Abstract
A new decapod crustacean faunule is described from the early Miocene of the Slovenian part of the Styrian Basin. The Ivnik Beds exposed at the Činžat locality contain seven species: Calliax michelottii (Axiidea: Callianassidae), Lepidophthalmus paratethyensis sp. nov. (Axiidea: Callianassidae), Jaxea kuemeli (Gebiidea: Laomediidae), Styrioplax exiguus (Brachyura: Chasmocarcinidae), Goneplax gulderi (Brachyura: Goneplacidae), Neopilumnoplax pohorjensis sp. nov. (Brachyura: Mathildellidae) and Retropluma slovenica sp. nov. (Brachyura: Retroplumidae). Numerous specimens of well-preserved Styrioplax exiguus permitted its redescription and re-assignment of its familial placement to Chasmocarcinidae. Neopilumnoplax pohorjensis sp. nov. constitutes the first fossil occurrence of the genus known to date. The decapod association, as well as other faunal elements, suggests low-energy deep-water depositional environment with epibathyal water depth of more than 125 m. The studied locality is situated in the Ribnica–Selnica graben filled with sediments once deposited in the Central Paratethys sea. Based on the affinities of decapod genera of the Central Paratethys and the Proto-Mediterranean, we conclude that the exchange of decapod faunas between these regions was probably regulated by an anti-estuarine circulation permitting an easier incursion of species from the Proto-Mediterranean into the Paratethys and simultaneous hindering the Paratethyan endemics (Styrioplax) from entering the Mediterranean.
Keywords: Decapoda, early Miocene, Central Paratethys, Slovenia, deep-water environment, palaeobiogeography
Decapod crustaceans from Neogene strata of northeastern Slovenia are poorly known. Records are limited to isolated specimens from occasional construction or earth works, and no decapod association has been previously recorded.
Previous records of Miocene decapods from the Slovenian part of the Styrian Basin (Table 1) are those by Glaessner (1928) and Mikuž (2003). Glaessner (1928) described Microplax exiguus on the basis of three specimens from the Langhian (‘Badenian’) mudstones of two different localities, the towns of St Egydi (Šentilj) and St Leonhard (Lenart) within the Kungota Formation. Almost a century later, Mikuž (2003) reported on remains of Portunus monspeliensis Milne Edwards, 1860, from the grey sandstone of the same formation cropping out near the town of Šentilj.
TABLE 1.
Synopsis of Miocene decapod Crustacea reported from Slovenia.
| Species | Age | Localities | References |
|---|---|---|---|
| ‘Callianassa’ jahringensis | Karpatian | Jarenina | Glaessner (1928) |
| Lepidophthalmus paratethyensis sp. nov. | Karpatian | Činžat | Herein |
| Jaxea kuemeli | Karpatian | Činžat | Herein |
| Goneplax gulderi | Karpatian | Činžat | Herein |
| Neopilumnoplax pohorjensis sp. nov. | Karpatian | Činžat | Herein |
| Retropluma slovenica sp. nov. | Karpatian | Činžat | Herein |
| Styrioplax exiguus | Karpatian | Šentilj, Lenart, Činžat | Glaessner (1928) and herein |
| Coeloma sp. | ?Karpatian | Kamnik | Križnar and Preisinger (2008) |
| Calliax michelottii | Karpatian, Badenian | Jarenina, Kamnik, Činžat | Glaessner (1928), Hyžný and Gašparič (2014) and herein |
| ‘Callianassa’ sismondai | Badenian | Šentilj | Glaessner (1928) |
| ‘Callianassa’ norica | Badenian | Šentilj | Glaessner (1928) |
| Portunus monspeliensis | Badenian | Šentilj | Glaessner (1928) and Mikuž (2003) |
| Cancer illyricus | Badenian | Zagorje | Bittner (1884) |
| Tasadia carniolica | Badenian | Kamnik | Bittner (1884), Mikuž and Pavšič (2003) and Mikuž (2010) |
| Macrophthalmus sp. | ?Badenian | Kamnik | Križnar (2006) |
This study describes the first decapod crustacean faunule from Slovenia. The assemblage was collected from the Ivnik Beds at the locality Činžat on the northern slopes of Mt Pohorje. Decapod remains are a dominant group in the grey to brownish siltstones of the early Miocene (‘Karpatian’) age.
GEOLOGICAL AND STRATIGRAPHICAL SETTING
The studied locality Činžat is situated in the Ribnica–Selnica graben, within the westernmost parts of the Styrian Basin, Slovenia. It is situated approximately 15 km west of Maribor (GPS coordinates: 46°32′35.05”N, 15°26′35.15”E), a city near the northern Slovenian border with Austria, along the steep valley of the Drava River (Fig. 1). Here, strata of the Ivnik Beds (Mioč 1972) are exposed in a belt from Maribor, on the northern slopes of the igneous Pohorje pluton, towards the town of Radlje. The decapod-bearing layers were exposed during renovation works on a local road connecting the towns of Ruše and Lovrenc na Pohorju.
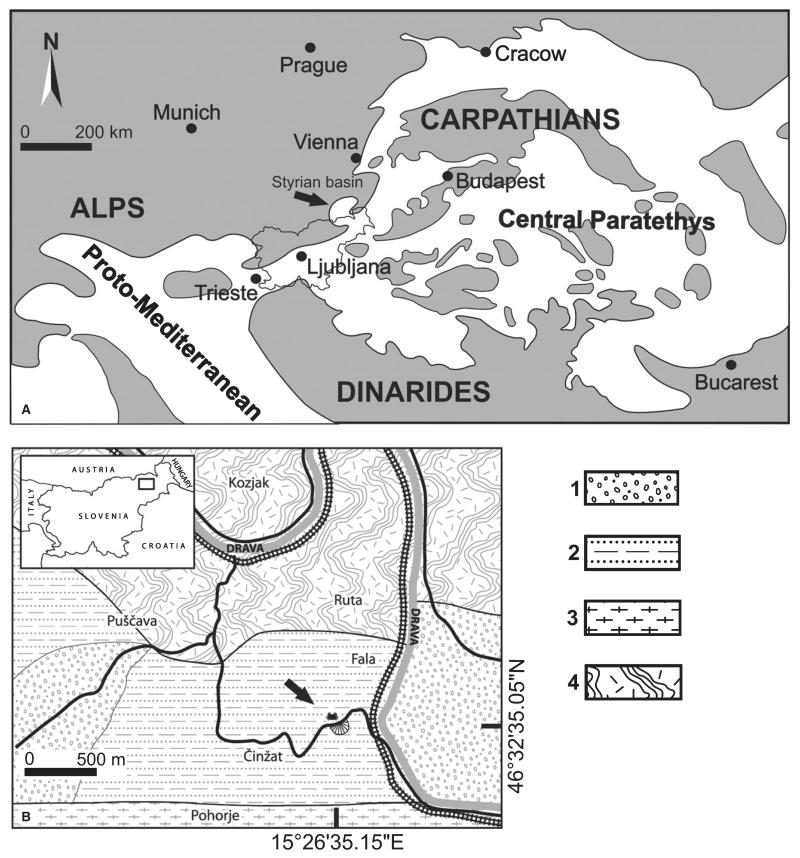
FIG. 1.
Location of the studied area. A, palaeogeographical situation of the Styrian Basin within the early–mid-Miocene Paratethys Sea (modified after Rögl 1998). B, simplified geological map and position of Činžat in north-eastern Slovenia. 1, early Miocene conglomerates; 2, early Miocene fossiliferous siltstones; 3, plutonic rocks of the Pohorje massif; 4, metamorphic rocks of the Kozjak series.
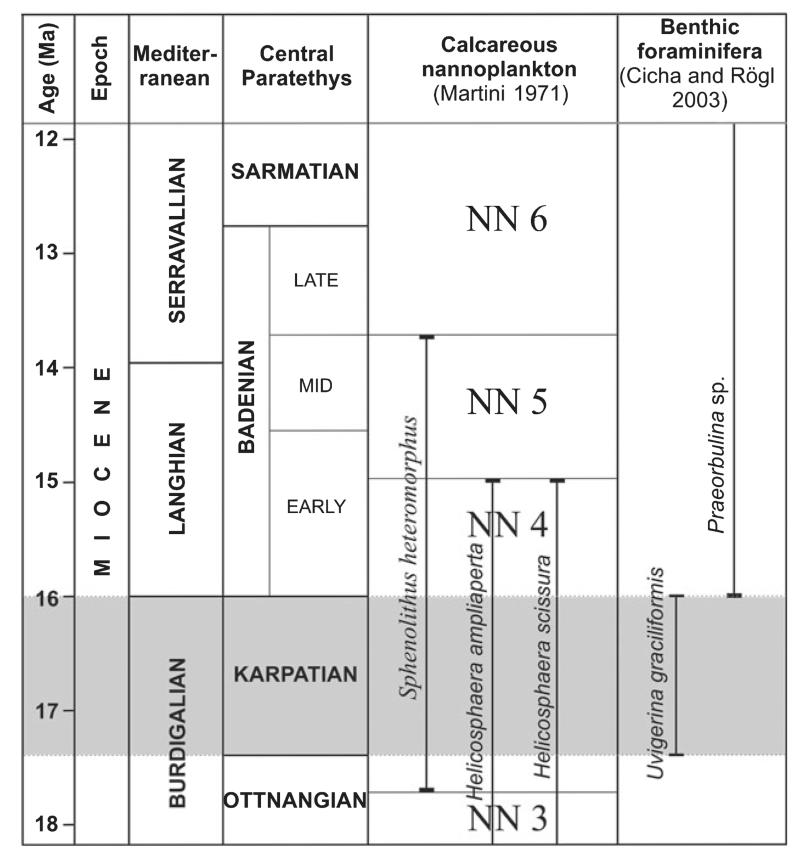
Fossil-bearing micaceous laminated siltstones cover older pre-Cenozoic rocks and sequences of loosely bound conglomerates, alternating with sandstones and siltstones of the Ivnik Beds (Pavšič and Horvat 2009). A late Burdigalian age coinciding with the ‘Karpatian’ stage of the regional scale (Piller et al. 2007) was identified based on a benthic foraminiferal association (Mioč 1977). The ‘Karpatian’ stage was identified based on the presence of the foraminiferal taxa Uvigerina graciliformis Papp and Turnovsky, 1953, and the absence of Praeorbulina Olsson, 1964. The lowest occurrence (LO) of U. graciliformis delineates the base of the Karpatian stage, and the LO of Praeorbulina occurs at the base of the Badenian stage (Cicha and Rögl 2003). Results of nannoplankton sampling (Bartol et al. 2013) confirm a ‘Karpatian’ age; based on the co-occurrence of Sphenolithus heteromorphus Deflandre, 1953, and Helicosphaera ampliaperta Bramlette and Wilcoxon, 1967, a nannoplankton zone NN4 sensu Martini (1971) which spans into early ‘Badenian’ was identified (Fig. 2).
FIG. 2.
Chronostratigraphic chart of Miocene, showing Mediterranean and Paratethyan (regional) stages. The age of the Činžat locality based on the co-occurrences of nannoplankton is highlighted.
The late Burdigalian (‘Karpatian’) marks the beginning of marine transgression into the Central Paratethys. During this time, a connection with the Mediterranean realm (via a Slovenian corridor) was reopened (Pavšič and Horvat 2009). The transgressing sea filled deep, narrow and elongated sedimentation basins, which subsided in the Ribnica–Selnica graben as a result of synrift tectonics with rapid tectonic block subsidence (Jelen and Rifelj 2002). The agglutinated foraminiferal Cyclammina–Bathysiphon assemblage (Mioč 1977) is indicative of the deepest sublittoral to upper bathyal environmental conditions (Murray 1991), with the basin depth most likely exceeding 125 m.
Despite the fact that similar sediments of the Ivnik Beds are exposed in outcrops in the wider region, macrofaunal fossil remains are so far limited only to layers of the Činžat locality studied herein.
MATERIAL AND METHODS
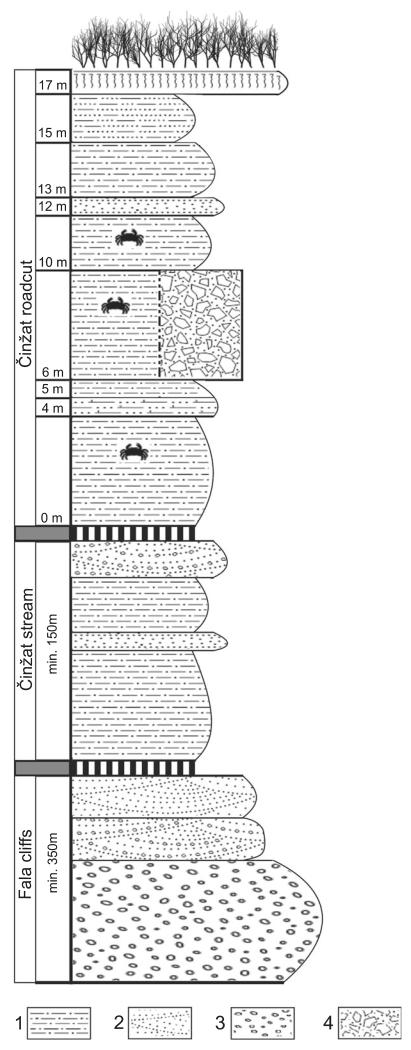
The studied material consists of 245 specimens (Table 2) of decapod crustaceans collected by the senior author from the years 1991 to 2012. The specimens are compressed, but retain most of the original cuticular surfaces. Many brachyurans are preserved as articulated specimens, whereas the ghost shrimp are preserved predominantly as claws. The decapod remains are randomly distributed within the siltstone layers of the studied section, although certain layers and variations in lithology are more likely to contain macrofaunal fossil remains, including decapods (Fig. 3). Interbedded layers of sandstones and conglomerates contain no macrofossils.
TABLE 2.
Representative share of individual species within a sample of decapod crustaceans from Činžat.
| Species | Specimens | Share in sample (%) |
|---|---|---|
| Calliax michelottii | 5 | 2.0 |
| Lepidophthalmus paratethyensis sp. nov. | 36 | 14.7 |
| Callianassidae indet. | 27 | 11.0 |
| Jaxea kuemeli | 1 | 0.4 |
| Styrioplax exiguus | 72 | 29.4 |
| Goneplax gulderi | 3 | 1.2 |
| Neopilumnoplax pohorjensis sp. nov. | 5 | 2.0 |
| Retropluma slovenica sp. nov. | 96 | 39.2 |
FIG. 3.
Simplified log of the early Miocene section at the Činžat locality. Fossil-bearing layers are located only in the uppermost ‘Činžat roadcut’ section. 1, laminated siltstones; 2, sandstones with occasional cross bedding; 3, conglomerates; 4, breccia.
The material was prepared by the authors manually, using fine needles under a Leica EZ-4D stereo microscope. If needed, the specimens were whitened with ammonium chloride prior to photography by a Nikon Coolpix P7800 digital camera. The photographs were not manipulated digitally; only levels and sharpness were adjusted prior to publishing.
SYSTEMATIC PALAEONTOLOGY
This published work and the nomenclatural acts it contains have been registered in Zoobank: http://www.zoobank.org/References/59258F04-5A1F-4464-BEB9-E9CB8-0A84241
The studied material is deposited in the ‘Rok Gašparič palaeontological collection’, part of the palaeontological collections of the Slovenian Museum of Natural History, Ljubljana, Slovenia (RGA/SMNH). Higher systematics follows Ng et al. (2008) and De Grave et al. (2009).
Abbreviations. Mxp 3, maxilliped 3; P1–P5, pereiopods 1–5; H, height; L, length; W, width.
Infraorder AXIIDEA de Saint Laurent, 1979
Family CALLIANASSIDAE Dana, 1852
Subfamily EUCALLIACINAE Manning and Felder, 1991
Genus CALLIAX de Saint Laurent, 1973
Type species
Callianassa (Callichirus) lobata de Gaillande and Lagardère, 1966.
Remarks
Due to changing concepts of the genus, its taxonomic history is rather complex (Hyžný 2012, table 1). Here, the genus concept as presented by Ngoc-Ho (2003) and Hyžný and Gašparič (2014) is adopted.
When dealing with isolated major propodi in the fossil record, the genus can be distinguished based on a rectangular propodus outline, relatively short fingers and possession of two more-or-less parallel ridges positioned at the base of the fixed finger and extending onto the manus (Hyžný and Gašparič 2014). The fossil record of the genus, including remarks on its palaeobiogeography, was recently discussed by Hyžný and Gašparič (2014).
