Abstract
Ulcerative dermatitis (UD) in C57BL/6 mice is poorly understood and challenging to treat. We sought to evaluate the evidence regarding commonly cited risk factors for UD and reported UD treatments. The terms ‘ulcerative dermatitis’ and ‘C57BL/6’ were used to search 3 electronic databases. The resulting 347 articles were screened to identify publications that compared the risk of spontaneous UD in wild-type C57BL/6 mice according to sex, season, diet, or age and those that compared the degree of healing or rate of lesion resolution according to the intervention used. Articles were evaluated by using published criteria for assessing methodologic quality, including study design, number of animals per study group, case definition, method of diagnosis, randomization, enrollment criteria, exclusion criteria, and outcomes. The search identified 11 publications on risk factors that met the inclusion criteria, and no publication on UD treatment met all of the criteria. Relaxing the inclusion criteria for reporting of risk factors and treatment outcomes to include both wild-type C57BL/6 mice and genetically engineered mice on a B6 background yielded 12 publications on risk factors and 3 publications on treatment. Dietary factors, particularly caloric restriction, appear to influence UD risk. Female sex was inconsistently associated with a higher risk of UD, which most often occurred in 13- to 24-mo-old mice in the studies that were reviewed. Only 1 of the 3 publications that evaluated UD treatments included an untreated group or alternative therapy control. Further research is needed to explore epidemiologic aspects of UD and to compare treatment options.
Abbreviations: B6, C57BL/6; GEM, genetically engineered mouse; UD, ulcerative dermatitis
Ulcerative dermatitis (UD) is a common condition of several strains of laboratory mice, especially C57BL/6 (B6) mice. The condition is characterized by intense scratching and ulcerative skin lesions of the dorsal cervicothoracic region that are notoriously resistant to treatment.1,13,16 Large numbers of mice are affected with UD each year, given that B6 mice and genetically engineered mice on a B6 background are some of the most commonly used mice in research.4,10,16 Although an overall UD prevalence or incidence is unknown for B6 mice, in some reports more than 30% of the mice developed UD during the study period.1,12 Mice with this disease experience distress related to the severe pruritus and the progressive nature of the lesions.1,6,12 Concerns regarding animal wellbeing and the potential confounding effects of this disease on research endpoints frequently lead to euthanasia of affected mice.16 However, despite the devastating effect of this condition in laboratory animal medicine, the pathogenesis of UD is poorly understood and, accordingly, a consistently effective treatment is unavailable.
The cause of UD is speculated to be multifactorial.6,28 The risk of UD reportedly is affected by sex, age, season, and various diets, although not all of these effects have consistently been associated with UD.13,24,29 For example, female mice have been found to be at increased risk in some studies13,23 but not in others.1,16,17 In addition to being clinically useful, identifying reliable risk factors may be advantageous in forming hypotheses regarding the etiology of UD. Therefore, the first aim of this systematic review is to identify peer-reviewed literature that compares the incidence or prevalence of spontaneous UD according to sex, age, season, or diet and to evaluate the scientific evidence for these risk factors.
In addition, various treatments for UD have had limited success.1,8,16,29 As a result, the treatment of UD is largely determined by clinician preference and personal experience. The second aim of this review is to identify studies that report treatments for UD and the quality of evidence supporting the use of the treatment.
Materials and Methods
Search strategy.
The PubMed, PubMed Central, and Google Scholar databases were searched using the terms “ulcerative dermatitis” and “C57BL/6.” The searches were performed during June 2015. Abstracts for the articles were screened for relevance. Some articles had no mention of ulcerative dermatitis in the abstract, and an electronic keyword search of full-text articles for the word “dermatitis” quickly identified articles that were unrelated to the research questions of this review. For example, some articles briefly mentioned an experimental animal that was removed from a study due to UD but lacked further discussion or comparison of UD prevalence among groups. When an electronic full text version of the article was unavailable for the keyword search, the complete full-text version of the article was reviewed for inclusion and exclusion criteria. Similarly when dermatitis was mentioned more than a few times (an arbitrary cut-off of 3 was selected), the article underwent a complete full-text review for eligibility. Eligible full-text articles as identified by the abstract and keyword searches were reviewed for content as described following. No date restrictions were used in any of the searches.
