metal-CCCN species but it might not be suitable for generating M-NCCC species, which is an important issue to consider in the non-observation of AlNCCC in our experiment. In addition, in the case of AlC3N species the cyanide isomer is 20 kcal/mol more stable than the isocyanide isomer. Hence, although AlNCCC was actually generated in our experiment it would not be enough populated to be detected.
The electronic quadrupole coupling constant for the Al nucleus was found to be eQq (Al) = −38.5993 (8) MHz. This value can be compared with those for other aluminum containing species to gain some insights about the bonding properties of Al-C union. The quadrupole constant of AlCCCN lies between those for AlCCH (−42.3917 (65) MHz)21 and AlCN (−37.2225 (29) MHz).33 These numbers suggest that the bond Al-C in AlCCCN is not as covalent as AlCCH and presents some ionic character like in the AlCN system. This fact is reflected in the Al-C bond lengths, shown in Table IV. The previous conclusion has been confirmed by using a topological analysis of the electronic density in the framework of the Bader’s Quantum Theory of Atoms in Molecules (QTAIM).38 This analysis was performed for each stationary point on the PES’s using the Keith’s AIMAll package39 including standard thresholds. Results for AlCN, AlCCCN, and AlCCH are shown in Table V and the corresponding contour maps of the Laplacian of the electron density are shown in Figure S1 of the supplementary material.45 In this context two limiting types of interactions can be identified namely shared and closed-shells interactions.40 In shared interactions, typical of covalent compounds, the nuclei are bound as a consequence of the lowering of the potential energy associated with the concentration of electronic charge shared between the nuclei; this is reflected in relatively large values of ρ(r) and negative values of the Laplacian, ∇2ρ(r) at the critical point. The second limiting type of atomic interaction is that occurring between closed-shell systems, such as those found in ionic bond or van der Waals molecules for instance. In these interactions, ρ(r) is relatively low in value and the Laplacian, ∇2ρ(r), is positive.
TABLE IV.
Nuclear quadrupole coupling constant for the Al nucleus and Al-C bond distances for some Al-containing species.
TABLE V.
Local topological properties (in au.) of the electronic charge density distribution calculated at the position of the bond critical points for the different (AIMAll) species.a
| Species | Bond | ρ(r) | ∇2ρ(r) | ∣V(r)∣/G(r) | H(r) |
|---|---|---|---|---|---|
| AlCN | Al-C | 0.069 | 0.242 | 1.214 | −0.016 |
| C-N | 0.487 | −0.457 | 2.142 | −0.919 | |
| AlCCCN | Al-C1 | 0.072 | 0.266 | 1.205 | −0.017 |
| C1-C2 | 0.413 | −1.357 | 3.018 | −0.672 | |
| C2-C3 | 0.313 | −1.043 | 4.488 | −0.366 | |
| C3-N | 0.482 | −0.333 | 2.102 | −0.898 | |
| AlCCH | Al-C1 | 0.075 | 0.289 | 1.204 | −0.019 |
| C1-C2 | 0.410 | −1.350 | 3.058 | −0.657 | |
| C2-H | 0.299 | −1.253 | 10.458 | −0.350 |
The electronic charge density [ρ(r)], the Laplacian [∇2ρ(r)], the relationship between the potential energy density V(r) and the lagrangian form of kinetic energy density G(r)) and the total energy density, [H(r)].
Another useful property to characterize the degree of covalence of a bond is the total energy density H(r). It is defined as the sum of the potential energy density, V(r) and the gradient kinetic energy density G(r) at a critical point. In covalent interactions H(r) is negative in value41 and the positive value of H(r) is characteristic of ionic interactions and van der Waals systems.40 The covalent character of an interaction can also be quantitatively analyzed by taking into account the ∣V(r)∣/G(r) ratio. In covalent interactions the value of this relationship is greater than 2. It is smaller than 1 for non-covalent interactions and between 1 and 2 for partially covalent bonds.