Calliax michelottii (Milne Edwards, 1860) Figures 4–5
1860 Callianassa Michelotti Milne Edwards, p. 341, pl. 14, fig. 3.
1871 Callianassa Michelottii Milne Edwards; Fritsch, p. 691, pl. 17, figs 5–13.
1886 Callianassa Michelottii Milne Edwards; Noetling, p. 84, pl. 5, fig. 4.
1895 Callianassa Michelottii Milne Edwards; Crema, p. 667, fig. 3.
1928 Callianassa Michelottii Milne Edwards; Glaessner, p. 167–168.
1984 Callianassa szobensis Müller, 1984, p. 53 (partim), pl. 7, figs 3–4.
2014 Calliax michelottii (Milne Edwards); Hyžný and Gašparič, p. 45, figs 5–10.
Material
RGA/SMNH 0282, two right major propodi associated with dactyli (Fig. 4A); RGA/SMNH 0588, a near-complete right major propodus associated with dactylus; RGA/SMNH 0622, deformed right major propodus; RGA/SMNH 0640, fragmentary right major propodus; RGA/SMNH 0671, a near-complete left major propodus (Fig. 4B).
FIG. 4.
Calliax michelottii (Milne Edwards, 1860). A, two right propodi associated with dactyli (RGA/SMNH 282). B, major left propodus (RGA/SMNH 671). The specimens were whitened with ammonium chloride prior to photography. Scale bars represent 5 mm.
Description
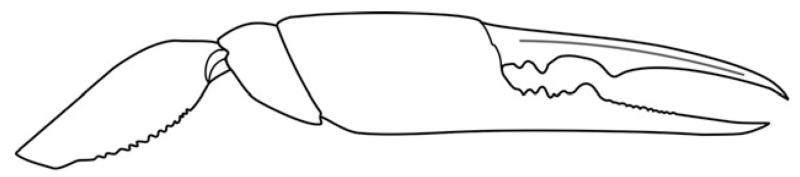
Major propodus rectangular, longer than high (L/H ~ 1.25), with parallel upper and lower margins; fixed finger short, with blunt tooth positioned at mid-point of the occlusal margin, denticulated proximally and possessing two ridges extending onto manus. Dactylus unarmed, with hooked tip (Fig. 5).
FIG. 5.
Calliax michelottii (Milne Edwards, 1860). Reconstruction of the outer side of right major propodus.
Remarks
The material shows strong affinities to Calliax michelottii (Milne Edwards, 1860) as redescribed by Hyžný and Gašparič (2014). However, the preservation of the cuticle is rather poor. In fact, only the fingers retain the cuticular surface; thus, the presence of two parallel ridges on the fixed finger extending onto manus, typical for C. michelottii, is obscured in the material from Činžat. Nevertheless, all other aspects of the chelae clearly point to attribution of the material to this species.
Occurrence
Calliax michelottii is known from numerous Oligocene and Miocene localities in Europe (Hyžný and Gašparič 2014, table 2). Regarding Slovenia, Calliax michelottii has so far been reported from the Miocene strata of Jarenina (Glaessner 1928), Kamnik-Košiše (Hyžný and Gašparič 2014) and Činžat (herein).
Subfamily CALLICHIRINAE Manning and Felder, 1991
Genus LEPIDOPHTHALMUS Holmes, 1904
Type species
Lepidophthalmus eiseni Holmes, 1904, by monotypy.
Remarks
Identification of the genus in the fossil record was discussed in detail by Hyžný and Dulai (in press). They argued that the distinctive shape of the major cheliped merus (tiny meral hook and presence of meral blade) can be used as a proxy character for the assignment of the fossil material to Lepidophthalmus. In the morphology of major chelipeds, the genus is rather similar to Callianopsis de Saint Laurent, 1973, which, however, does not possess a meral blade. Moreover, the morphology of the minor chela is strikingly different in both genera (Hyžný and Dulai in press). Whereas Callianopsis has a sharp distally oriented tooth situated on the occlusal margin of the fixed finger (Schweitzer Hopkins and Feldmann 1997; Hyžný and Schlögl 2011), Lepidophthalmus lacks this. The general shape of the minor chela is also different; that is, in Lepidophthalmus, the minor propodus is more massive (Sakai 1970; Felder and Manning 1997; Felder 2003; Dworschak 2007).
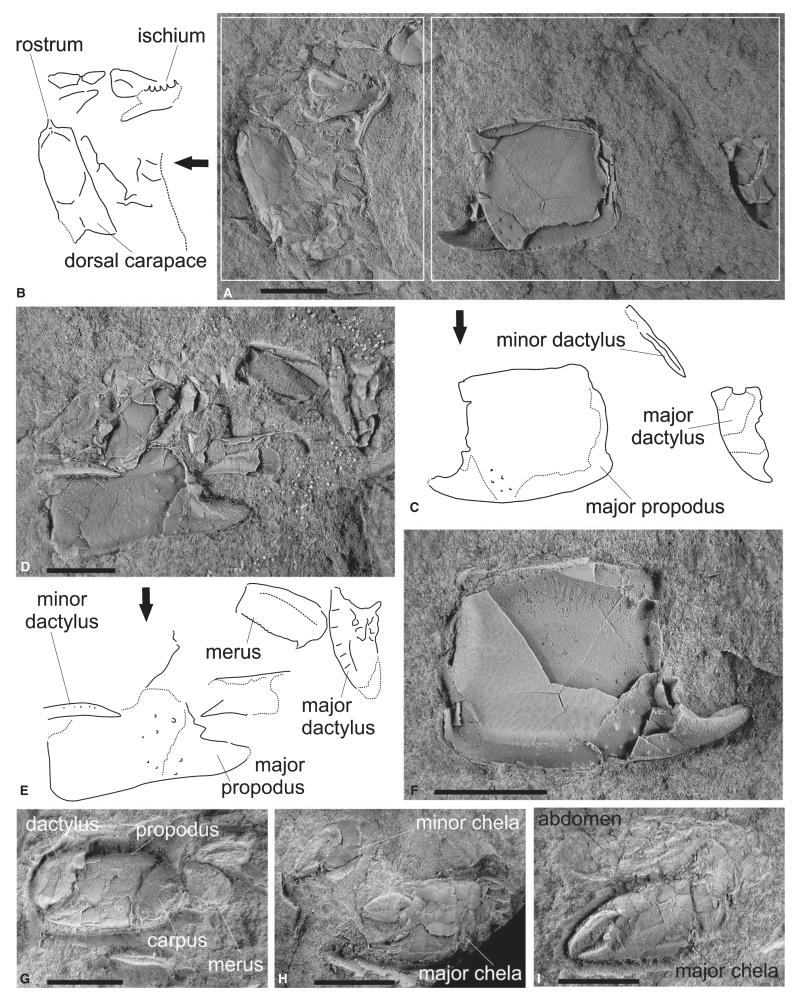
Lepidophthalmus paratethyensis sp. nov. Figures 6–7
LSID
urn:lsid:zoobank.org:act:2DA0BE28-DEFE-465C-82FA-1BA748A0993B
Derivation of name
The epithet refers to the Paratethys, a network of inland seas that spread across much of Central and Eastern Europe during the Oligocene and Miocene.
Holotype
RGA/SMNH 1153–1155, two individuals showing partial carapace and remains of both chelipeds (Fig. 6A–F).
FIG. 6.
Lepidophthalmus paratethyensis sp. nov. A–F, holotype (RGA/SMNH 1153–1155) consisting of two individuals. Note the preservation of dorsal carapace (line drawing in B), remains of both chelae (line drawing C), including major merus with diagnostic features on its lower margin in D (SMNH 1154; line drawing E); major propodus in F (RGA/SMNH 1153) is counterpart of the same structure shown in A (RGA/SMNH 1155). G, paratype (RGA/SMNH 0638) with preserved supposed male major chela. H, paratype (RGA/SMNH 0612) with both chelae of supposed male. I, paratype (RGA/SMNH 0606) with major chela and abdomen of supposed female. All specimens were whitened with ammonium chloride prior to photography. All scale bars represent 5 mm.
Paratypes
RGA/SMNH 0133, right major propodus; RGA/SMNH 0603 (part) and 0614 (counterpart), fragmentary individual; RGA/SMNH 0604 (part) and 0644 (counterpart), right major chela; RGA/SMNH 0606 (part; Fig. 6I) and 0637 (counterpart), left major chela with other remains; RGA/SMNH 0612, major and minor chela in close association (Fig. 6H); RGA/SMNH 0613, right minor propodus; RGA/SMNH 0638 (part; Fig. 6G) and 0639 (counterpart), left major chela; RGA/SMNH 0731, two individuals with both chelipeds; RGA/SMNH 0883, a near-complete major cheliped; RGA/SMNH 1489, right major chela associated with left minor one; RGA/SMNH 1491, right major chela associated with left minor one.
Additional material
RGA/SMNH 0132, isolated right major propodus; RGA/SMNH 0327, merus associated with minor propodus; RGA/SMNH 0572, internal meral plate; RGA/SMNH 0601, left major propodus; RGA/SMNH 0602, right minor chela with carpus; RGA/SMNH 0605, two right major propodi; RGA/SMNH 0607, isolated left major propodus; RGA/SMNH 0611, isolated minor chela; RGA/SMNH 0615, fragments of minor and major chela; RGA/SMNH 0621, isolated merus associated with cheliped fragments; RGA/SMNH 0623, cheliped fragments; RGA/SMNH 0632, major cheliped fragments; RGA/SMNH 0653, remains of major chela; RGA/SMNH 0712, cheliped fragments; RGA/SMNH 0714, cheliped fragments; RGA/SMNH 0736, cheliped fragments associated with a burrow structure; RGA/SMNH 0892, right major propodus associated with left minor one; RGA/SMNH 1220, fragmentary propodus.
Type locality
Činžat, Slovenian part of the Styrian Basin; Ivnik Beds (‘Karpatian’, late Burdigalian in the Mediterranean scale).
Diagnosis
Carapace with dorsal oval. Rostrum long and narrow. Major cheliped ischium with spinose lower margin. Merus ovoid, lower margin with small sharp hook proximally and rounded finely serrated blade distally, lateral surface keeled. Carpus shorter than high, proximo-lower margin rounded. Propodus massive. Manus rectangular, outer lateral surface with several rounded tubercles positioned at base of the fixed finger; distal margin exhibiting two morphotypes: (1) with subtriangular, distally directed tooth undercut by broad notch at base of fixed finger; and (2) without tooth and notch with denticulated occlusal margin of the fixed finger. Dactylus high and robust, occlusal margin armed with one or several broad teeth, lateral surface with longitudinal keel and large setal pits oriented perpendicular to the occlusal surface. Manus of minor cheliped approximately as long as high, distal margin smoothly passing into fixed finger; both fingers long and slender; dactylus unarmed.
Description
Carapace with dorsal oval. Rostrum long, narrow, projecting from rather broad base (Fig. 6B). Major cheliped of presumed male massive. Ischium robust, lower margin armed with large spines (Fig. 6B). Merus ovoid, length about two times height, upper margin straight, lower margin with small sharp hook proximally and rounded finely serrated blade distally (Fig. 6E), lateral surface with keel at midline or closer to the upper margin. Carpus shorter than high, upper margin straight, proximo-lower margin rounded, both margins terminated distally in angular corners. Propodus broad, heavy, articulation with carpus takes place along the whole proximal margin. Manus rectangular (L/H = 0.7–1.1), outer lateral surface with several rounded tubercles positioned at base of the fixed finger; lower margin of propodus distinctly keeled; proximal margin straight; distal margin with subtriangular, distally directed tooth, the tooth usually undercut by broad notch at base of fixed finger. Fixed finger triangular with sharp tip, tip bent gently upward. Dactylus high and robust, upper margin convex, occlusal margin usually armed with several broad teeth, lateral surface of dactylus with longitudinal keel and large setal pits oriented perpendicular to the occlusal surface.
Outline of major cheliped of presumed female similar to presumed male in virtually all aspects. Differences concern mainly the shape of propodus: distal margin of propodus without tooth and notch, smoothly passing into fixed finger, occlusal margin armed with well-developed denticles; lateral surface of propodus less armed. Dactylus less armed, occlusal margin usually forming only one large molariform tooth.
Manus of minor cheliped approximately as long as high, distal margin smoothly passing into fixed finger; both fingers long and slender; dactylus unarmed (Figs 6H, 7C). Abdomen and other appendages unknown, poorly preserved (Fig. 6I) or not preserved at all.
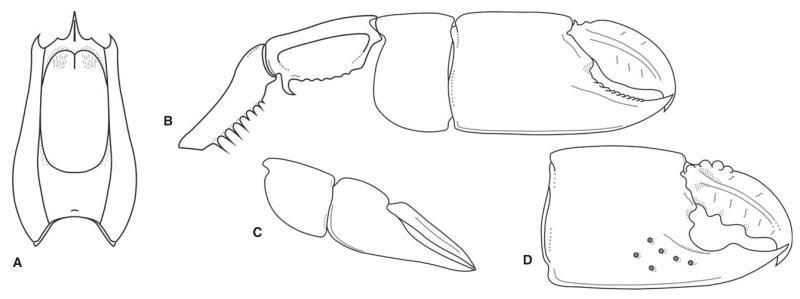
FIG. 7.
Lepidophthalmus paratethyensis sp. nov. reconstruction. A, dorsal carapace (based on the holotype RGA/SMNH 1155). B, major cheliped of supposed female; composite figure based on several specimens. C, minor cheliped. D, outer side of the major propodus and dactylus of supposed male; composite figure based on several specimens; note the presence of tubercles on the upper margin of dactylus.
Variations
The intraspecific variation largely concerns major P1 propodus length/height ratio and the armature of the major P1 dactylus. These two characters partly correlate; deep dactylus with strongly hooked tip and ornamented with tubercles proximally is present mostly in rather short propodi of the supposed male morphotype (with notch, see Fig. 7D), whereas proportionately longer propodi possess less armed dactyli and usually belong to the supposed female morphotype (without notch, see Fig. 7B). This phenomenon is seen also in some extant taxa, where sexually mature males often exhibit a short propodus with large notch at the base of the fixed finger and massive dactylus sometimes with high profile (Sakai 1969). Similar morphological change with maturity has been postulated also for the Late Cretaceous Mesostylus faujasi (Desmarest, 1822) by Mourik et al. (2005).
Remarks
The new species differs from the only known fossil species Lepidophthalmus crateriferus (Lőrenthey in Lörenthey and Beurlen, 1929) from the Oligocene of Hungary, in several aspects. It does not have large longitudinal setal pores on the mesial surface of propodus oriented perpendicular to the upper margin, and the major P1 merus possesses serrated blade, which is rather smooth in L. crateriferus (Hyžný and Dulai in press, fig. 2D–E). The serration on the meral blade is present in many extant species as well (Felder and Manning 1997; Felder 2003; Dworschak 2007). Lepidophthalmus paratethyensis sp. nov. differs from all its extant congeners in the nature of occlusal margin in the major P1 propodus of the supposed female which possesses denticles, often elongated in proximodistal direction.
The rostrum is rather variable in Lepidophthalmus. It can be relatively short, as in L. tridentatus (von Martens, 1868; Sakai 1970, fig. 1b; Dworschak 2007, figs 19–20), or long, as in L. bocourti (Milne Edwards, 1870; Felder 2003, figs 11–12) and L. eiseni Holmes, 1904 (Felder 2003, figs 20–21). The new species has a long rostrum, and in this aspect, it is morphologically close to the type species of the genus.
The studied material clearly exhibits two morphotypes differing in the shape of major propodus and dactylus. They are tentatively interpreted herein as sexual dimorphs (Hyžný and Dulai in press). In extant congeners, similar dimorphs are present. In males, the major P1 propodus often possesses a notch at the base of fixed finger and is heavily armed with tubercles, whereas the dactylus has numerous teeth on the occlusal margin (Sakai 1970, fig. 2a, c; Felder and Manning 1997, figs 1b, 2h, i, 3a, b). Females are armed to a somewhat lesser extent (Sakai 1970, fig. 2d; Felder and Manning 1997, fig. 3c–e). However, it should be noted that animals do not always follow the same pattern; that is, de Man (1928, fig. 21a–f) figured female specimens of L. turneranus (White, 1861) exhibiting armed chelae typical for males and Dworschak (2007, figs 11, 13) figured a female of L. tridentatus exhibiting a large notch at the base of the fixed finger, otherwise characteristic for males. More research is needed to fully understand the sexual dimorphism in extant Lepidophthalmus.
Infraorder GEBIIDEA de Saint Laurent, 1979
Family LAOMEDIIDAE Borradaile, 1903
Genus JAXEA Nardo, 1847
Type species
Jaxea nocturna Nardo, 1847, by original designation.
Jaxea kuemeli Bachmayer, 1954 Figures 8–9
1954 Jaxea kümeli Bachmayer, 1954, p. 64, pl. 1, figs 1–2.
1984 Jaxea kuemeli Bachmayer; Müller, p. 49.
1993 Jaxea kuemeli Bachmayer; Müller, p. 5.
1998 Jaxea kümeli Bachmayer; Mayoral et al., p. 508.
1998 Jaxea kuemeli Bachmayer; Müller, p. 9.
2011a Jaxea kuemeli Bachmayer; Hyžný, p. 176, figs 2–6.