For inclusion in this review, articles had to meet the following criteria: 1) the article was an original research paper that 2) was published in a peer-reviewed section of the journal and 3) compared the risk of spontaneous UD in wild-type C57BL/6 mice according to sex, season, diet, or age or compared the healing or resolution of UD lesions according to intervention. In addition, 4) measurements of the compared factors had to be stated, and 5) the full-text article was available in English. Reports that described mite-associated ulcerative skin lesions and those that focused on ulcerative skin lesions of genetically engineered or mutant mice were excluded from analysis.
The exclusion of genetically engineered mice (GEM) was intended to omit reports of GEM that had lesions that appeared grossly similar to UD but had a differing underlying pathogenesis. Practically speaking, however, many mice in modern vivaria are GEM on a B6 background, and an epidemiologic study in this setting might include a mix of wild-type B6 mice and GEM on a B6 background (for example, the study reported in reference 13). This caveat became evident after the initial literature search, and the exclusion criteria subsequently were relaxed to include studies that describe B6 mice and GEM on a B6 background. This process is described in more detail in the Results section.
Quality analysis.
Risk factors.
For articles reporting potential risk factors for UD, information was collected regarding study type, blinding, the number of animals per study group, definition of UD, and method of UD diagnosis and whether an aim of the study was to assess UD risk factors.
Study type was categorized as previously described:25
A: Blinded randomized controlled trial comparing 2 interventions
B: Controlled trial lacking either blinding or randomization
C: Prospective cohort study
D: Prospective case–control study
E: Retrospective cohort or case–control study
F: Prospective study with single intervention
G: Retrospective case series with single intervention
The definition given for UD was classified as follows (adapted from reference 30): ‘not defined’ indicates that the words ‘ulcerative dermatitis’ were used without further explanation; ‘partially defined’ indicates that the article contained a brief description of ulcers with scratching or pruritus; and ‘well defined’ means that there was a complete description of the lesions and their locations in mice that had appropriate signalment.
Similarly, whether an aim of the article was to assess UD risk factors was scored as previously described.30 That is, ‘no’ indicates that the aims of the article were unrelated to epidemiologic aspects of UD; ‘partially’ denotes articles that were epidemiologic studies into aspects of UD; and ‘yes’ is assigned when the primary aim of an article was the identification of UD risk factors.
Treatments.
Reports of treatments or interventions for UD were analyzed by using previously published guidelines for methodologic quality.14 Information regarding study type, number of animals per study group, randomization, blinding, intervention studied, enrollment criteria, exclusion criteria, and outcomes were tabulated. Outcome measures of particular interest included the percentage reduction in lesion size and the percentage of mice with complete resolution.
Reporting of results.
Variation in outcome reporting between studies precluded quantitative analysis. Information regarding study design and the reported findings were compiled into tables.
Results
Search results.
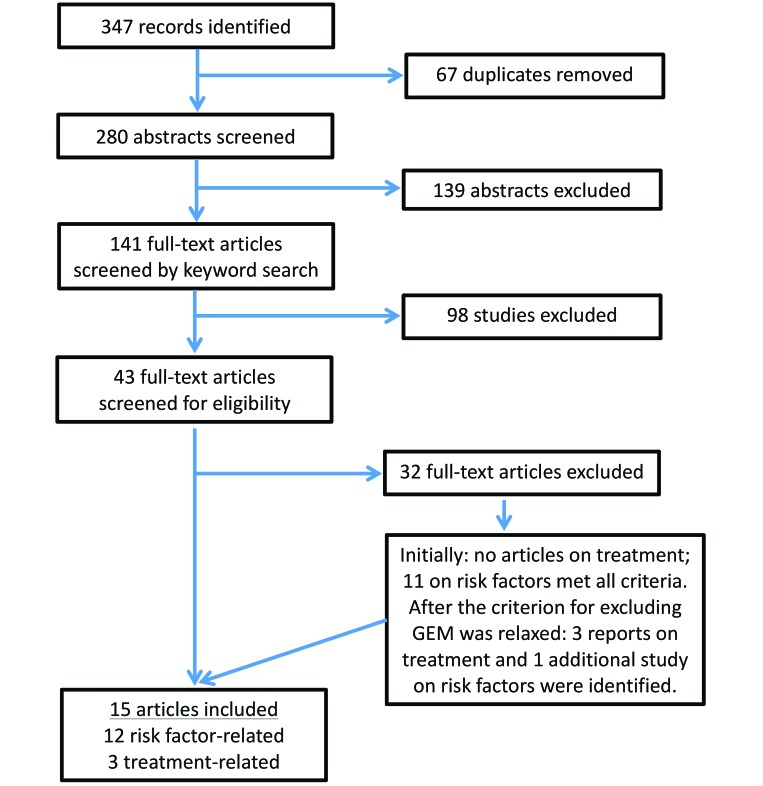
Initial database searches identified 347 articles. After removing duplicate publications, the total number of reports for abstract review was 280. Abstract-level review excluded 139 studies, 98 of which were excluded because they were not original research papers published in peer-reviewed journals (n = 98). An additional 14 studies were excluded because they described mite-associated ulcerative dermatitis, and 10 described experimentally induced ulcerative skin lesions. Another 10 studies were unrelated to UD in B6 mice and instead described ulcerative skin disease in other species, such as humans and dogs. There were 5 studies that described an ulcerative dermatopathy as part of a mutant mouse phenotype and 2 studies that described dermatitis in other inbred strains of mice (NC and C3H, specifically).