The local topological properties of the carbon-carbon and carbon-nitrogen bond critical points are indicative of shared interactions: large values of electron density, negative values of its Laplacian, ∣V(r)∣/G(r)∣ ratios greater than 2 and negative values of the total energy densities H(r). On the opposite, the aluminum-carbon bond critical points show low values of ρ(r) and positive values of its Laplacian. The ∣V(r)∣/G(r)∣ ratios are between 1 and 2 and H(r) is negative with a low value. Thus these interactions can be classified as closed shell interactions (typical of ionic compounds) with a small degree of covalence.
On the other hand, the quasi identical eQq values for 14N nucleus in AlCCCN and HCCCN, −4.2475 (6) MHz and −4.31924 (1) MHz42 respectively, indicate that the electronic environment around the 14N nucleus is very similar in both species. Thus, one can infer that the nature of the bond in the C≡N group in AlCCCN should be the same to that found in HCCCN.
Finally, the rotational constants derived from the observed laboratory spectrum of AlCCCN allow performing a search in the millimeter domain using the 3 mm line survey of IRC + 10216 taken with the 30-m IRAM radiotelescope.43,44 The values of the experimental constants obtained in the present investigation have been used to predict its transitions in the 3 mm domain with uncertainties of 0.15–0.4 MHz, which is enough to search for lines having total frequency coverage in IRC + 10216 of ~10 MHz at these frequencies (total linewidth ~29 km/s). Unfortunately, we have not detected any of the lines covered in the line survey, from J = 30–29 up to J = 43–42. From the most sensitive spectra we obtain an upper limit (3σ) to the column density of AlCCCN in front of IRC + 10216 of <3 × 1012 cm−2. Note that, AlNC has been detected6 in this source with a column density of 9 × 1011 cm−2. The upper limit obtained for the column density of AlCCCN is 3 times higher than that of AlNC. Although the dipole moment of AlCCCN is higher than that of AlNC, its partition function is much higher and it could be much better to search for this species in the 7 mm domain.
IV. CONCLUSIONS
The first metal cyanoacetylide, AlCCCN, has been produced and characterized in the laboratory using a combination of laser ablation techniques and Fourier transform microwave spectroscopy. A newly constructed ablation-heating source has been proved as an effective method to create metallic cyanoacetylides, using solid samples of BrCCCN as main precursor.
The predictions for the rotational constant at both CCSD(T)/aug-cc-pVQZ and composite methods are in good agreement with the experimental value. Since the composite method is more affordable in terms of computational demands than the CCSD(T)/aug-cc-pVQZ level, our results suggest that the composite method corrected with vibration-rotation interaction can provide good estimates of rotational constants for this type of compounds.
We have searched for AlCCCN species towards the carbon-rich evolved star IRC + 10216 and obtained only upper limits. Important information on the chemistry of metal cyanides and isocyanides could be obtained if M-CCCN species are detected in the circumstellar medium. Mg- and Na-C3N are the most obvious candidates after AlC3N and will be studied soon in our laboratory. Although their detection seems to be difficult within the state-of-the-art observations of evolved stars, the sensitivity that will be provided by the ALMA interferometer in the next years could open the possibility to detect these species in circumstellar envelopes.
Supplementary Material
ACKNOWLEDGMENTS
This research has been supported by the Ministerio de Ciencia e Innovación of Spain (Grants CTQ2010-19008, Consolider-Ingenio 2010 CSD2009-00038, and CTQ2010-16864), by European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC-2013-SyG (Grant Agreement No. 610256 NANOCOSMOS) and by the Junta de Castilla y León (Grants VA175U13 and VA077U13). C.C. thanks the Junta de Castilla y León for a postdoctoral contract (Grant CIP13/01). J.-C.G. thanks the Centre National d’Etudes Spatiales (CNES) and the Program PCMI (INSU-CNRS) for financial support. J.-C.G. and J.C. received the support of the contract ANR-13-BS05-0008 IMOLABS. We thank Marcelino Agúndez for useful comments and suggestions.