Material
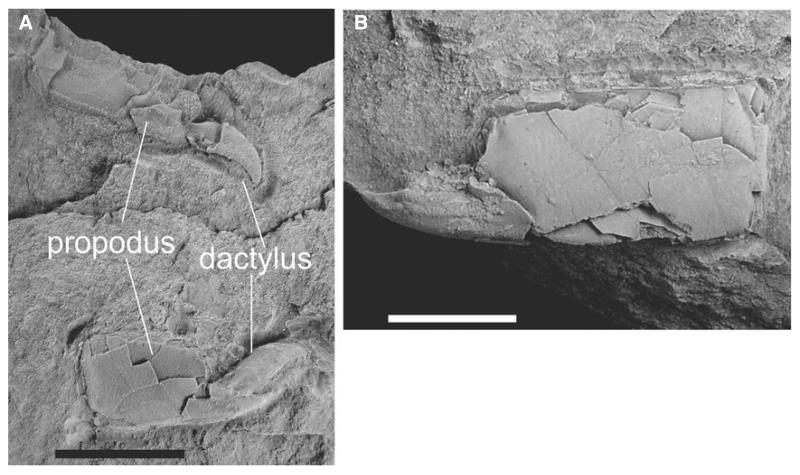
A single specimen (RGA/SMNH 1149) consisting of two chelae, with fragmentary remains of associated carpus and merus (Fig. 8).
FIG. 8.
Jaxea kuemeli Bachmayer, 1954. Two chelipeds of the same specimen (RGA/SMNH 1149). Specimen was whitened with ammonium chloride prior to photography. Scale bar represents 5 mm.
Description
Chelae equal in size, dactylus slightly longer than the fixed finger and cutting edge of both fingers armed with teeth. Polex with three round teeth positioned proximally, followed by a notch and more proximally positioned triangular median tooth, distal half of polex with smaller serrations. Dactylus with two round teeth proximally, followed by a broad notch and a distal median tooth. Manus rectangular, much longer than wide (Fig. 9).
FIG. 9.
Jaxea kuemeli Bachmayer, 1954. Reconstruction of the right cheliped.
Remarks
Despite the small size and poor preservation of the described specimen, the dentition of the chelae is typical for Jaxea kuemeli Bachmayer, 1954, as redescribed by Hyžný (2011b).
Occurrence
The species was originally described from the middle Miocene of Austria (Bachmayer 1954). Hyžný (2011b) revised the taxon and reported further occurrences from the early and middle Miocene of Slovakia and Hungary. The occurrence from Činžat is the first report of the species from Slovenia.
Infraorder BRACHYURA Linnaeus, 1758
Superfamily GONEPLACOIDEA MacLeay, 1838
Family CHASMOCARCINIDAE Serène, 1964
Subfamily CHASMOCARCININAE Serène, 1964
Genus STYRIOPLAX Glaessner, 1969
Type species
Microplax exiguus Glaessner, 1928, by monotypy.
Emended diagnosis
As for species.
Styrioplax exiguus (Glaessner, 1928) Figures 10–12
1928 Microplax exiguus Glaessner, 1928, p. 195, pl. 3, figs 14, 14a; text-fig. 8.
1929 Microplax exiguus Glaessner; Glaessner, p. 258.
1969 Styrioplax exiguus (Glaessner); Glaessner, p. R527, fig. 335/2 (= copy of Glaessner, 1928, text-fig. 8).
2003 Styrioplax exiguus (Glaessner); Karasawa and Kato, p. 145.
2011 Styrioplax exiguus (Glaessner); Hyžný and Schlögl, p. 338, text-figs 12–15.
Material
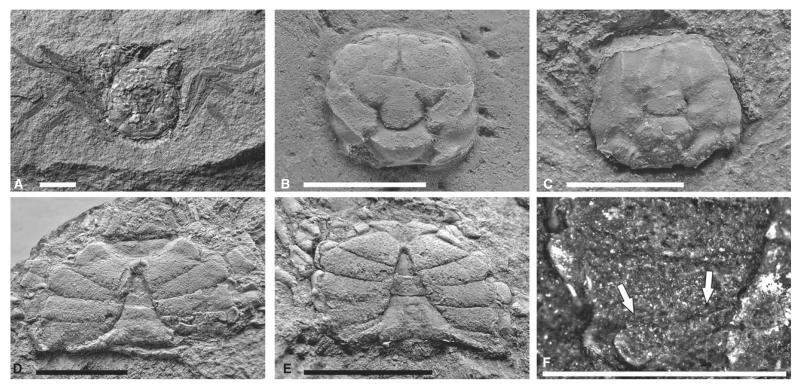
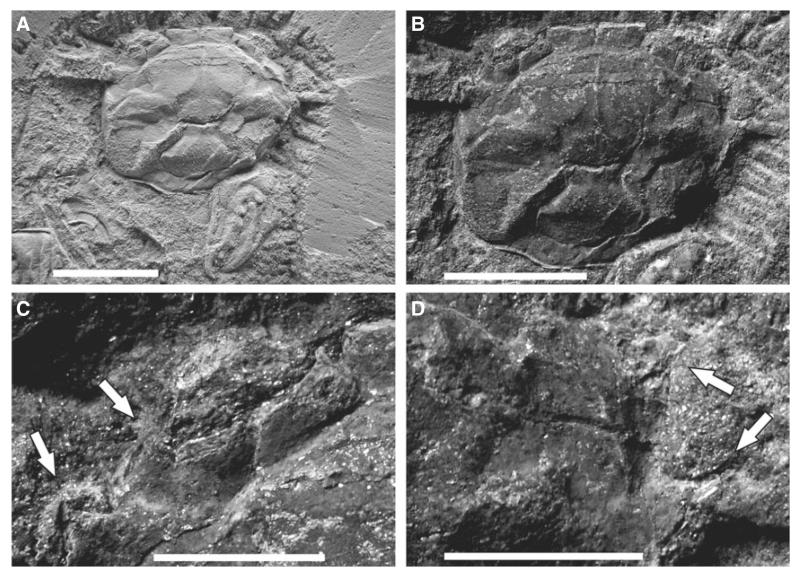
Altogether, 72 specimens preserved in various modes, mostly dorsoventrally compressed, rarely preserved in three dimensions. Almost all specimens are articulated. Diagnostically distinct specimens (Fig. 10A–E): RGA/SMNH 0286, RGA/SMNH 0291, RGA/SMNH 0582, RGA/SMNH 0667, RGA/SMNH 0726, RGA/SMNH 0889, RGA/SMNH 1148, RGA/SMNH 1208, RGA/SMNH 1490, RGA/SMNH 1493, RGA/SMNH 1494 and RGA/SMNH 1497.
FIG. 10.
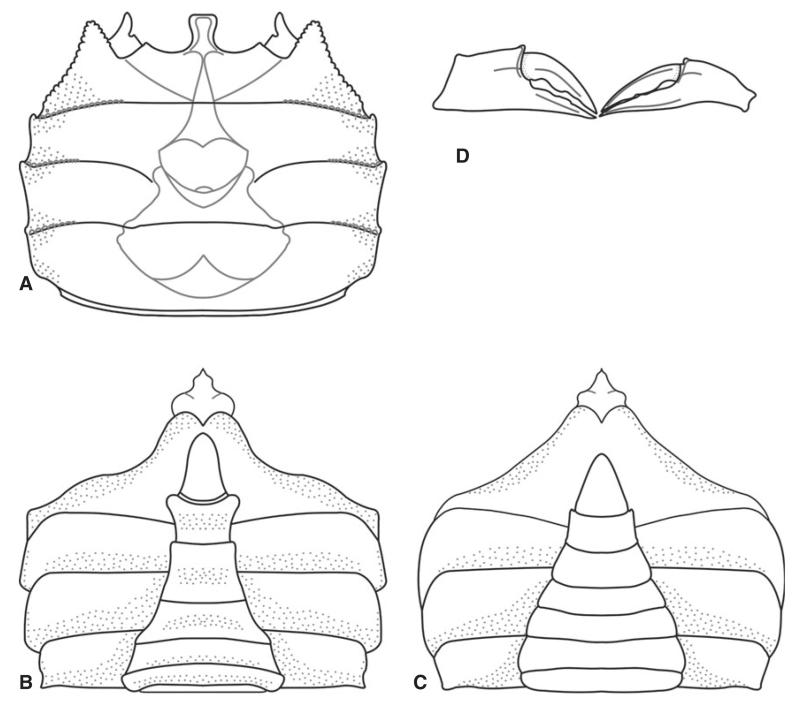
Styrioplax exiguus (Glaessner, 1928). A, complete specimen (RGA/SMNH 0889); note the fragmented and flattened dorsal carapace. B, dorsal carapace of specimen (RGA/SMNH 1490) showing three-dimensional preservation. C, dorsal carapace of specimen (RGA/SMNH 1208). D, male specimen (RGA/SMNH 0291) showing venter and merus of Mxp 3. E, male specimen (RGA/SMNH 1493) showing venter and supplementary coxosternal plate on sternite 8. F, close-up of supplementary coxosternal plate (arrows) on specimen (RGA/SMNH 1493). Specimens in B, C, D and E were whitened with ammonium chloride prior to photography. All scale bars represent 5 mm.
Emended diagnosis
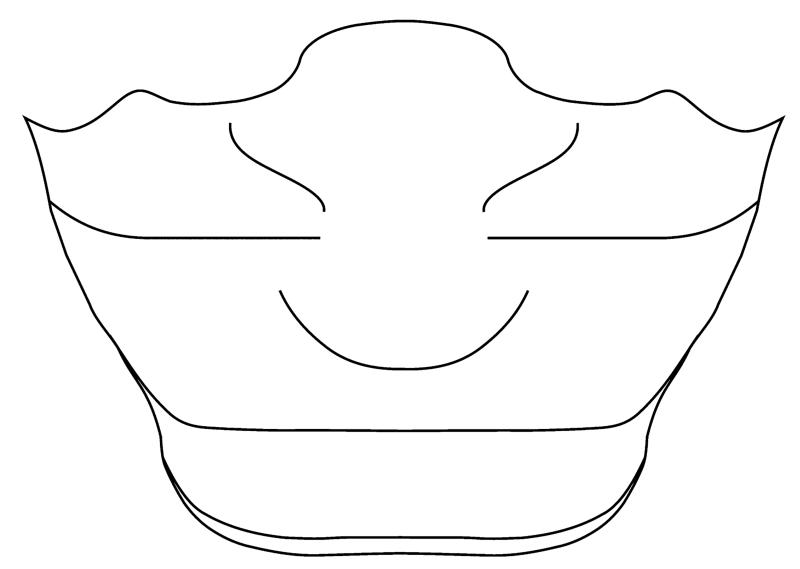
Carapace subrectangular to trapezoid, slightly wider than long (W/L ~ 1.10), widest at mid carapace (Fig. 12). Front bilobed, downturned and protruding beyond the orbits, which are shallow and oriented forward. Sinuous front margin with postorbital protrusion, anterolateral margin rounded. Lateral margin progressively steeper posteriorly, sinuous at first and straight after the posterolateral flange. Posterior margin centrally concave and rimmed, with developed re-entrants for fifth pereiopods. Defined gastric and cardiac regions with diagonal mesobranchial ridge. Male abdominal somites 3–5 fused, genital groove covered by a supplementary coxosternal plate between sternites 7 and 8. Chelipeds unequal, right cheliped larger. Pereiopods P2–P5 long and slender, P4 longest.
Description
Small subrectangular to trapezoid carapace, almost as long as wide (width/length ratio c. 1.10), widest at posterolateral margins and narrowing anteriorly. Carapace transversely and longitudinally convex, high domed, higher posteriorly with steep posterolateral margins. Protruding, bilobed and rimmed rostrum, broader at the base. The furrow between lobes deeper in juveniles, juvenile rostrum consisting of two separated round protrusions, in adults distal rostrum border almost straight or even slightly concave with shallow notch. Small, shallow orbits are directed forward and followed by a postorbital sinuous margin, which forms an indistinct postorbital tooth in adult specimens. Anterolateral margin rounded and convex. Anterior half of lateral margin sinuous, widening posteriorly, with median projection. Carapace widest at mid-point, where lateral margin continues into a defined mesobranchial ridge and forms a linear lateral projection (Fig. 10B). Lateral margin progressively steeper posteriorly. Posterolateral margin straight with observed concave re-entrants in P5 (Fig. 10C). Posterior margin sinuous and centrally concave with rimmed posterior border.
Dorsal carapace mostly smooth to granulose with occasional raised tubercles, which are more common in frontal and gastric region. Gastric region clearly defined, posterior border defined by cervical groove, deeply incised and concave, fainter lateral borders converging towards front, terminating with depressed furrow, which ends just behind the centrally bilobed front. Posterior part of gastric region swollen, anterior part flat. Protogastric regions flat and broadly triangular. Border to epibranchial region defined only centrally, near gastric region, by continuing cervical groove, deep and incised at first, but disappearing anteriorly. Cardiac region well-defined ovate to trapezoid, swollen and raised centrally (Fig. 11A). Cardiac region laterally separated from branchial region by sinuous groove, its posterior border straight. Epibranchial region wide, posteriorly limited by mesobranchial ridge extending diagonally from lateral border, where it forms a blunt projection, halfway towards cardiac region. Mesobranchial region long, narrow and sickle shaped, not fully separated, but posterior border marked with a fainter metabranchial ridge running parallel to mesobranchial ridge (Fig. 10B). Metabranchial region trapezoid shaped, lateral sides steep, slightly swollen centrally.
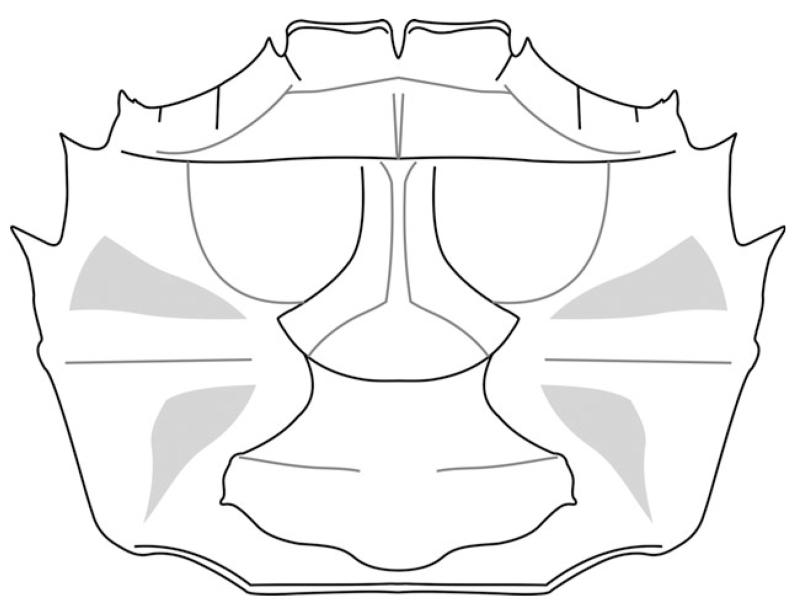
FIG. 11.
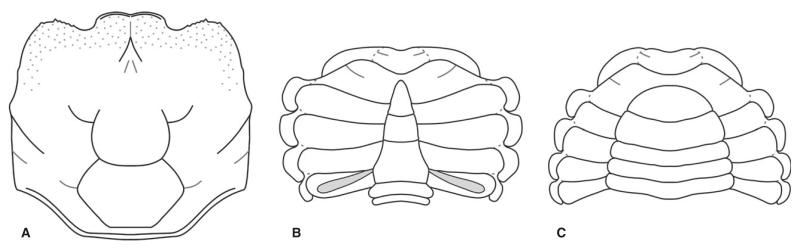
Styrioplax exiguus (Glaessner, 1928), reconstruction. A, dorsal carapace. B, male venter. C, female venter.