Keyword searches of full-text articles revealed an additional 98 studies that failed to meet the review criteria. The majority of these did not compare the incidence of UD or response to treatment. Specifically, 28 studies mentioned dermatitis briefly as a mutant phenotype, 25 studies mentioned UD briefly as a source of animal loss during the experimental period, 17 articles briefly mention UD in the background or discussion section without making comparisons between groups, and 3 studies described a change in study design because of ulcerative dermatitis. In addition, 19 studies either included the words ‘ulcerative dermatitis’ only in the references (n = 10) or ‘cited by’ (n = 9) section. The remaining articles were excluded for briefly describing an ulcerative dermatopathy in other species (n = 3) or because they described an experimentally induced dermatitis (n = 3).
Full-text review identified another 32 articles that failed to meet the inclusion criteria. Some of these described UD but did not compare risk factors or treatments (n = 11) or made comparisons but did not quantify their observations (n = 2). Others described dermatitis in hybrid mice or strains other than the B6 (n = 7), described ulcerative lesions as a mutant phenotype (n = 6), combined B6 mice and GEM mice on a B6 background for measures of interest (n = 4), or grouped several causes of dermatitis (UD, fight wounds, and so forth) in their reported measures (n = 2). At this step of the literature search, no article that compared treatments met all of the criteria, but 3 articles from peer-reviewed journals compared treatments among wild-type B6 mice and GEM on a B6 background. After relaxing exclusion criteria, one additional study that compared UD incidence among B6 mice and GEM on a B6 background was identified. The 3 articles that compared treatments and a revised total of 12 articles that addressed potential risk factors were reviewed for content (Figure 1).
Figure 1.
Flow chart of the search strategy used to identify articles for inclusion.
Overview of UD risk factors.
The 12 publications that met the inclusion criteria for the assessment of UD risk factors are detailed in (Table 1). Of these, one compared risk relative to sex, age, and season. Another 6 of the articles compared risk according to diet group only; 2 studies assessed UD risk in relation to sex and diet; and another analyzed risk by sex, age, and diet. The final 2 studies evaluated UD risk by sex and age.
Table 1.