References
- 1.Cernicharo J, Guélin M. Astron. Astrophys. 1987;183:L10. [Google Scholar]
- 2.Agúndez M, Fonfría JP, Cernicharo J, Kahane C, Daniel F, Guelin M. Astron. Astrophys. 2012;543:A48. [Google Scholar]
- 3.Ziurys LM, Apponi AJ, Phillips TG. Astrophys. J. 1994;433:729. [Google Scholar]
- 4.Tenenbaum ED, Ziurys LM. Astrophys. J. 2009;694:L59. [Google Scholar]
- 5.Tenenbaum ED, Ziurys LM. Astrophys. J. 2010;712:L93. [Google Scholar]
- 6.Ziurys LM, Savage C, Highberger JL, Apponi AJ, Guélin M, Cernicharo J. Astrophys. J. 2002;564:L45. [Google Scholar]
- 7.Turner BE, Steimle TC, Meerts L. Astrophys. J. 1994;426:97. [Google Scholar]
- 8.Pulliam RL, Savage C, Agúndez M, Cernicharo J, Guélin M, Ziurys LM. Astrophys. J. 2010;725:L181. [Google Scholar]
- 9.Kawaguchi K, Kagi E, Hirano T, Takano S, Saito S. Astrophys. J. 1993;406:L39. [Google Scholar]
- 10.Ziurys LM, Apponi AJ, Guélin M, Cernicharo J. Astrophys. J. 1995;445:L47. [Google Scholar]
- 11.Zack LN, Halfen DT, Ziurys LM. Astrophys. J. 2011;733:L36. [Google Scholar]
- 12.Guélin M, Muller S, Cernicharo J, Apponi AJ, McCarthy MC, Gottlieb CA, Thaddeus P. Astron. Astrophys. 2000;363:L9. [Google Scholar]
- 13.Guélin M, Muller S, Cernicharo J, McCarthy MC, Thaddeus P. Astron. Astrophys. 2004;426:L49. [Google Scholar]
- 14.Cabezas C, Cernicharo J, Alonso JL, Agúndez M, Mata S, Guélin M, Peña I. Astrophys. J. 2013;775:133. [Google Scholar]
- 15.Bell MB, Feldman PA, Travers MJ, McCarthy MC, Gottlieb CA, Thaddeus P. Astrophys. J. 1997;483:L61. [Google Scholar]
- 16.Herbst E. Chem. Soc. Rev. 2001;30:168. [Google Scholar]
- 17.Alonso JL, Pérez C, Sanz ME, López JC, Blanco S. Phys. Chem. Chem. Phys. 2009;11:617. doi: 10.1039/b810940k. and references therein. [DOI] [PubMed] [Google Scholar]
- 18.Bermúdez C, Mata S, Cabezas C, Alonso JL. Angew. Chem., Int. Ed. 2014;53:6655. doi: 10.1002/anie.201405347. [DOI] [PubMed] [Google Scholar]
- 19.Sanz ME, Cabezas C, Mata S, Alonso JL. J. Chem. Phys. 2014;140:204308. doi: 10.1063/1.4876001. [DOI] [PubMed] [Google Scholar]
- 20.Kloster-Jensen E. Acta Chem. Scand. 1963;17:1862–1865. [Google Scholar]
- 21.Cabezas C, Mata S, Daly AM, Martín A, Alonso JL, Cernicharo J. J. Mol. Spectrosc. 2012;278:31. [Google Scholar]
- 22.Petrie S. Mon. Not. R. Astron. Soc. 1999;302:482. [Google Scholar]
- 23.Plesset C. MØller and M. Phys. Rev. 1934;46:618. [Google Scholar]
- 24.Dunning TH. J. Chem. Phys. 1989;90:1007. [Google Scholar]
- 25.Raghavachari K, Trucks GW, Pople JA, Head-Gordon M. Chem. Phys. Lett. 1989;157:479. [Google Scholar]
- 26.Heckert M, Kalay M, Gauss J. Mol. Phys. 2005;103:2109. [Google Scholar]
- 27.Heckert M, Kalay M, Tew DP, Klopper W, Gauss J. J. Chem. Phys. 2006;125:044108. doi: 10.1063/1.2217732. [DOI] [PubMed] [Google Scholar]