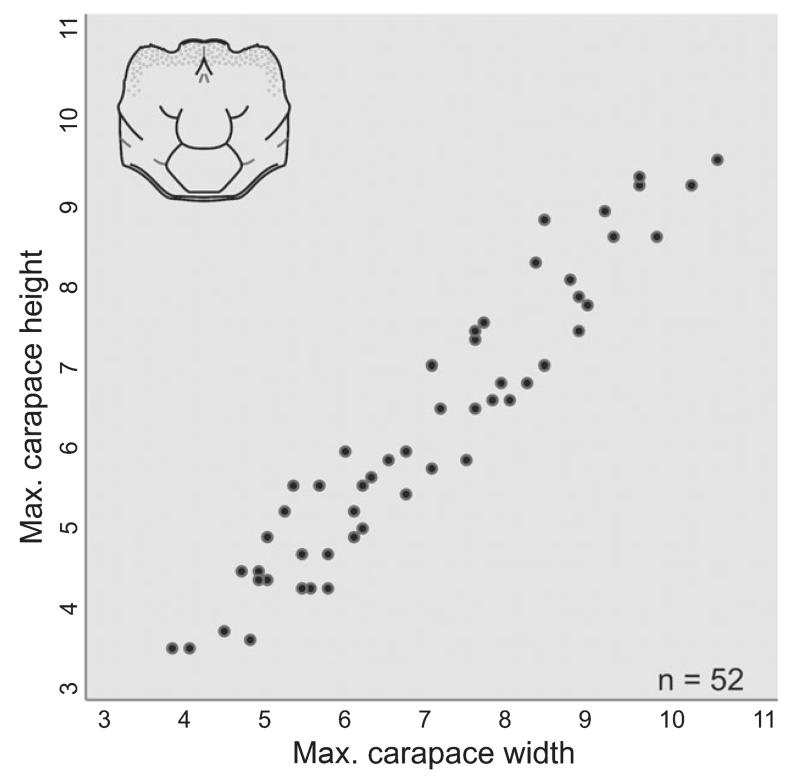
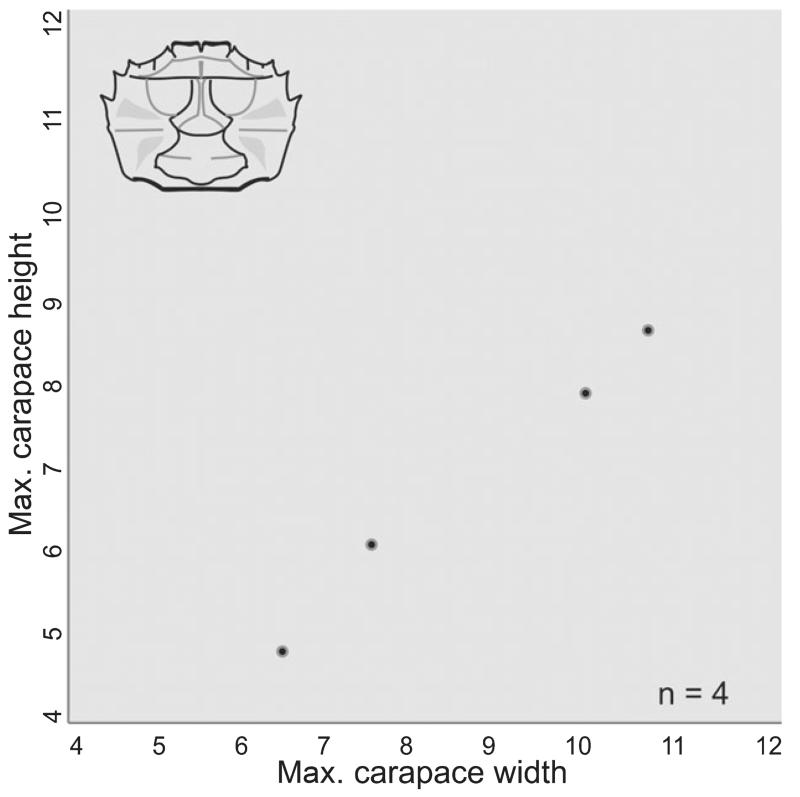
FIG. 12.
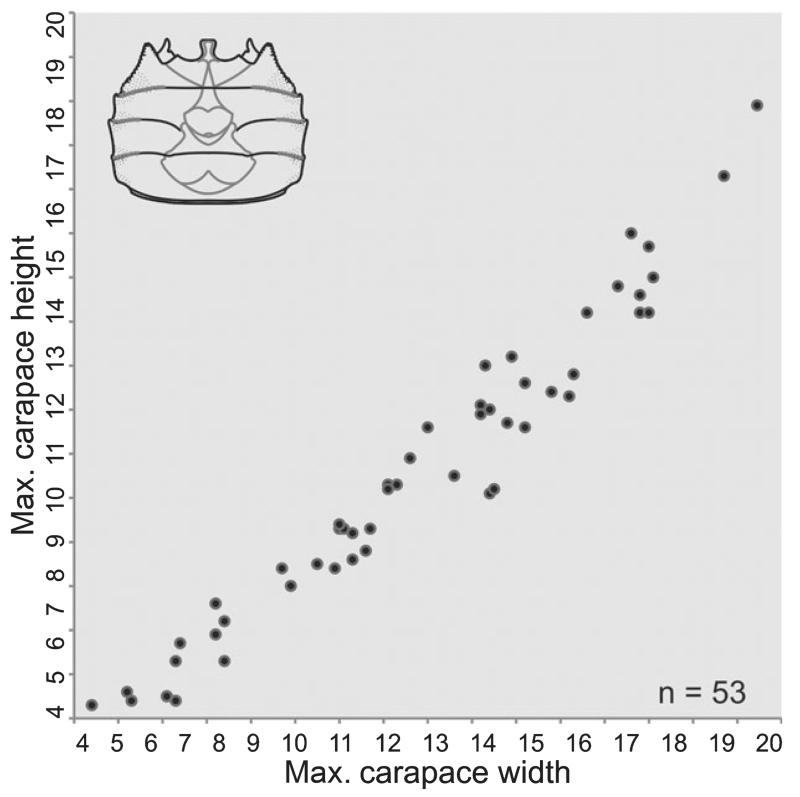
Diagram of maximum carapace width and height measurements in mm for Styrioplax exiguus (Glaessner, 1928).
Sternum broadening posteriorly with concave sutures of sternites; sutures between sternites 1 and 2 fused, sutures between sternites 3 and 4 fused, sutures between sternites 4 and 8 distinct. Sternite 8 visible ventrally, partly overlies sternite 7. Sternites 4–7 form prominently expanded lateral episternites (Figs 10D, 11C). Female pleon not fused, all somites free, narrowing anteriorly in ventral view lateral margin convex. Female telson wider than long, semicircular in shape (Fig. 11C). Male pleon narrow and pointed, lateral margins concave, third somite being the widest, slightly convex laterally. Telson longer than wide and triangular, somite 6 about as wide as long. Somites 3–5 fused with faint median ridge; posterior portion of somites 3–5 widest. Somites 2 and 1 short, not always preserved (Fig. 10D–E). Visible median ridge on the posterior part of pleon. Sternite 8 in males slightly folded with a deep groove in the middle, which is covered by an intercalated supplementary coxosternal plate. Coxosternal plate almost as long as sternite 8 and half as wide, expanding laterally and terminating with a concave margin. Merus of Mxp 3 suboval (Fig. 10F).
Chelipeds unequal, right cheliped stouter and larger. Carpus quadrate, propodus and dactylus longer and curved. Cutting edge of polex and dactylus dentate with pointed tips. Pereiopods P2–P5 achelate, long and slender, P4 longest, surface finely granulose and flattened, ending in straight styliform dactyli (Fig. 10A).
Remarks
Hyžný and Schlögl (2011) recognized rhizopine pilumnid characters in Styrioplax exiguus, which was previously assigned to Goneplacidae by Glaessner (1969). The newly collected material allows the generic diagnosis to be revised as well as placement of the genus in Pilumnidae.
All Styrioplax exiguus material so far described originates from fine-grained clastic rocks, mostly mudstones and siltstones, which have a very high compaction rate during lithification (Harms et al. 1982). Therefore, most of the specimens exhibit a set of key carapace features that are somewhat distorted due to the dorsoventral compression. This is not obvious during first examination, because the specimens are small and their cuticle seems poorly calcified and easily deformable. This also explains the lack of fragmentation during compression. What can be observed from several new three-dimensionally preserved specimens is that the carapace was originally highly vaulted posteriorly, but not distinctly wider, and after compaction, this height is distorted in posteriorly wider lateral flange. The same is true for dorsal carapace regions; on compressed specimens, all regions except the crescent-shaped cervical groove are obliterated. In better preserved specimens, one can observe the true shape of the frontal and lateral margin, as well as inflated dorsal regions with mesobranchial ridge.
Well-preserved male abdominal details with fused abdominal somites 3–5 and intercalated supplementary coxosternal plate on sternite 8 (Fig. 10F) question the position of Styrioplax exiguus in Rhizopinae as suggested by Hyžný and Schlögl (2011). As Karasawa and Kato (2003) noted (although they did not study the type material first hand), assignment to the chasmocarcinid subfamily Chasmocarcininae seems more appropriate.
Members of Chasmocarcininae are defined by trapezoidal carapace, a bilobed or flared front, orbits with sinuous upper margins, a fronto-orbital width that occupies almost the entire frontal margin, well-developed posterolateral re-entrants and a fronto-orbital width to posterior width ratio of about 1.0 (Schweitzer and Feldmann 2001). Additionally, they typically have male abdominal somites 3–5 fused and possess long, narrow pereiopods (Feldmann et al. 2010). Styrioplax exhibits all of the abovementioned characteristics and also possesses a supplementary coxosternal plate on sternite 8 (Guinot et al. 2013); thus, no doubt remains that it should be reassigned among Chasmocarcininae.
Of similar fossil and extant Chasmocarcininae, Styrioplax has a unique set of morphological characters. On the one hand, the front carapace shape including straight, forward-facing orbits is similar to Chasmocarcinus Rathbun, 1898, but the defined dorsal carapace regions with mesobranchial ridge are similar to the features of the members of Falconoplax Van Straelen, 1933. On the other hand, the supplementary coxosternal plate covering the genital groove is similar, but different in shape to that in Chasmocarcinus, and it differs from Falconoplax, which has an open genital groove on sternite 8. Additionally, Styrioplax differs from Chasmocarcinus by its narrower length-to-width ratio, which is 0.9, rounded anterolateral margin and sinuous lateral margin with projection where it intersects with mesobranchial ridge. Therefore, Styrioplax represents a clearly differentiated genus within the Chasmocarcininae.
Family GONEPLACIDAE MacLeay, 1838
Genus GONEPLAX Leach, 1814
Type species
Ocypoda bispinosa Lamarck, 1801, by original designation.
Goneplax gulderi Bachmayer, 1953 Figures 13–14
1953 Goneplax gulderi Bachmayer, 1953, p. 143, pl. 9, figs 1–3.
1984 Goneplax gulderi; Müller, p. 96, pl. 93, figs 2–3.
1993 Goneplax gulderi; Müller, p. 23, fig. 11.
1998 Goneplax gulderi; Mayoral et al., p. 508, fig. 2 (5).
1998 Goneplax gulderi; Müller, p. 38.
2006 Goneplax gulderi; De Angeli and Garassino, p. 65.
2009 Goneplax gulderi; De Angeli et al., p. 187, fig. 18A, B.
2012 Goneplax gulderi; Garassino et al., p. 46.
2013 Goneplax gulderi; Pasini and Garassino, p. 321.
2013 Goneplax gulderi; Garassino et al., p. 357, fig. 1A.
Material
Three specimens exhibiting a partially preserved carapace, RGA/SMNH 0635 (Fig. 13), RGA/SMNH 0649, RGA/SMNH 0733.
FIG. 13.
Goneplax gulderi Bachmayer, 1953. Partially preserved carapace of specimen (RGA/SMNH 0635). Specimen was not whitened with ammonium chloride prior to photography. Scale bar represents 5 mm.
Description
Carapace trapezoidal, wider than long, with two distinct transverse ridges. Carapace widest at position of anterolateral teeth, then converging posteriorly. Front protruding, truncate, slightly rounded. Wide and sinuous supraorbital margin, with a small, rounded postorbital tooth. Anterolateral margin wide, convex, ending anteriorly with forward-facing, pointy, postorbital tooth. Lateral margin poorly preserved, but a protrusion is noticeable at lateral border where it meets the lowermost median transverse ridge (Fig. 14).
FIG. 14.
Goneplax gulderi Bachmayer, 1953. Reconstruction of dorsal carapace.
Remarks
The specimens differ from Goneplax rhomboides (Linnaeus, 1758) (Garassino et al. 2012), in having more convex anterolateral margins with shorter anterolateral teeth. Relatively wide front is reminiscent of published figure of Goneplax sacci Crema, 1895; the species was recently considered a nomen dubium by Garassino et al. (2013).
Family MATHILDELLIDAE Karasawa and Kato, 2003
Genus NEOPILUMNOPLAX Serene, 1969
Type species
Pilumnus heterochir Studer, 1883, by original designation.
Diagnosis
Carapace depressed, flat, a little wider than long, more or less hexagonal; regions faintly indicated. Front straight; anterolateral margin oblique, toothed. Supraorbital margin with one or two incisions. Antennal flagellum standing in orbital hiatus without or with a small distal lateral lobule on basal joint, only partly filling the orbital hiatus. Chelipeds slightly unequal. Pleon with seven segments, 1–3 of male covering whole sternal width between 5th coxae. Male pleopod 2 short (after Miers 1886 based on the type specimen of Pilumnus heterochir).
Remarks
Six extant species are known so far (Table 3). Concerning fossil representatives, Pilumnoplax hannibalanus Rathbun, 1926, from the Eocene of the North American West Coast was re-assigned to Neopilumnoplax by Tucker and Feldmann (1990). However, Schweitzer (2000) revised the species and synonymized it with Branchioplax washingtoniana Rathbun, 1916; leaving Neopilumnoplax pohorjensis sp. nov., the only known fossil member of the genus. Several Paleocene to Oligocene members of the Mathildellidae have been described from North America (Karasawa et al. 2008), but no Neogene representatives of the family were known so far.
TABLE 3.
Geographical distribution of extant Neopilumnoplax species.
| Species | Occurrence | Depth (m) |
|---|---|---|
| Neopilumnoplax americana (Rathbun, 1898) | South-east USA, Mexico, Cuba, Brazil | 130–800 |
| Neopilumnoplax gervaini Tavares and Guinot, 1996 | Carribean | 350 |
| Neopilumnoplax heterochir (Studer, 1883) | Australia, Tasmania | 500 |
| Neopilumnoplax nieli Ahyong, 2008 | New Zealand, South Australia | 430–1050 |
| Neopilumnoplax sinclairi (Alcock and Anderson, 1899) | South Africa, India | 285 |
| Neopilumnoplax lipkeholthuisi Tavares and De Melo, 2010 | Brazil, Argentina | 125–200 |
Neopilumnoplax pohorjensis sp. nov. Figures 15–17
LSID
urn:lsid:zoobank.org:act:B1489723-C603-45B0-99DA-AA218DE40016
Derivation of name
The species name refers to the type locality in the Pohorje Mountains range in north-eastern Slovenia.
Holotype
RGA/SMNH 1147 (part; Fig. 15) and 1151 (counterpart), a complete carapace with associated cheliped.
FIG. 15.
Neopilumnoplax pohorjensis sp. nov.; holotype (RGA/SMNH 1147). A, dorsal carapace with associated cheliped. B, details of anterolateral margin. C, close-up of left anterolateral margin, with arrows pointing to 1st and 3rd anterolateral teeth. D, close-up of right anterolateral margin, with arrows pointing to 3rd and 4th anterolateral teeth. Specimen in A was whitened with ammonium chloride prior to photography. Scale bars represent 5 mm in A and B and 2 mm in C and D.
Paratypes
RGA/SMNH 0142 and RGA/SMNH 0645, both isolated carapaces and dorsoventrally compressed.
Additional material
An isolated cheliped RGA/SMNH 0142.
Type locality
Činžat, Slovenian part of the Styrian Basin; Ivnik Beds (‘Karpatian’, late Burdigalian in the Mediterranean scale).
Diagnosis
Subhexagonal carapace, wider than long (W/L ~ 1.25). Dorsal carapace surface finely granulose, with well-defined regions, strong branchial ridges, deflected front and well-developed mesogastric anterior process. Front truncate, bimarginate and divided by a distinct median notch. First and second anterolateral tooth fused, rounded, third and fourth anterolateral tooth spine-like and forward facing. Fifth anterolateral tooth obsolete.
Description
Carapace subhexagonal, 1.25 times wider than long (Fig. 17), maximum width at third anterolateral tooth. Front truncate, bimarginate, formed by transverse anterior groove and divided by a distinct median notch (Fig. 15B). Supraorbital margin low and divided from front with a V-shaped notch, thus forming a small pointed supraorbital tooth at its end. Orbits wide, incised by a shallow fissure centrally and again before first anterolateral tooth. First (postorbital) and second anterolateral tooth basally fused, rounded, second tooth apex somewhat larger and pointed (Fig. 15C). Third and fourth anterolateral tooth spine-like and facing slightly outwards (Fig. 15D). Fifth anterolateral tooth obsolete, reduced to a raised lateral tubercle. Posterolateral margin with noticeable concavity, whole carapace narrowing posteriorly, posterior margin almost straight.