Characteristics of publications that compare UD risk by sex, diet, age, or season
| Reference | Study typea | n | Blinding | Definition of UD | Confirmation of UD diagnosis | Aim to identify UD risk factors | Comparison according to |
|||||
| Sex | Age | Diet | Season | |||||||||
| 1 | Andrews and colleagues (1994) | C | 18 | Not described | Well described | Histology | Partially | X | X | |||
| 2 | Bender and colleagues (2008) | B | 81 | Yes (behavioral assessment) | Not defined | Histology | No | X | ||||
| 3 | Blackwell and colleagues (1995) | B | 266 | Not described | Not defined | Unclear | No | X | X | |||
| 7 | Dufour and colleagues (2010) | A | 12 | Yes (experiment 1) | Well described | Identification by veterinarian | Partially | X | X | |||
| 12 | Hampton and colleagues (2012) | C | 25 | Not described | Well described | Histology | Partially | X | X | |||
| 13 | Kastenmayer and colleagues (2006) | E | 1352 | Not described | Well described | Histology, bacterial culture | Yes | X | X | X | ||
| 15 | Krugner-Higby and colleagues (2012) | B | 11 | Not described | Well described | Histology, bacterial culture | Partially | X | ||||
| 19 | Neuhaus and colleagues (2012) | B | 10 | Not described | Partially defined: age and gross findings | Lesion character | Yes | X | ||||
| 20 | Pugh and colleagues (1999) | B | 75 | Not described | Partially defined: open wounds and excessive scratching | Unclear | No | X | ||||
| 21 | Sell and colleagues (2000) | B | 31 | Not described | Not defined | Lesion, response to treatment | No | X | ||||
| 26 | Turturro and colleagues (2002) | B | 56 | Not described | Partially defined: ulceration and self-mutilation | Unclear, possibly histology | No | X | X | X | ||
| 31 | Zhang and colleagues (2015) | B | 16 | Not described | Partially defined: ulcerated inflammatory lesions | Histology | Partially | X | ||||
Study types: A, blinded, randomized, controlled trial comparing 2 interventions; B, controlled trial lacking either blinding or randomization; C, prospective cohort study; E, retrospective cohort or case-control study
Diet as a risk factor.
Of the studies reviewed for content, 9 compared UD risk in mice that received various diets (Table 2). Caloric restriction, either by quantity restriction or feeding of a calorie-reduced diet, was associated with a lower risk of UD in 4 studies, 3 of which showed a strong, significant (P < 0.005) effect of caloric restriction.20,21,26 The fourth study3 did not report a P value but described a “notable” difference between groups fed free-choice (26% lifetime prevalence in female mice, 13.5% in male mice) and those that were diet-restricted (2% for female mice, 0.3% for male mice).
Table 2.
Publications that compared UD risk according to dietary intervention group
| Reference | Dietary intervention | Control diet | Outcome | P | |
| 2 | Bender and colleagues (2008) | “Standard rodent diet” supplemented with 1% creatinine | Equicaloric standard diet | Nonsignificantly increased risk of UD in the creatinine-fed group | >0.05 |
| 3 | Blackwell and colleagues (1995) | 60% caloric restriction by limiting quantity | NIH31 fed free-choice | Effects of diet and sex reported, with UD risk highest in unrestricted female mice and lowest in calorie-restricted male mice | not reported |
| 7 | Dufour and colleagues (2010) | Custom serotonin-promoting diet | Custom purified- ingredient diet formulated to emulate standard rodent chow | Significantly higher risk of UD in mice fed the seratonin-promoting diet | 0.001 |
| 15 | Krugner-Higby and colleagues (2012) | AIN76A supplemented with 1% conjugated linoleic acid fed free-choice | AIN76A fed free-choice | No UD in any group of WT B6 | not applicable |
| 19 | Neuhaus and colleagues (2012) | High-fat (35%) diet fed free-choice | “Normal chow diet,” 3.3% fat | Nonsignificantly increased risk of UD in high-fat–diet group | 0.14 |
| 19 | Neuhaus and colleagues (2012) | “Normal chow diet” supplemented with lithium chloride (1g/kg diet) | “Normal chow diet” with no added lithium | Higher risk of UD in lithium-supplemented group | <0.01 |
| 20 | Pugh and colleagues (1999) | 26% CR by feeding of a limited quantity of a reduced calorie diet starting at 1 y of age | Limited quantity of a higher calorie control diet | Lower risk of UD in caloric-restriction group | <0.005 |
| 21 | Sell and colleagues (2000) | 60% caloric restriction by free-choice feeding of reduced-calorie diet | NIH31 fed free-choice | Lower risk of UD risk in caloric-restriction group | <0.0001 |
| 26 | Turturro and colleagues (2002) | 60% CR by quantity restriction | NIH31 fed free-choice | Significantly lower risk of UD in caloric-restriction group | <0.001 |
| 31 | Zhang and colleagues (2015) | High-fat (60% or 33%) diets fed free-choice | Low-fat (10%) diet fed free-choice | Significantly higher risk of UD in high-fat–diet groups | <0.05 (33% fat), <0.01 (60% fat) |
Among the remaining 5 studies, one19 compared the risk of UD in mice fed a high-fat or normal control diet, as well as the effect of lithium supplementation. The risk of UD was higher (albeit nonsignificantly) in the high-fat group (P = 0.14). In addition, lithium supplementation significantly(P < 0.01) increased UD risk.19 In another study, mice fed a high-fat diet (either 60% or a 33% fat content) had a significantly (P < 0.01 and P < 0.05, respectively) higher risk of UD than did mice fed a 10% fat control diet.31 A study assessing UD risk in mice receiving a creatinine supplement found that 47% of mice on the creatinine-supplemented diet and 44% of mice on the control diet developed UD, a nonsignificant difference.2 Another group7 evaluated the use of a serotonin-promoting diet for the treatment of barbering and learned that mice on the serotonin-promoting diet had a significantly increased risk of UD. The remaining report15 compared the risk of UD in female mice fed either a semipurified diet or the same diet with added conjugated linoleic acid. The trial was of short duration (4 wk) and used relatively young mice (age, 4 mo); none of the wild-type B6 mice developed UD during the experiment.