- 28.Barone V, Biczysko M, Bloino J, Puzzarini C. J. Chem. Theory Comput. 2013;9:1533. doi: 10.1021/ct3010672. [DOI] [PubMed] [Google Scholar]
- 29.Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 09, Revision B.01. Gaussian, Inc.; Wallingford, CT: 2010. [Google Scholar]
- 30.CFOUR, a quantum chemical program package written by J. F. Stanton, J. Gauss, M. E. Harding, and P. G. Szalay with contributions from A. A. Auer, R. J. Bartlett, U. Benedikt, C. Berger, D. E. Bernholdt, Y. J. Bomble, L. Cheng, O. Christiansen, M. Heckert, O. Heun, C. Huber, T.-C. Jagau, D. Jonsson, J. Jusélius, K. Klein, W. J. Lauderdale, D. A. Matthews, T. Metzroth, L. A. Mück, D. P. O’Neill, D. R. Price, E. Prochnow, C. Puzzarini, K. Ruud, F. Schiffmann, W. Schwalbach, C. Simmons, S. Stopkowicz, A. Tajti, J. Vázquez, F. Wang, J. D. Watts and the integral packages MOLECULE (J. Almlöf and P. R. Taylor), PROPS (P. R. Taylor), ABACUS (T. Helgaker, H. J. Aa. Jensen, P. Jørgensen, and J. Olsen), and ECP routines by A. V. Mitin and C. van Wüllen, 2013.
- 31.Pickett HM. J. Mol. Spectrosc. 1991;148:371. [Google Scholar]
- 32. Gordy W, Cook RL. Microwave Molecular Spectra. 3rd ed. Wiley; New York: 1984. [Google Scholar]
- 33.Walker KA, Gerry MCL. Chem. Phys. Lett. 1999;301:200. [Google Scholar]
- 34.Robinson JS, Apponi AJ, Ziurys LM. Chem. Phys. Lett. 1997;278:1. [Google Scholar]
- 35.Ma B, Yamaguchi Y, Schaefer HF., III Mol. Phys. 1995;86:1331. [Google Scholar]
- 36.Petrie S. J. Phys. Chem. 1996;100:11581. [Google Scholar]
- 37.Sun M, Halfen DT, Clouthier DJ, Ziurys LM. Chem. Phys. Lett. 2012;553:11–16. doi: 10.1063/1.3696893. [DOI] [PubMed] [Google Scholar]
- 38.Bader RWF. Atoms in Molecules: A Quantum Theory. Clarendon Press; New York: 1990. [Google Scholar]
- 39.Keith TA. AIMAll, version 13.11.04, Professional, TK Gristmill Software: Overland Park, KS. 2013 see http://aim.tkgristmill.com.
- 40.Bader RWF. Chem. Rev. 1991;91:893–928. [Google Scholar]
- 41.Cremer D, Kraka E. Angew. Chem., Int. Ed. 1984;23:627–628. [Google Scholar]
- 42.Deleonb RL, Muenter JS. J. Chem. Phys. 1985;82:1702. [Google Scholar]
- 43.Cernicharo J, Guélin M, Agúndez M, McCarthy MC, Thaddeus P. Astrophys. J. 2008;688:L83–L86. [Google Scholar]
- 44.Cernicharo, et al. A sensitive line survey at 3 mm with the 30 m IRAM radio telescope of IRC+10216. (unpublished)
- 45.See supplementary material at http://dx.doi.org/10.1063/1.4894501 for Table S1, with the anharmonic vibrational frequencies and IR intensities for AlCCCN evaluated at the MP2/aug-cc-pVTZ level and Figure S1, with the contour maps of the Laplacian distribution of the electron density for the AlCN, AlCCCN, and AlCCH species.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.