Dorsal carapace surface finely granulose, with granules arranged in rows along anterolateral margin and orbits. Mesogastric region clearly defined, posterior border, with sinuous lateral borders and with mesogastric anterior process somewhat down-bent anteriorly. Centrally and posteriorly mesogastric region flat and raised, with depressed furrow near the protogastric border. Protogastric regions flat and subovate. Posterior margin crescent-shaped, anterior straight, then dropping slightly to the deeper set, subrectangular and wide epigastric region. Cardiac region well defined, somewhat swollen and separated from metabranchial region by a raised ridge, posterior margin sinusoidal with a central concavity. Cardiac region wide, occupying almost half of carapace width, posteriorly even wider. Anterior part of cardiac region depressed, then it swells posteriorly with its highest part forming a ridge at two-thirds length. Branchial region well defined and wide, prominent epibranchial and metabranchial ridges extending diagonally from antero-/posterolateral border inwards, with flat triagonal mesobranchial region in between. Intestinal region flat, narrow and wide, following the outline of posterior carapace margin. Both frontal and orbital region deflected and rimmed anteriorly (Fig. 16).
FIG. 16.
Neopilumnoplax pohorjensis sp. nov. Reconstruction of dorsal carapace based on the holotype (RGA/SMNH 1147).
Propodus and dactylus of cheliped stout, cutting edge bluntly dentate. Finger apices pointy and crossing, leaving a gap between fingers (Fig. 15A). P2–P5 unknown.
Remarks
Of the six extant species listed in Table 3, Neopilumnoplax pohorjensis sp. nov. most resembles N. lipkeholthuisi Tavares and De Melo, 2010. The newly described species is similar to N. lipkeholthuisi in the dorsal aspect of the carapace, but can be easily separated by a more pronounced V-shaped notch between front and supraorbital margin, first and second anterolateral tooth fused, and retaining separate apices with second tooth terminating at a point. Third and fourth anterolateral teeth resembling spines and pointing slightly outwards, unlike in N. lipkeholthuisi, where they are forward facing. Dorsal carapace regions of N. pohorjensis are well defined with strong branchial ridges and a well-developed mesogastric anterior process, which is not present in N. lipkeholthuisi. The frontal and orbital regions are more depressed in N. pohorjensis.
FIG. 17.
Diagram of maximum carapace width and height measurements in mm for Neopilumnoplax pohorjensis sp. nov.
Neopilumnoplax pohorjensis shares a similar arrangement of anterolateral teeth and prominent branchial ridge with extant N. nieli Ahyong, 2008. It differs from the extant species by much more clearly defined dorsal regions, especially in posterior part of the carapace. The cardiac region is raised and swollen, and posteriorly depressed in N. pohorjensis, the epibranchial ridge is stronger, and the carapace possesses a strong metabranchial ridge. Additionally, the posterolateral border of N. pohorjensis has a wider concavity and the anterolateral teeth point slightly outwards, with the second tooth being pointed and not rounded as in N. nieli.
Superfamily RETROPLUMOIDEA Gill, 1894
Family RETROPLUMIDAE Gill, 1894
Genus RETROPLUMA Gill, 1894
Type species
Archaeoplax notopus Alcock and Anderson, 1894, by monotypy.
Remarks
All species of the genus Retropluma share a set of characteristic carapace features including carapace more or less quadrangular flattened and traversed by carinae, front truncate, exposed orbits, narrow single-lobed rostrum, pincer-shaped chelipeds, long slender P2–P4 and diminutive setose P5, which are linked to the reduced size of sternite 8 (McLay 2006a). The known Retropluma species are separated by differences in the ornamentation of the carapace, position of anterolateral teeth and rostrum shape. Other distinctive features like relative size of the ocular peduncles (de Saint Laurent 1989) are not present in fossil material.
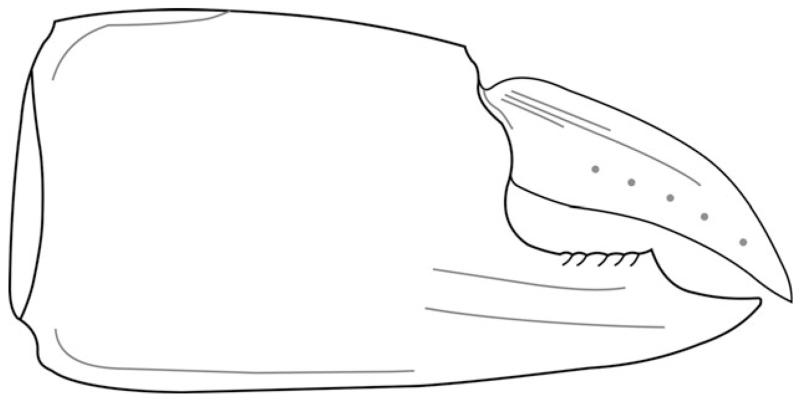
Retropluma slovenica sp. nov. Figures 18–21
LSID
urn:lsid:zoobank.org:act:E2EF5869-DA16-485E-A36F-74973B8695D6
Derivation of name
The species name refers to Slovenia, the country where the type locality is situated.
Holotype
RGA/SMNH 1203 (Fig. 18A), an isolated well-preserved carapace without rostrum.
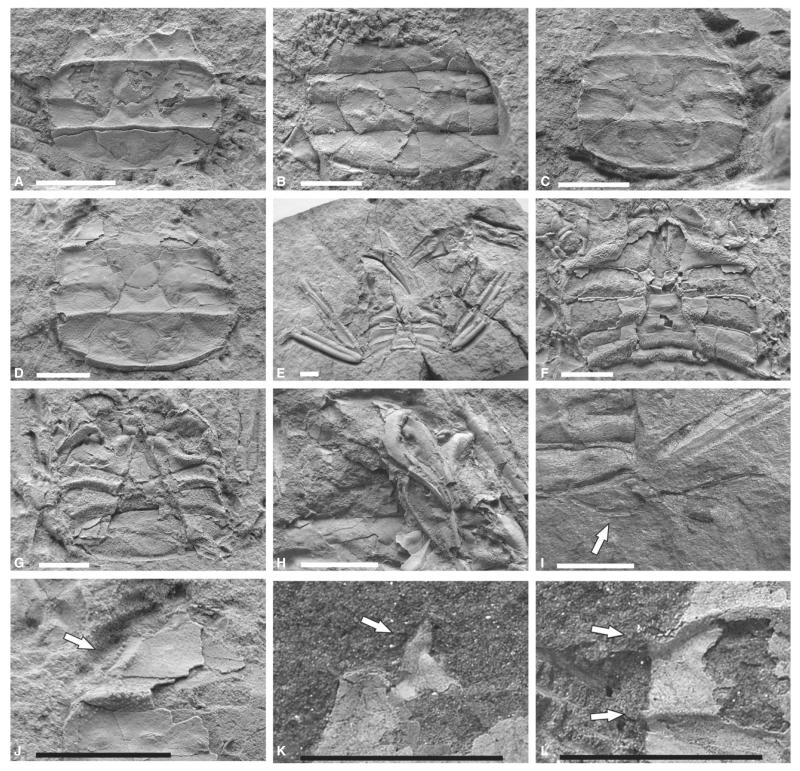
FIG. 18.
Retropluma slovenica sp. nov. A, dorsal carapace of the holotype (RGA/SMNH 1203). B, dorsal carapace of the paratype (RGA/SMNH 0554), showing preserved rostrum. C, complete dorsal carapace of the paratype (RGA/SMNH 0642). D, complete dorsal carapace of the paratype (RGA/SMNH 0705) with good lateral and posterior details. E, complete male specimen (RGA/SMNH 0674) showing venter, chelipeds P2–P5 (with reduced P5). F, male specimen (RGA/SMNH 0673) showing venter. G, female specimen (RGA/SMNH 0318) showing venter. H, chelipeds of a male specimen RGA/SMNH 0659. I, significantly reduced P5 (arrow) of the specimen RGA/SMNH 0290. J, anterolateral margin of the specimen (RGA/SMNH 0705) showing serrated edge (arrow). K, anterolateral margin of the specimen RGA/SMNH 1203 showing preserved infraorbital tooth (arrow). L, lateral margin of the specimen (RGA/SMNH 1203) showing preserved lateral teeth on contacts with carinae (arrows). Specimens A–J were whitened with ammonium chloride prior to photography. All scale bars represent 5 mm.
Paratypes
RGA/SMNH 0270 (part) and RGA/SMNH 0290 (counterpart), complete dorsal specimen, with infraorbital teeth and fifth pereiopods; RGA/SMNH 0554 (part; Fig. 18B) and RGA/SMNH 0559 (counterpart), internal cast of dorsal carapace with well-developed rostrum; RGA/SMNH 0642 (part; Fig. 18C) and RGA/SMNH 0619 (counterpart), dorsal carapace with rostrum; RGA/SMNH 0658 (part) and RGA/SMNH 0659 (counterpart; Fig. 18H), male ventral detail and chelipeds; RGA/SMNH 0673 (part; Fig. 18F) and RGA/SMNH 0674 (counterpart; Fig. 18E), complete ventral specimen with male pleon and chelipeds; RGA/SMNH 0707, partial dorsal carapace with rostrum; RGA/SMNH 0710, dorsal carapace.
Additional material
RGA/SMNH 0173, RGA/SMNH 0267, RGA/SMNH 0318 (part) and RGA/SMNH 0319 (counterpart), RGA/SMNH 0320, RGA/SMNH 0334 (part) and RGA/SMNH 0335 (counterpart), RGA/SMNH 0555 (part) and RGA/SMNH 0556 (counterpart), RGA/SMNH 0558 (part) and RGA/SMNH 0553 (counterpart), RGA/SMNH 0656, RGA/SMNH 0657, RGA/SMNH 0662, RGA/SMNH 0663, RGA/SMNH 0705, RGA/SMNH 0724, RGA/SMNH 0735, RGA/SMNH 1142 (part) and RGA/SMNH 1143 (counterpart), RGA/SMNH 1208 (part) and RGA/SMNH 1207 (counterpart), RGA/SMNH 1216, RGA/SMNH 0139, RGA/SMNH 0145, RGA/SMNH 0275, RGA/SMNH 0287, RGA/SMNH 0322, RGA/SMNH 0548, RGA/SMNH 0549, RGA/SMNH 0557, RGA/SMNH 0561, RGA/SMNH 0564, RGA/SMNH 0631, RGA/SMNH 0633, RGA/SMNH 0641, RGA/SMNH 0646, RGA/SMNH 0654, RGA/SMNH 0655, RGA/SMNH 0660, RGA/SMNH 0664, RGA/SMNH 0672, RGA/SMNH 0725, RGA/SMNH 0732, RGA/SMNH 0886 (part) and RGA/SMNH 0887 (counterpart), RGA/SMNH 0890, RGA/SMNH 1206, RGA/SMNH 1209, RGA/SMNH 1212, RGA/SMNH 1213, RGA/SMNH 0129, RGA/SMNH 0135, RGA/SMNH 0147, RGA/SMNH 0153, RGA/SMNH 0156, RGA/SMNH 0163, RGA/SMNH 0166, RGA/SMNH 0239, RGA/SMNH 0264, RGA/SMNH 0284, RGA/SMNH 0298, RGA/SMNH 0321, RGA/SMNH 0551, RGA/SMNH 0560, RGA/SMNH 0562, RGA/SMNH 0563, RGA/SMNH 0625, RGA/SMNH 0526, RGA/SMNH 0629, RGA/SMNH 0636, RGA/SMNH 0647, RGA/SMNH 0651, RGA/SMNH 0661, RGA/SMNH 0668, RGA/SMNH 0669, RGA/SMNH 0717, RGA/SMNH 1145, RGA/SMNH 1204 and RGA/SMNH 1217.
Type locality
Činžat, Slovenian part of the Styrian Basin; Ivnik Beds (‘Karpatian’, late Burdigalian in the Mediterranean scale).
Diagnosis
Subrectangular carapace wider than long (W/L ~ 1.20), well-developed anterolateral tooth, pointing slightly outwards. Rostrum curved downward, the same length as anterolateral tooth, with concave sides and significantly wider at distal end. Ventrally positioned infraorbital tooth, closely followed and partly overlapping with anterolateral tooth. Dorsal carapace with three distinct transverse carinae, forming variably pronounced blunt teeth at contact with lateral carapace margin. The tooth on anterior carina border most pronounced.
Description
Subrectangular carapace, 1.20 times wider than long (Fig. 21), maximum width at the level of median carina. Carapace gently convex longitudinally and transversely nearly flat. Rostrum, as long as anterolateral tooth, downward depressed, with concave sides and significantly wider distal end (Fig. 18B). Supraorbital margin is wide with pronounced supraorbital tooth, deep and marked orbits. Infraorbital tooth almost as long as postorbital tooth, but positioned ventrally and rarely observed in fossil material (Fig. 18K). Infraorbital tooth is closely followed and partly overlapping with well-developed postorbital tooth, with concave outer lateral side, pointing outward by 2–3 degrees. Long anterolateral margin, concave till anterior ridge. Postorbital tooth appears almost tuberculated and anterolateral margin densely granulose in adult specimens (Fig 18J). Lateral and posterior margins convex, with well-developed concave re-entrant in P5 at their posterolateral junction.
Dorsal carapace with three distinct transverse carinae, forming variably pronounced blunt teeth at contact with lateral carapace margin (Fig. 18L). Anterior carina almost straight, continuously parallel to anterior border, slightly curving posteriorly in last third towards lateral margin, where it forms a projection, which is most pronounced of the three. Median carina strongly developed at flanks only, interrupted by well-marked urogastric region. Posterior carina sinuous and well formed, convex laterally, then slightly concave centrally after intersecting with cardiac region, its lateral projections least distinct and completely absent in juveniles. Whole dorsal carapace surface is finely granulose. Anterolateral and lateral margins bear bigger tubercles, which extend for a short distance along anterior carina. Tubercles on anterolateral margin larger than those more posteriorly on lateral margin. Posterior margin well-marked, with distinct, fine striation marks on the cuticle of posterior part of dorsal carapace (Fig. 18D).
The mesogastric region is pointedly pentagonal and continues over anterior carina in a long anterior process with bilobed ending close to the rostrum. All sides of mesogastric region are concave, and posterior border is divided into two lobes. The median part, continuing into anterior process and posteriorly into urogastric region, is raised. Protogastric regions are not clearly defined; they form a rectangular outline together with hepatic region and are diagonally divided by the anterior carina. The urogastric region is well defined and semicircular or crescent shaped, anteriorly separated from the mesogastric region by two lobes; posterior border is marked by a distinct cervical groove, running from lateral border of anterior carina to the concave lobe delimiting the urogastric region. At its most posterior position, almost centrally, a cervical groove forms a slight convexity. The cardiac region is large and well formed; the anterolateral margins are slightly concave, ending in rounded anterolateral angles just behind the median carina and similarly rounded concave posterolateral angles at intersection with posterior carina. The posterior margin is long and concave, and the whole cardiac region is divided by the posterior carina. In the anterior part, a crescent-shaped groove represents the deepest set part of cardiac region. The posterior part of cardiac region is divided by two smaller crescent lobes into three parts, two lateral semicircular concave regions and a triangular, broader posterior one. The intestinal region is wide and narrow, with concave anterior margin and straight lateral margin. The metabranchial, mesobranchial and epibranchial regions less defined, elongated and subrectangular in outline (Fig. 19A).
FIG. 19.
Retropluma slovenica sp. nov. reconstruction. A, dorsal carapace. B, male venter. C, female venter. D, chelipeds.
Female pleon broadly triangular, narrowing anteriorly, six free somites and a telson visible, each becoming narrower and longer anteriorly, telson longer than wide (Fig. 18G). Transverse crest observed on pleonites 1–4 (Fig. 19C). Adult male pleon with tuberculated surface, narrower than female. Somite 1 wide and short, lying posteriorly and hard to observe in ventrally preserved fossil material. Somite 2 wider and longer than somite 1; faint transverse crest present. Somites 3–5 fused (Fig. 19B), sutures 3/4 and 4/5 faintly visible. Male pleon narrows significantly after somite 3, lateral borders of fused 3–5 segment concave, narrowest at 4/5 suture, the slightly widening distally. Strong transverse crest on somite 6, with pronounced granulated surface. Telson narrow and longest, with rounded termination (Fig. 18F). Sternal plate in females rounder in outline with broadly convex sternites 7–5. Male sternites 7–5 long, overlapping, with squarer termination. Sternite 8 in both sexes obsoletely reduced and covered by pleon.