Age as a risk factor.
Of the 4 studies that compared UD risk according to age,1,12,13,26 2 used prospective cohorts euthanized at predetermined time points and compared the rate of UD among these cohorts. In one study,12 the risk of UD in the cohort euthanized at 19 mo of age (26.7% for male mice, 35% for female mice) was higher than that for the 13-mo cohort (6% for both sexes); no P value was reported, however. In another prospective cohort study,1 no clear trend was observed at necropsy, with 37% mice affected at 17 mo of age, 11% affected at 19 mo, 0% at 21 mo, and 20% at 23 mo. In the same facility, clinical cases presented to veterinary staff members had an average age of onset of UD of 20 mo. In a retrospective study,13 the mean age of UD onset was 13 mo, with 13% of cases in mice younger than 6 mo; in addition, the authors noted that the percentage of affected mice tended to increase with increasing age. The authors of the remaining study26 presented a graph that portrayed the “cumulative probability of death from dermatitis” and noted that the disease occurred in mice between 550 d and approximately 2 y of age. Mice with UD in the cited study26 were euthanized due to poor prognosis at the time of diagnosis.
Sex as a risk factor.
Of the 6 studies that compared the risk of UD in female and male mice, 43,7,13,26 reported a greater risk in female mice, whereas the remaining 21,12 reported a similar risk of UD for both sexes. In particular, 2 of the studies that reported a higher rate of UD in female mice involved free-choice feeding compared with caloric restriction, and both papers reported a significant effect of both diet and sex.3,26 A serotonin-promoting diet with added tryptophan and an increased carbohydrate:protein ratio increased the risk of UD, particularly among female mice.7 In comparison, 6% of female B6 mice developed UD compared with only 2% of male mice in another study population.13 The remaining 6 of the 12 studies that assessed UD risk factors used mice of only one sex (male only, references 20, 21, and 31; female only, references 2, 15, and 19), thus preventing the comparison of prevalence or incidence of UD between sexes in these reports.
Two articles compared the age at UD onset relative to sex. One study26 found that affected female mice were younger than male mice with UD, but the peak incidence for female mice occurred slightly later (600 to 750 d of age) than did that for male mice (550 to 700 d of age). In comparison, the mean age at onset did not differ by sex in the other study.12 However, all the mice in that study were euthanized by 19 mo of age (approximately 570 d), so differences that might have emerged later would not have been detected.
Quality analysis of studies identifying potential risk factors for UD.
The majority of the evaluated publications (9 of 12) described controlled trials in which mice were grouped according to dietary intervention. Two papers described prospective cohort studies of mice of different ages at the time of necropsy and tissue collection. Blinding was only described in 2 of the publications.2,7 The minimal experimental group size ranged from 10 to 266, with a median of 25 mice per group. One study13 was a retrospective study of UD cases observed in 1352 mice housed in a particular animal room over a 2 y span. Retrospective studies pose various limitations, given that the sex, age, and genotypes represented are determined by investigator needs, perhaps leading to the underrepresentation of specific subsets (for example, geriatric male mice).