P1 (chelipeds) long, slender and subequal (Fig. 19D), left cheliped somewhat longer and more slender, right cheliped shorter and stouter in some specimens (Fig. 18E). Propodus and dactylus curved, narrowing distally and with smooth surface. Both fingers adorned by several blunt rounded teeth proximally and pincer like distally (Fig. 18H). Carpus and merus stout and adorned with tubercles. P2–P4 achelate, longer than chelipeds, P3 longest, surface finely granulose and flattened, ending in blade-like dactyli. P5 strongly reduced (Fig. 18I), the length about one quarter P3, thin and fragile.
Variations
Intraspecific variation appears to influence mostly dorsal carapace features like density of tubercles, forming of lateral projections on median carinae and shape of the rostrum. All these variable features can be attributed to ontogenetic stages and sexual dimorphism. Smaller juvenile specimens exhibit shorter rostrum with straight sides and rounded distal end (Fig. 20A). Rostrum is longer with concave sides and rounded bulbous termination in medium size subadult and possibly female specimens (Fig. 20C). The largest specimens are male, and these exhibit the straight wide distal end of rostrum with concave sides and spatulate shape (Fig. 20E). On the other hand, the lateral protrusions on anterior and median carinae are clearly an ontogenetic trait as they are only observed on larger adult specimens, presumably of both sexes.
FIG. 20.
Reconstructive drawing showing variability of rostrum shape and size in Retropluma slovenica sp. nov. A, juvenile specimen (RGA/SMNH 0656). B, subadult specimen (RGA/SMNH 0643). C, subadult specimen (RGA/SMNH 0710). D, adult specimen (RGA/SMNH 0707). E, adult specimen (RGA/SMNH 0554). Scale bar represents 5 mm.
FIG. 21.
Diagram of maximum carapace width and height measurements in mm for Retropluma slovenica sp. nov.
Remarks
Until now, no fossil retroplumids have been recorded from Slovenia. Nevertheless, Retropluma is known from the Miocene of Europe, including Denmark (Fraaije et al. 2005) and Slovakia (Hyžný 2011a).
Retropluma slovenica sp. nov. differs from its fossil congeners by having well-marked regions, strong carinas forming blunt lateral projections, strong outward facing postorbital teeth, the presence of infraorbital teeth and long distally widening rostrum. Of the five previously known fossil members of Retropluma, R. slovenica is morphologically close to R. borealis Fraaije, Hansen and Hansen, 2005, and R. craverii Crema, 1895. It differs from R. borealis in having a well-formed, distally wider and down curved rostrum, wider supraorbital margin with pronounced supraorbital tooth and deeper marked orbits. Additionally, R. slovenica has a well-developed and tuber-culated postorbital tooth with concave sides. It also exhibits stronger projections at junctions of carinae with lateral margin than R. borealis. R. slovenica differs from R. craverii by having a wider and shorter rostrum, which never extends further than the postorbital tooth. Postorbital teeth in R. slovenica point outward and are tuberculated, and the whole dorsal carapace is granulose to profoundly tuberculated in anterolateral area and around carinas. The carinae on R. craverii are less pronounced and not as sharply crested as in R. slovenica. The new species also exhibits a concave re-entrant on P5, projections at junctions of carinas with lateral margin, and well-defined gastric and cardiac regions.
The genus is represented by seven extant forms mostly from the Indo-Pacific region (Table 4). Retropluma slovenica resembles R. serenei de Saint Laurent, 1989, more closely in general outline of the carapace, possession of wide rostrum, outward pointing postorbital tooth and pointy projections of junctions of carinae with lateral border. The anterior carina of R. serenei is straighter, and both median and posterior carinae are much less pronounced. R. slovenica has better defined regions, and the carapace surface is granulose and ornamented with tubercles in anterior and anterolateral carapace and striations in its posterior part.
TABLE 4.
Geographical distribution of extant and fossil Retropluma species.
| Species | Age | Occurence | Depth |
|---|---|---|---|
| Retropluma denticulata Rathbun, 1932 | Recent | Japan, China, Philippines | 70–285 m |
| Retropluma notopus (Alcock and Anderson, 1894) | Recent | India, Indonesia, Fiji | 180–450 m |
| Retropluma plumosa Tesch, 1918 | Recent | Indonesia, Fiji | 310–390 m |
| Retropluma quadrata de Saint Laurent, 1989 | Recent | South China, Philippines, Indonesia | 100–300 m |
| Retropluma serenei (de Saint Laurent, 1989) | Recent | Philippines, Fiji, New Caledonia | 180–440 m |
| Retropluma solomonensis McLay, 2006b | Recent | Indonesia, Philippines, New Caledonia | 220–350 m |
| Retropluma planiforma (Kensley, 1969) | Recent | Mozambique, Madagascar | 175–400 m |
| Retropluma gallica (Artal, van Bakel and Castillo, 2006) | Early Eocene | Spain | Outer shelf |
| Retropluma eocenica (Vía Boada, 1959) | Mid-Eocene–Oligocene | Spain, Italy | Outer shelf |
| Retropluma slovenica sp. nov. | Early Miocene | Slovenia | Epibathyal |
| Retropluma borealis (Fraaije, Hansen and Hansen, 2005) | Mid–Late Miocene | Denmark, Slovakia | Offshore |
| Retropluma laurentae (Collins, Lee and Noad, 2003) | Late Miocene | Borneo: Sabah | Shelf |
| Retropluma craverii (Crema, 1895) | Pliocene | Italy | Epibathyal |
DISCUSSION
Palaeoenvironment and bathymetry
The fossiliferous Ivnik Beds represent a deep-water depositional environment as documented by its decapod crustacean faunule and associated assemblage of foraminifers. The decapod fauna suggests an epibathyal water depth of more than 125 m. In modern environments, the genus Retropluma, which is the most abundant component of the studied assemblage (Table 2), usually inhabits muddy or sandy bottoms in the depth interval of 100–450 m (de Saint Laurent 1989; McLay 2006a). Artal et al. (2006) argued for adaptation to the soft bottoms on inner and outer continental shelf, although some fossil species are known also from shallow water settings (Beschin et al. 1996; Hyžný and Müller 2010). The second most abundant decapod species, Styrioplax exiguus, has so far been found exclusively in fine-grained clastic rocks of deep-water facies (Hyžný and Schlögl 2011). Extant species of Neopilumnoplax inhabit bathyal muddy bottoms and cold seeps in a depth range of 125–1050 m (Ahyong 2008). For extant Goneplax species reported from 50 to 750 m (Neumann et al. 2010), the depth range is wider.
Regarding ghost and mud shrimps, the palaeobathy-metric significance is less clear. Jaxea is known from a wide range of depths (Ngoc-Ho 2003), and the fossil record confirms that also for the Miocene occurrences (Hyžný 2011b). Similarly, Calliax ranges from very shallow settings (Ngoc-Ho 2003) to cold seepages at 800 m depth (Taviani et al. 2013). Lepidophthalmus today inhabits exclusively very shallow environments (Felder and Lovett 1989; Dworschak 2007), but it has been reported from deeper water settings in the Oligocene of Hungary (Hyžný and Dulai in press).
The foraminiferal assemblage at Činžat is dominated by Bathysiphon sp., Cyclammina sp. and Sigmoilinella sp. These taxa form the so-called agglutinated foraminiferal assemblage typical for bathyal depths (Kender et al. 2008). Bathysiphon Sars, 1872, is commonly associated with decapods in the collected material; due to the fact that it can reach significant size, specimens over 5 cm in length are not uncommon. Bathysiphon usually inhabits depths of 500–2000 m, whereas larger individuals actually favour greater depth (Saja et al. 2009).
The low diversity of calcareous nannoplankton assemblage consisting of Coccolithus pelagicus Wallich, 1877, Cyclicargolithus floridanus Roth and Hay in Hay et al. 1967, Helicospharera ampliaperta Bramlette and Wilcoxon, 1967, Helicosphaera scissura Miller, 1981, Helicosphaera carteri Wallich, 1877, Reticulofenestra pseudoumbilicus Gartner, 1967, and Reticulofenestra haquii Backman, 1978, and without any discoasterid species (Bartol et al. 2013) indicates a hemipelagic cold water basin of epibathyal depth with poor connection to nutrient-rich oceanic waters (Chira 2004).
Vertebrate remains are rare, represented only by shark teeth from three genera, which most likely floated down the water column. The shark teeth belong to Isistius triangulus Probst, 1879, Galeocerdo aduncus Agassiz, 1843, and Odontaspis sp. These sharks inhabit continental slope waters to a depth of 450 m (Galeocerdo Müller and Henle, 1837 and Odontaspis Agassiz, 1838), however, in the case of Isistius Gill, 1865, epipelagic to bathypelagic depths of 85–3000 m (Kocsis 2007). If we postulate that these small and fragile teeth were not transported, they further indicate epibathyal depth of the deposited sediments.
Deep-water decapod crustacean assemblages from the Neogene fossil record are sparse (Hyžný and Schlögl 2011 and references therein), although recently several reports from the Pliocene and Pleistocene of Italy were published (Garassino et al. 2012; Baldanza et al. 2013; Pasini et al. 2014).
Taphonomy
The fine silty sedimentological composition of the layers with crustacean remains favours the preservation of fine details of this small-bodied fauna. Many decapod remains are still articulated which, taking into account their fragility, implies a very calm depositional environment with low water energy on the bottom and excludes any major post-mortem transport. Some remains are partly disarticulated or show flow displacement, but are almost never damaged, which would indicate that the decapods were only exposed for a short period of time and not transported far before being deposited in the sediment. Most articulated brachyurans are likely preserved as corpses.
Soft, silty to muddy substrate, calm low-energy bottom conditions and a high rate of sedimentation enabled the organisms to be embedded in the sediment and not disturbed after death. Despite the favourable preservation conditions, the specimens display extreme compaction, which is caused by the high compaction index of soft muddy substrate when it lithifies into siltstones under considerable weight and pressure. Most of the specimens are preserved almost two dimensionally with rare exceptions.
Extant species of Retropluma and Neopilumnoplax are reported from deep-water muddy bottom waters (de Saint Laurent 1989), where they spend most of the time buried in soft substrate (Ahyong 2008). Ghost and mud shrimps typically inhabit burrows (Felder 2001). All this supports the hypothesis that most of the fossil remains represent an endobenthic infaunal community that spent most of the time already buried in the sediment, which enhanced the preservational potential.
Palaeobiogeography
The decapod fauna from Činžat exhibits mixed affinities. Jaxea, Calliax and Goneplax are currently known also from the Mediterranean (Ngoc-Ho 2003; Castro 2007), and all have been reported from various Miocene localities of the former Central Paratethys (Hyžný 2011b; Hyžný and Gašparič 2014; Müller 1984, respectively). Calliax michelottii is also known from older strata, namely the early Oligocene of Germany, but the genus itself apparently has an even longer history (Hyžný and Gašparič 2014 and references therein). Goneplax is known today from the Atlantic Ocean and the Mediterranean; for all supposed Indo-West Pacific species, new genera were erected (Castro 2007). The revised fossil record of Goneplax is restricted to the Mediterranean and Paratethys (Garassino et al. 2013). The same is true for Jaxea, whose fossils are restricted to the same area as for Goneplax (Hyžný 2011b). Lepidophthalmus is absent in the recent Mediterranean, but it is known from the Oligocene (Kiscellian) of Hungary (Hyžný and Dulai in press). Today, it is restricted to tropics and subtropics and its extinction in the Proto-Mediterranean, where it probably lived during Neogene, and may be linked to the cooling of climate at the end of Miocene. The deep-water character of Lepidophthalmus paratethyensis sp. nov. suggests a close relationship to its older congener from the Oligocene of Hungary, L. crateriferus. Neopilumnoplax today exhibits wide distribution from the West Atlantic to Indian Ocean (Table 3). As only one fossil representative is known, we consider further discussion on the origin of Neopilumnoplax as speculative. However, today, the genus is absent in the Mediterranean.
Styrioplax is an extinct genus known only from the early Miocene of Central Paratethys (Glaessner 1928; Hyžný and Schlögl 2011; herein).
Retropluma is considered to be of Tethyan origin, as the oldest species is Retropluma gallica Artal, van Bakel and Castillo, 2006, from the early Eocene of France (Artal et al. 2006). In fact, numerous retroplumid taxa were described from the Eocene strata of Europe (Vía Boada 1959; Beschin et al. 1996; Artal et al. 2006, 2013; van Bakel et al. 2010; Hyžný and Müller 2010), and Retropluma survived in the Mediterranean until the Pliocene and Pleistocene with R. craverii known from Italy (Crema 1895; De Angeli et al. 2011; Baldanza et al. 2013). The Miocene occurrences comprise Retropluma borealis from Denmark (Fraaije et al. 2005) and Slovakia (Hyžný 2011a) and the newly described species herein.
To conclude, the decapod assemblage from Činžat is close to the Mediterranean suggesting close biogeographical affinities between Central Paratethys and the Proto-Mediterranean in the early Miocene. At least three genera, Calliax, Lepidophthalmus and Retropluma, were present in both areas during the late Eocene – Oligocene when Paratethys was born (Rögl 1998, 1999; Popov et al. 2004; Piller et al. 2007). Calliax still lives in the Mediterranean today and Retropluma survived there until the Pleistocene, whereas Lepidophthalmus disappeared completely and is today restricted to the tropics. Styrioplax appears to be endemic to Central Paratethys. For Jaxea and Goneplax, the fossil record does not go back before the origin of the Paratethys; nevertheless, both genera are well known from the present Mediterranean Sea (Ngoc-Ho 2003; Castro 2007).
The exchange of decapod faunas between Proto-Mediterranean and Central Paratethys was probably regulated by an anti-estuarine circulation permitting an easier incursion of species from the Proto-Mediterranean into the Paratethys, and simultaneous hindering the Paratethyan endemics (e.g. Styrioplax) from entering the Mediterranean. This would explain the absence of Styrioplax in the Proto-Mediterranean, although collecting bias is also possible. The faunal exchange regulated by an anti-estuarine circulation was already proposed for the mid-Miocene Mediterranean and Paratethyan bryozoans (Moisette et al. 2006). Similarly, Kroh (2007) concluded that the overwhelming majority of the Central Paratethys echinoid species were immigrants from the Proto-Mediterranean; the same was postulated for gastropods and bivalves (Harzhauser et al. 2003). The fossil record of decapod crustaceans corroborates with these results.
CONCLUSIONS
From the description of this Miocene decapod crustacean fauna, the following conclusions can be drawn:
The Ivnik Beds of the Činžat locality situated in the Slovenian part of the Styrian Basin contain a rich decapod association consisting of seven species, of which three are new: Lepidophthalmus paratethyensis, Neopilumnoplax pohorjensis and Retropluma slovenica.
The studied material includes numerous specimens of Styrioplax exiguus permitting its redescription and reconsideration of its familial placement; it is placed within Chasmocarcinidae herein.
Evaluation of decapod crustaceans and other faunal elements from Činžat suggests low-energy deep-water depositional environment with epibathyal water depth of more than 125 m and a muddy bottom.
Based on the affinities of decapod genera of the Central Paratethys and Proto-Mediterranean, it is concluded that the exchange of decapod faunas between these regions was probably regulated by an anti-estuarine circulation permitting an easier incursion of species from the Proto-Mediterranean into the Paratethys, and simultaneously hindering the Paratethyan endemics (Styrioplax) from entering the Mediterranean.
Acknowledgements
We thank the following people, who have kindly assisted us by providing copies of their work, other items of literature and for their helpful assistance and comments: P. Artal (Museo Geologico del Seminario de Barcelona) for helpful input on fossil Retroplumidae, M. Bartol (Ivan Rakovec Institute of Paleontology, Scientific Research Centre of the Slovenian Academy of Sciences and Arts) for preliminary nannoplankton age determination, B. W. M. van Bakel and R. H. B. Fraaije (Oertijdmuseum De Groene Poort, Boxtel) for helpful suggestions, R. M. Feldmann and C. E. Schweitzer (Department of Geology, Kent State University) for support and valuable literature, A. Garassino (Museo di Storia Naturale Milano) for items of literature, D. Guinot (Muséum national d’Histoire naturelle, Paris) for help and literature on chasmocarcinid morphology and systematics, N. Hudáčková (Comenius University, Bratislava) for advice on foraminiferal assemblages, P. K. L. Ng (Department of Biological Sciences, National University of Singapore) for literature items and valuable insight into the Mathildellidae, T. Nyborg (Loma Linda University, Department of Earth and Biological Sciences) for proofreading the manuscript, M. Elstrup Steeman and T. Sørensen (Museum of Southern Jutland, Gram) for providing information on the type material of Retropluma borealis and A. Žibrat Gašparič (University of Ljubljana, Faculty of Arts, Department of Archeology) for continuous support and help with proofreading. The manuscript benefited from constructive reviews of R. M. Feldmann and R. H. B. Fraaije. The research of MH has been supported by Austrian Science Fund (FWF): Lise Meitner Program M 1544-B25. This work was supported by the Slovak Research and Development Agency under the contract Nos APVV-0640-10 and APVV-0436-12.
REFERENCES
- AGASSIZ L. Recherches sur les poissons fossiles. Tome I. Imprimérie de Petitpierre; Neuchatel: 1838. p. 177. [Google Scholar]
- — . Recherches sur les poissons fossiles. Tome III (livr. 15–16). Imprimérie de Petitpierre; Neuchatel: 1843. pp. 157–390. [Google Scholar]
- AHYONG ST. Deepwater crabs from seamounts and chemosynthetic habitats off eastern New Zealand (Crustacea: Decapoda: Brachyura) Zootaxa. 2008;1708:1–72. [Google Scholar]
- ALCOCK A, ANDERSON AR. Natural History notes from H.M. Indian Marine Survey Steamer “Investigator”, Commander C.F. Oldham, R.N., commanding. Series II, No. 14. An account of a recent collection of deep-sea Crustacea from the Bay of Bengal and Laccadive Sea. Journal of the Asiatic Society of Bengal. 1894;63:141–185. [Google Scholar]
- — — Natural history notes from H. M. Royal Indian Marine Survey Ship ‘Investigator,’ Commander T. H. Heming, R.N., commanding. Series 3, number 2. An account of the deep-sea Crustacea dredged during the surveying-season of 1897–98. The Annals & Magazine of Natural History, series 7. 1899;3:278–292. [Google Scholar]
- ARTAL P, VAN BAKEL BWM, CASTILLO J. Retropluma Gill, 1894 (Crustacea, Decapoda) from the Eocene of the eastern Pyrenees (Spain, France) Cainozoic Research. 2006;5:65–71. [Google Scholar]
- — — , FRAAIJE RHB, JAGT JWM. New retroplumid crabs (Crustacea, Brachyura, Retroplumidae Gill, 1894) from the Eocene of Huesca (Aragón, Spain) Zootaxa. 2013;3652(3):343–352. doi: 10.11646/zootaxa.3652.3.3. [DOI] [PubMed] [Google Scholar]
- BACHMAYER F. Goneplax gulderi, eine neue Crustaceen-Species aus dem tortonischen Tegel desWiener Beckens. Paläontologische Zeitschrift. 1953;27(3/4):143–145. [Google Scholar]
- — Zwei bemerkenswerte Crustaceen Funde aus dem Jungtertiar des Wiener Beckens. Sitzungsberichte der Österreichischen Akademie der Wissenschaften in Wien. 1954;I:63–70. [Google Scholar]
- BACKMAN J. Late Miocene – Early Pliocene nannofossil biochronology and biogeography in the Vera Basin, SE Spain. Stockholm Contributions in Geology. 1978;32:93–114. [Google Scholar]
- VAN BAKEL BWM, ARTAL P, FRAAIJE RHB, JAGT JWM. Loerenthopluma danielae, a new crab (Decapoda, Brachyura, Retroplumidae) from the Lower Eocene of northwest Belgium. In: NG P, CASTRO P, DAVIE P, RICHER DE FORGES B, editors. Studies on Brachyura: a homage to Danièle Guinot. Vol. 11. 2010. pp. 41–49.pp. 366 Crustaceana Monographs. [Google Scholar]
- BALDANZA A, BIZZARRI R, FAMIANI F, GARASSINO A, HYŽNÝ M, PASINI G. The bathyal decapod crustacean community from the Poggio i Sodi quarries (Siena Basin, Tuscany, Italy) Boletín de la Sociedad Geológica Mexicana. 2013;65(2):335–353. [Google Scholar]
- BARTOL M, MIKUŽ V, GAŠPARIČ R. Nekaj primerov datiranja makrofosilov s kalcitnim nanoplanktonom. Geološki Zbornik. 2013;22:11–12. [Google Scholar]
- BESCHIN C, BUSULINI A, DE ANGELI A, TESSIER G. Retroplumoidea (Crustacea, Brachyura) nel Terziario del Vicentino (Italia settentrionale) Lavori – Società Veneziana di Scienze Naturali. 1996;21:83–102. [Google Scholar]
- BITTNER A. Beiträge zur Kenntniss Tertiärer Brachyuren Faunen. Denkschriften der Akademie der Wissenschaften, Mathematik und Naturwissenschaften. Cl. 1884;48(2):3–18. 15–30. [Google Scholar]
- BORRADAILE LA. On the classification of the Thalassinidea. The Annals and Magazine of Natural History. 1903;12:534–551. [Google Scholar]
- BRAMLETTE MN, WILCOXON JA. Middle Tertiary calcareous nannoplankton of the Cipero section, Trinidad. Studies in Geology and Paleontology. 1967;5:93–131. [Google Scholar]
- CASTRO P. A reappraisal of the family Goneplacidae MacLeay, 1838 (Crustacea, Decapoda, Brachyura) and revision of the subfamily Goneplacinae, with the description of 10 new genera and 18 new species. Zoosystema. 2007;29:609–774. [Google Scholar]
- CHIRA C. Early Miocene calcareous nannofossils assemblages from Transylvania. Acta Palaeontologica Romaniae. 2004;4:81–88. [Google Scholar]
- CICHA I, RǑGL F. Definition of the Karpatian Stage. In: BRZOBOHATÝ R, CICHA I, KOVÁC M, RÖGL F, editors. The Karpatian: a Lower Miocene stage of the Central Paratethys. Masaryk University; Brno: 2003. pp. 15–20.pp. 360 [Google Scholar]
- COLLINS JSH, LEE C, NOAD L. Miocene and Pleistocene crabs (Crustacea, Decapoda) from Sabah and Sarawak. Journal of Systematic Palaeontology. 2003;1(3):187–226. [Google Scholar]
- CREMA C. Sopra alcuni decapodi terziario del Piemonte. Atti della Reale Accademia di Scienze di Torino. 1895;30:664–681. [Google Scholar]
- DANA JD. Conspectus of the Crustacea of the exploring expedition under Capt. Wilkes, U.S.N. Proceedings of the Academy of Natural Sciences of Philadelphia. 1852;6:6–28. [Google Scholar]
- DE ANGELI A, GARASSINO A. New reports of decapod crustaceans from the Mesozoic and Cenozoic of Friuli-Venezia Giulia (NE Italy) Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2006;147:267–294. doi: 10.4081/nhs.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- — — , PASINI G. New reports of anomurans and brachyurans from the Cenozoic of Tuscany (Italy) Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano. 2009;150(2):163–196. doi: 10.4081/nhs.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- — — — Retropluma craverii (Crema 1895) (Crustacea, Decapoda, Brachyura, Retroplumidae) from the Pliocene of Reggio Emilia (N Italy) Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano. 2011;152:37–44. [Google Scholar]
- DE GRAVE S, PENTCHEFF ND, AHYONG ST, CHAN TY, CRANDALL KA, DWORSCHAK PC, FELDER DL, FELDMANN RM, FRANSEN CHJM, GOULDING LYD, LEMAITRE R, LOW MEY, MARTIN JW, NG PKL, SCHWEITZER CE, TAN SH, TSHUDY D, WETZER R. A classification of living and fossil genera of decapod crustaceans. The Raffles Bulletin of Zoology. 2009;21(Supplement):1–109. [Google Scholar]
- DEFLANDRE G. Hétérogénéité intrins equeèt pluralité des éléments dans les coccolithes actuels et fossiles. Comptes Rendus de l’Académie des Sciences. 1953;237:1785–1787. [Google Scholar]
- DESMAREST AG. Histoire naturelle des crustacés fossils, sous les rapports zoologiques et les crustacés proprement dites. F.-G. Levrault; Paris: 1822. p. 685. [Google Scholar]
- DWORSCHAK PC. First record of Lepidophthalmus tridentatus (von Martens, 1868) (Callianassidae) from the Philippines. Annalen des Naturhistorischen Museums in Wien. 2007;108B:121–130. [Google Scholar]
- FELDER DL. Diversity and ecological significance of deep-burrowing macrocrustaceans in coastal tropical waters of the Americas (Decapoda: Thalassinidea) Interciência. 2001;26(10):440–449. [Google Scholar]
- — Ventrally sclerotized members of Lepidophthalmus (Crustacea: Decapoda: Callianassidae) from the Eastern Pacific. Annalen des Naturhistorischen Museums in Wien. 2003;104B:429–442. [Google Scholar]
- — , LOVETT DL. Relative growth and sexual maturation in the estuarine ghost shrimp Callianassa louisianensis Schmitt, 1935. Journal of Crustacean Biology. 1989;9:540–553. [Google Scholar]
- — , MANNING RB. Ghost shrimps of the genus Lepidophthalmus from the Caribbean region, with description of L. richardi, new species, from Belize (Decapoda: Thalassinidea: Callianassidae) Journal of Crustacean Biology. 1997;17(2):309–331. [Google Scholar]
- FELDMANN RM, SCHWEITZER CE, ENCIN AS A. Neogene Decapod Crustacea from southern Chile. Annals of Carnegie Museum. 2010;78(4):337–366. [Google Scholar]
- FRAAIJE RHB, HANSEN J, HANSEN T. Late Miocene decapod fauna from Gram, Denmark. Palaeontos. 2005;7:51–61. [Google Scholar]
- FRITSCH KVON. Ueber einige fossile Crustaceen aus dem Septarienthon des Mainzer Beckens. Zeitschrift der Deutschen geologischen Gesselschaft. 1871;23:679–701. [Google Scholar]
- DE GAILLANDE D, LAGARDÈRE JP. Description de Callianassa (Callichirus) lobata nov. sp. (Crustacea Decapoda Callianassidae) Recueil des Travaux de la Station Marine d’Endoume. 1966;40:259–265. [Google Scholar]
- GARASSINO A, PASINI G, DE ANGELI A, CHAR-BONNIER S, FAMIANI F, BALDANZA A, BIZZARRI R. The decapod community from the Early Pliocene (Zanclean) of “La Serra” quarry (San Miniato, central Italy): sedimentology, systematics, and palaeoenvironmental implications. Annales de Paléontologie. 2012;98:1–61. [Google Scholar]
- — — , CASTRO P. Revision of the fossil species of Goneplax Leach, 1814 (Crustacea, Decapoda, Brachyura, Goneplacidae) Boletín de la Sociedad Geol ogica Mexicana. 2013;65(2):355–368. [Google Scholar]
- GARTNER S. Paleontological Contributions. Vol. 29. University of Kansas; 1967. Calcareous nannofossils from Neogene of Trinidad, Jamaica, and Gulf of Mexico; pp. 1–7. [Google Scholar]
- GILL T. Synopsis of the Eastern American sharks. Proceedings of the Academy of Natural Sciences of Philadelphia. 1865;16(5):258–265. [Google Scholar]
- — A new bassalian type of crabs. The American Naturalist. 1894;28:1043–1045. [Google Scholar]
- GLAESSNER MF. Die Dekapodenfauna des Österreichischen Jungtertiars. Jahrbuch der Geologischen Bundesanstalt. 1928;78:161–219. [Google Scholar]
- — . Crustacea Decapoda. In: POMPECKJ JF, editor. Fossilium Catalogus I: Animalia. Vol. 41. W. Junk; Berlin: 1929. pp. 1–464.pp. 464 [Google Scholar]
- — . Decapoda. In: MOORE RC, editor. Treatise on invertebrate paleontology, Part R, Arthropoda 4. Geological Society of America. Boulder, CO and University of Kansas Press; Lawrence, KS: 1969. pp. 400–533.pp. 651 [Google Scholar]
- GUINOT D, TAVARES M, CASTRO P. Significance of the sexual openings and supplementary structures on the phylogeny of brachyuran crabs (Crustacea, Decapoda, Brachyura), with new nomina for higher-ranked podotreme taxa. Zootaxa. 2013;3665(1):1–414. doi: 10.11646/zootaxa.3665.1.1. [DOI] [PubMed] [Google Scholar]
- HARMS JC, SOUTHARD JB, SPEARING DR, WALKER RG. Depositional environments as interpreted from primary sedimentary structures and stratification sequences. Society of Economic Paleontologists and Mineralogists, Short Course. 1982;2:161. [Google Scholar]
- HARZHAUSER M, MANDIC O, ZUSCHIN M. Changes in Paratethyan marine molluscs at the Early/Middle Miocene transition – diversity, paleogeography and paleoclimate. Acta Geologica Polonica. 2003;53:323–339. [Google Scholar]
- HAY WW, MOHLER HP, ROTH PH, SCHMIDT RR, BOUDREUX JE, editors. Calcareous nannoplankton zonation of the Cenozoic of the Gulf Coast and Caribbean-Antillean area, and transoceanic correlation. Transactions of the Gulf Coast Association of Geological Societies. 1967;17:428–480. [Google Scholar]
- HOLMES SJ. On some new or imperfectly known species of West American Crustacea. Proceedings of the California Academy of Sciences. 1904;3:307–328. [Google Scholar]
- HYŽNÝ M. Revision of Jaxea kuemeli Bachmayer, 1954 (Decapoda: Gebiidea: Laomediidae) from the Miocene of Europe, with remarks on the palaeobiogeography of the genus Jaxea Nardo, 1847. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen. 2011a;260:173–184. [Google Scholar]
- — Synopsis of fossil decapod crustaceans from Slovakia (Western Carpathians) Neues Jahrbuch für Geologie und Paläontologie Abhandlungen. 2011b;260:165–171. [Google Scholar]
- — Calliaxina chalmasii (Brocchi, 1883) comb. nov. (Decapoda: Axiidea: Callianassidae: Eucalliacinae), a ghost shrimp from the Middle Miocene of Europe, with reappraisal of the fossil record of Eucalliacinae. Zootaxa. 2012;3492:49–64. [Google Scholar]
- — , DULAI A. Deep-water fossorial shrimps from the Oligocene Kiscell Clay of Hungary: taxonomy and palaeoecology. Acta Palaeontologica Polonica. doi: 10.4202/app.2012.0078. in press. doi: http://dx.doi.org/10.4202/app.2012.0078. [DOI] [PMC free article] [PubMed]
- — , GAŠPARIČ R. Ghost shrimp Calliax de Saint Laurent, 1973 (Decapoda: Axiidea: Callianassidae) in the fossil record: systematics, palaeoecology and palaeobiogeography. Zootaxa. 2014;3821:37–57. doi: 10.11646/zootaxa.3821.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- — , MÜLLER PM. Loerenthopluma Beschin, Busulini, De Angeli & Tessier, 1996 (Decapoda: Brachyura: Retroplumidae) from the Oligocene of Hungary. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano. 2010;151(2):129–140. [Google Scholar]
- — , SCHLÖGL J. An early Miocene deep-water decapod crustacean faunule from the Vienna basin (Western Carpathians, Slovakia) Palaeontology. 2011;54(2):323–349. [Google Scholar]
- JELEN B, RIFELJ H. Stratigraphic structure of the B1 Tertiary tectonostratigraphic unit in Eastern Slovenia. Geologija. 2002;45(1):115–138. [Google Scholar]
- KARASAWA H, KATO H. The family Goneplacidae MacLeay, 1838 (Crustacea: Decapoda: Brachyura): systematics, phylogeny, and fossil records. Paleontological Research. 2003;7:129–151. [Google Scholar]
- — — , KASE T, MAAC-AGUILAR Y, KURIHARA Y, HAYASHI H, HAGINO K. Neogene and Quaternary ghost shrimps and crabs (Crustacea: Decapoda) from the Philippines. Bulletin of the National Science Museum (Japan), Series C. 2008;34:51–76. [Google Scholar]
- KENDER S, KAMINSKI MA, JONES RW. Early to middle Miocene foraminifera from the deep-sea Congo Fan, offshore Angola. Micropaleontology. 2008;54:477–568. [Google Scholar]
- KENSLEY BF. Decapod Crustacea from the south-west Indian Ocean. Annals of the South African Museum. 1969;52(7):149–181. [Google Scholar]
- KOCSIS L. Central Paratethyan shark fauna (Ipolytarnóc, Hungary) Geologica Carpathica. 2007;58:27–40. [Google Scholar]
- KRIŽNAR M. Najdba ostankov rakovic rodu Macrophthalmus (Decapoda, Brachyura) iz miocenskih plasti Tuhinjske doline. Kamniški Zbornik. 2006;18:309–314. [Google Scholar]
- — , PREISINGER D. Rak Coeloma iz govške formacije Tunjiškega gričevja. Kamniški Zbornik. 2008;19:335–338. [Google Scholar]
- KROH A. Climate changes in the Early to Middle Miocene of the Central Paratethys and the origin of its echinoderm fauna. Palaeogeography, Palaeoclimatology, Palaeoecology. 2007;253:185–223. [Google Scholar]
- DE LAMARCK C. Système des animaux sans vertèbres: ou tableau général des classes, deséne ordres et des genres de ces animaux. Deterville; Paris: 1801. p. 432. [Google Scholar]
- LEACH WE. Crustaceology. In: BREWSTER D, editor. Edinburgh Encyclopaedia. Vol. 7. Blackwood; Edinburgh: 1814. pp. 383–437.pp. 767 [Google Scholar]
- LINNAEUS C. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentis, synonymis, locis. Laurentii Salvii; Stockholm: 1758. p. 824. [Google Scholar]
- LÖRENTHEY E, BEURLEN K. Die fossilen Dekapoden der Länder der Ungarischen Krone. Geologica Hungarica, Series Palaeontologica. 1929;3:1–421. [Google Scholar]
- MACLEAY WS. On the brachyurous decapod Crustacea brought from the Cape by Dr. Smith. In: SMITH A, editor. Illustrations of the Annulosa of South Africa; being a portion of objects of natural history chiefly collected during an expedition into the interior of South Africa, under the direction of Dr. Andrew Smith, in the years 1834, 1835, and 1836; fitted out by ‘The Cape of Good Hope Association for Exploring Central Africa’. Smith, Elder and Co.; Cornhill, London: 1838. pp. 53–71.pp. 75 [Google Scholar]
- de MAN JG. The Decapoda of the Siboga-Expedition, Part 7: the Thalassinidae and Callianassidae collected by the Siboga-Expedition with some remarks on the Laomediidae. Siboga Expéditie. 1928;39(A6):1–187. [Google Scholar]
- MANNING RB, FELDER DL. Revision of the American Callianassidae (Crustacea: Decapoda: Thalassinidea) Proceedings of the Biological Society of Washington. 1991;104:764–792. [Google Scholar]
- MARTENS EVON. Über eine neue Art und Untergattung der Cyprinoiden, Homaloptera (Octonema) rotundicauda, über einige neue Crustaceen und über die neuholländischen Süsswasserkrebse. Monatsberichte der Königlichen Akademie der Wissenschaften. 1868;1868:607–619. [Google Scholar]
- MARTINI E. Standard Tertiary and Quaternary calcareous nannoplankton zonation. In: FARINACCI A, editor. Proceedings of the II Planktonic Conference; Roma. 1970; Rome: Edizioni Tecnoscienza; 1971. pp. 739–785.pp. 1369 [Google Scholar]
- MAYORAL E, MÜLLER P, MUÑIZ F. Lower Pliocene Decapod crustaceans from the Southwestern Iberian Peninsula (Guadalquivir Basin, Sevilla, Spain) Geobios. 1998;31(4):505–510. [Google Scholar]
- McLAY CL. Retroplumidae (Crustacea, Decapoda) from the Indo-Malayan archipelago (Indonesia, Philippine) and the Melanesian arc islands (Solomon Islands, Fiji and New Caledonia), and paleogeographical comments. In: RICHER DE FORGES B, JUSTINE JL, editors. Tropical deep-sea benthos. Vol. 24. Vol. 193. Mémoires du Muséum National d’Histoire Naturelle; 2006a. pp. 375–391.pp. 417 [Google Scholar]
- — Retropluma solomonensis nom. nov., a replacement name for Retropluma laurentae McLay, 2006 (Crustacea: Decapoda: Brachyura: Retroplumidae) Zootaxa. 2006b;1358:67–68. [Google Scholar]
- MIERS EJ. MURRAY J, editor. Report on the Brachyura collected by H.M.S. Challenger during the years 1873–76 Part II. The Cyclostomata, Ctenostomata and Pedicellinea. Report on the scientific results of the voyage of H.M.S. Challenger during the years 1873–76, Zoology. 1886;17(49):1–47. 362. [Google Scholar]
- MIKUŽ V. Miocenske rakovice iz okolice Šentilja v Slovenskih Goricah. (The Miocene crabs from vicinity Šentilj in Slovenske Gorice, Slovenia) Razprave IV. Razreda SAZU. 2003;44:187–199. [Google Scholar]
- — Rakovice iz srednjemiocenskih plasti kamnolomov nad Trbovljami. Folia Biologica et Geologica. 2010;51(1):13–20. [Google Scholar]
- — , PAVŠIČ J. ‘Kranjska rakovica’ iz srednjemiocenskih – badenijskih skladov kamnoloma Lipovica nad Brišami. Geologija. 2003;46(2):245–250. [Google Scholar]
- MILLER PL. Tertiary calcareous nannoplankton and benthic foraminifera biostratigraphy of the Point Arena area, California. Micropaleontology. 1981;27:419–443. [Google Scholar]
- MILNE EDWARDS A. Histoire des Crustacés podophthalmaires fossiles et Monographie des Décapodes Macroures de la famille des Thalassinens. (4th Series).Annales des Sciences Naturelles. 1860;14:129–357. [Google Scholar]
- — Révision du genre Callianassa (Leach) et description de plusieurs espéces nouvelles de ce groupe. Nouvelles Archives du Muséum d’Histoire Naturelle. 1870;6:75–102. [Google Scholar]
- MIOČ P. Tolmac za list Slovenj Gradec. Osnovna geološka karta SFRJ 1:100,000. Zvezni geološki zavod; Ljubljana: 1972. p. 74. [Google Scholar]
- — Geološka zgradba Dravske doline med Dravogradom in Selnico. (Geological Structure of the Drava Valley between Dravograd and Selnica) Geologija. 1977;20:193–230. [Google Scholar]
- MOISETTE P, DULAI A, MÜLLER P. Bryozoan faunas in the Middle Miocene of Hungary: biodiversity and biogeography. Palaeogeography, Palaeoclimatology, Palaeoecology. 2006;233:300–314. [Google Scholar]
- MOURIK AA, FRAAIJE RHB, VAN DER ZWAAN GJ, SCHEER U. The burrowing shrimp, Protocallianassa faujasi (Crustacea, Decapoda, Thalassinoidea), from the lower Campanian at Dülmen, Germany. Bulletin of the Mizunami Fossil Museum. 2005;32:1–12. [Google Scholar]
- MÜLLER P. Decapod Crustacea of the Badenian. Geologica Hungarica, Series Palaeontologica. 1984;42:3–317. [Google Scholar]
- — Neogene decapod crustaceans from Catalonia. Scripta Musei Geologici Seminarii Barcinonensis. 1993;225:1–39. [Google Scholar]
- — . Crustacea Decapoda. In: FLUGÜEL HW, editor. Catalogus Fossilium Austriae. Verlag der Österreichischen Akademie der Wissenschaften; Wien: 1998. pp. 1–48.pp. 48 [Google Scholar]
- MÜLLER J, HENLE FGJ. Sitzungsberichte der Königlich Preussischen Akademie der Wissenschaften zu. Vol. 2. Berlin: 1837. Gattungen der Haifische und Rochen, nach ihrer Arbeit: Ueber die Naturgeschichte der Knorpelfische; p. 111. [Google Scholar]
- MURRAY JW. Ecology and palaeoecology of benthic foraminifera. Longman Scientific and Technical; London: 1991. p. 397. [Google Scholar]
- NARDO GD. Sinonimia moderna delle specie registrate nell’opera intitolata: Descrizione de’Crostacei, de’Pesci che abitano Ie lagune e gulfo Veneto rappresentari in fugure, a chiaro-scuro ed a colori dell’ Abate Stefano Chiereghini, Ven. XI. Venezia: 1847. p. 127. [Google Scholar]
- NEUMANN H, EHRICH S, KRÖNCKE I. Establishment of the angular crab Goneplax rhomboides (Linnaeus, 1758) (Crustacea, Decapoda, Brachyura) in the southern North Sea. Aquatic Invasions. 2010;5(1):27–30. [Google Scholar]
- NG PKL, GUINOT D, DAVIE PJF. Systema Brachyurorum Part I: an annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology. 2008;17:1–286. [Google Scholar]
- NGOC-HO N. European and Mediterranean Thalassinidea (Crustacea, Decapoda) Zoosystema. 2003;25:439–555. [Google Scholar]
- NOETLING F. Über Crustaceenreste aus dem Oberoligocänen Sternberger Gestein. Sitzingsberichte der Gesselschaft Naturforschender Freunde Berlin. 1886;1886:32–34. [Google Scholar]
- OLSSON RK. Praeorbulina Olsson, a new foraminiferal genus. Journal of Paleontology. 1964;4:770–771. [Google Scholar]
- PAPP A, TURNOVSKY K. Die Entwicklung der Uvigerinen im Vindobon (Helvet und Torton) des Wiener Beckens. Jahrbuch der Geologischen Bundesanstalt. 1953;96:117–142. [Google Scholar]
- PASINI G, GARASSINO A. Records of brachyuran crabs from the Pliocene (Piacenzian) of Reggio Emilia (Emilia Romagna, N Italy) Boletín de la Sociedad Geologíca Mexicana. 2013;65(2):319–328. [Google Scholar]
- — — , HYŽNY̌ M, BALDANZA A, BIZZARRI R, FAMIANI F. The bathyal decapod crustacean community from the early Pleistocene of Volterra (Pisa, Tuscany, central Italy) Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2014;271/3:243–259. [Google Scholar]
- PAVŠIČ J, HORVAT A. Eocene, Oligocene and Miocene in central and eastern Slovenia. In: PLENIČAR M, editor. The geology of Slovenia. Geološki zavod Slovenije; Ljubljana: 2009. pp. 373–426.pp. 612 [Google Scholar]
- PILLER WE, HARZHAUSER M, MANDIC O. Miocene Central Paratethys stratigraphy – current status and future directions. Stratigraphy. 2007;4:151–168. [Google Scholar]
- POPOV SV, RÖGL F, ROZANOV AY, STEININGER FF, SHCHERBA IG, KOVÁC M. Lithological paleogeographic maps of Paratethys. 10 Maps Late Eocene to Pliocene. Courier Forschungsinstitut Senckenberg. 2004;250:1–46. [Google Scholar]
- PROBST J. Beiträge zur Kenntniss der fossilen Fische aus der Molasse von Baltringen. Hayfische. Jahreshefte des Ve-reins für Vaterländische Naturkunde in Württemberg. 1879;35:127–191. [Google Scholar]
- RATHBUN MJ. The Brachyura of the biological expedition to the Florida Keys and the Bahamas in 1893. Bulletin from the Laboratories of Natural History of the State University of Iowa. 1898;4(3):294. [Google Scholar]
- — Description of a new genus and species of fossil crab from Port Townsend, Washington. American Journal of Science. 1916;41:344–346. [Google Scholar]
- — The fossil stalk-eyed Crustacea of the Pacific slope of North America United States. National Museum Bulletin. 1926;138:155. [Google Scholar]
- — Fossil Pinnotherids from the California Miocene. Journal of the Washington Academy of Sciences. 1932;22:411–413. [Google Scholar]
- RÖGL F. Paratethys Oligocene – Miocene stratigraphic correlation. In: CICHA I, RÖGL F, RUPP C, CTYROKA J, editors. Oligocene – Miocene foraminifera of the central Paratethys. Vol. 549. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft; 1998. pp. 3–7.pp. 325 [Google Scholar]
- — Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene Paleogeography (short overview) Geologica Carpathica. 1999;59:339–349. [Google Scholar]
- DE SAINT LAURENT M. Sur la systématique et la phylogénie des Thalassinidea: définition des familles des Callianassidae et des Upogebiidae et de cinq genres nouveaux (Crustacea Decapoda) Comptes Rendus Hebdomadaires de S eances de I’Acad emie des Sciences Paris. 1973;D277:513–516. [Google Scholar]
- — Sur la classification et la phylogénie des Thalassinides: définitions de la superfamille des Axioidea, de la sous-famille des Thomassiniinae et de deux genres nouveaux (Crustacea Decapoda) Comptes Rendus Hebdomadaires de Séances de l’Acad emie des Sciences. 1979;288:1395–1397. [Google Scholar]
- — . La nouvelle superfamille des Retroplumoidea Gill, 1894 (Decapoda, Brachyura): systématique, affinités et évolution. In: FOREST J, editor. Résultats des campagnes MUSORSTOM. Volume 5. Vol. 144. Mémoires du Muséum national d’Histoire naturelle; 1989. pp. 103–179.pp. 385 [Google Scholar]
- SAJA DB, PFEFFERKORN HW, PHIPPS SP. Bathysiphon (Foraminiferida) at Pacheco Pass, California: a geopetal, paleocurrent, and paleobathymetric indicator in the Franciscan Complex. Palaios. 2009;24:181–191. [Google Scholar]
- SAKAI K. Revision of Japanese callianassids based on the variations of larger cheliped in Callianassa petalura Stimpson and C. japonica Ortmann (Decapoda: Anomura) Publications of the Seto Marine Biological Laboratory. 1969;17(4):209–252. [Google Scholar]
- — Supplementary description of Callianassa (Callichirus) tridentata Von Martens (Crustacea, Thalassinidea) Publications of the Seto Marine Biological Laboratory. 1970;17(6):393–401. [Google Scholar]
- SARS GO. Undersøgelser over Handargerfjordens Fauna. Kongelige Danske Videnskabernes Selskab Forhandlinger; Christiania: 1872. p. 251. [Google Scholar]
- SCHWEITZER CE. Tertiary Xanthoidea (Crustacea: Decapoda: Brachyura) from the West Coast of North America. Journal of Crustacean Biology. 2000;20(4):715–742. [Google Scholar]
- — , FELDMANN RM. Differentiation of the fossil Hexapodidae Miers, 1886 (Decapoda: Brachyura) from similar forms. Journal of Paleontology. 2001;75:330–345. [Google Scholar]
- SCHWEITZER HOPKINS C, FELDMANN RM. Sexual dimorphism in fossil and extant species of Callianopsis de Saint Laurent. Journal of Crustacean Biology. 1997;17:236–252. [Google Scholar]
- SERÈNE R. Redescription du genre Magasthesius Rathbun et definition des Chasmocarcininae, nouvelle sous-famille des Goneplacidae (Decapoda, Brachyura) Crustaceana. 1964;7:175–187. [Google Scholar]
- — Description de deux especes nouvelles et redecouverte d’une espece de Brachyoure (Decapoda, Crustacea) dans le eaux d’lndonesie. Cahiers du Pacifique. 1969;13:281–293. [Google Scholar]
- STUDER T. Verzeichniss der während der Reise S.M.S. ‘Gazelle’ an der Westküste von Afrika, Ascension und dem Cap der Guten Hoffnung gesammelten Crustaceen. Abhandlungen der Akademie der Wissenschaften in Berlin. 1883;2:1–32. [Google Scholar]
- TAVARES M, DE MELO G. A new species of Neopilumnoplax Serène in Guinot, 1969 (Decapoda, Brachyura, Mathildellidae) from the southwestern Atlantic. In: FRANSEN C, DE GRAVE S, NG P, editors. Studies on Malacostraca: Lipke Bijdeley Holthuis memorial volume. Vol. 14. Crustaceana Monographs; 2010. pp. 685–691.pp. 754 [Google Scholar]
- — , GUINOT D. Description de Neopilumnoplax gervaini sp. nov. des Caraïbes (Crustacea, Decapoda, Brachyura, Goneplacidae) Bulletin du Muséum National d’Histoire Naturelle, Séries 4, Section A, Zoologie. 1996;18(1–2):225–232. [Google Scholar]
- TAVIANI M, ANGELETTI L, CEREGATO A, FOGLINI F, FROGLIA C, TRINCARDI F. The Gela Basin pockmark field in the strait of Sicily (Mediterranean Sea): chemosymbiotic faunal and carbonate signatures of postglacial to modern cold seepage. Biogeosciences. 2013;10:4653–4671. [Google Scholar]
- TESCH JJ. The Decapoda Brachyura of the Siboga Expedition. I. Hymenosomidae, Retroplumidae, Ocypodidae, Grapsidae and Gecarcinidae. Siboga Expedition Monographs. 1918;39c:148. [Google Scholar]
- TUCKER AB, FELDMANN RM. Fossil decapod crustaceans from the lower Tertiary of the Prince William Sound region, Gulf of Alaska. Journal of Paleontology. 1990;64:409–427. [Google Scholar]
- VAN STRAELEN V. Sur des Crustacées Decapodes Cenozoiques du Venezuela. Bulletin du Musée Royal d’Histoire Naturelle de Belgique. 1933;9(10):1–14. [Google Scholar]
- VÍA BOADA L. Décapodos fósiles del Eoceno español (Resumen – avance de la tesis doctoral) Boletin del Instituto Geológico y Minero de España. 1959;70:313–402. [Google Scholar]
- WALLICH GC. Observations on the coccosphere. Annals and Magazine of Natural History. 1877;19:342–350. [Google Scholar]
- WHITE A. Descriptions of two species of Crustacea belonging to the families Callianassidae and Squillidae. Proceedings of the Scientific Meetings of the Zoological Society of London. 1861;1861:42–44. [Google Scholar]