The definition used for UD and the means by which the condition was diagnosed varied widely among the studies examined (Table 1). In regard to the features by which a case was defined, 3 publications2,3,21 provided no definition, 4 publications19,20,26,31 offered a brief description of features (including ulcers and self-trauma), and 5 publications1,7,12,13,15 provided a thorough description of UD. Likewise, the techniques used to diagnose or confirm UD differed among studies and included gross lesion characteristics, physical examination by a veterinarian, and histology; in some cases, no diagnostic criteria or confirmatory testing was reported.
Identifying risk factors for UD was a primary aim of only 2 of the studies analyzed.13,19 Another 5 publications1,7,12,15,31 were partially designed to assess epidemiologic aspects of UD. The remaining 5 studies2,3,20,21,26 quantified the risk of UD in the various experimental groups, although the aim of these studies was to assess the effects of dietary manipulations.
Reported treatments and quality analysis.
Three reports evaluated treatments for UD (Table 3). One study8 compared the palatability and efficacy of 2 different formulations of ibuprofen in drinking water and concluded that the liquid-gel formulation was more effective at reducing pruritus and UD lesion size. The second article16 assessed the resolution of UD wounds after vitamin E supplementation, and the third study29 evaluated maropitant citrate as a potential treatment and found that the 1-mg/kg dose was significantly more effective than either the 5-mg/kg dose or saline control. In terms of assessing the quality of the evidence provided, 2 of these 3 studies lack a control group. In the first,8 all mice received ibuprofen, and there was no untreated or alternative-therapy control group. The study of vitamin E treatment16 reported a rate of response to treatment and referred to historical recovery rates in other published reports but included no untreated or alternative-therapy control.
Table 3.
Characteristics of publications that compare healing or resolution of UD lesions according to intervention
| Ezell and colleagues (2012) | Lawson and colleagues (2005) | Williams-Fritze and colleagues (2011) | |
| Study type | Prospective study with single interventiona | Prospective study with single intervention | Blinded, randomized, controlled trial |
| n | 14 | 71 | 30 |
| Randomization | Adequate | Not applicable | Adequateb |
| Blinding | Unclear | Not applicable | Adequate for pruritus scoring, unclear for lesion scoring |
| Intervention | Ibuprofen (pediatric suspension or liquid-gel formula) at 1 mg/mL for 9 d in drinking water | NIH31 diet + 3000 IU vitamin E for 8 wk | Maropitant citrate at 1 or 5 mg/kg IP for 5 or 10 d |
| Enrollment criteria | B6 or B6 background, exam by veterinarian, defined as “single or multiple areas of the skin with excoriation, dermal ulceration with serocellular crusts or granulation and located on the dorsal scapular, dorsal thorarcolumbar, or lumbosacral regions” | B6 or B6 background; spontaneous UD lesion diagnosis by clinical appearance during routine health checks; not used in another study | Identified during routine health checks, veterinarian confirmed UD based on “physical exam and characteristic appearance of lesions” |
| Exclusion criteria | Severe UD: active bleeding, contracture, deep ulcers | None | Deep ulcers, other disease, deficiency in inducible nitric oxide synthase |
| Lesion size reduction | 65% reduction (liquid gel) compared with 29% (suspension), mean reduction in lesion size over 9 d | 25% had 50% to 99% reduction, 9% had 12% to 30% reduction, 21% had no reduction in lesion size | 10% reduction: 78% by day 15, 94% by day 57; 25% reduction: 59% by day 15 and 83% by day 43 (in 1 mg/kg group) |
| Complete resolution rate | Not reported (50% to 100% resolution rates reported) | 45% | Not reported |
| Other outcome measures | Food intake, scoring of pruritus, locomotor activity, and presence of grooming | Necropsy: lymphadenopathy, splenomegaly | Pruritus score: days 29-50, 1 mg/kg group lower pruritus score |
No nonibuprofen control
Randomized and sex-matched but no mention of matching for genotype or age
Discussion
Ulcerative dermatitis of B6 mice is one of the most common and frustrating conditions managed by laboratory animal veterinarians. Efforts to manage this disease are undermined by a lack of knowledge about its etiology and pathogenesis. Characterizing reliable risk factors can aid in the recognition of susceptible animals and provide clues regarding the etiology of UD.
Caloric restriction, accomplished by either a reduction in food quantity or the feeding of a lower calorie diet, was consistently associated with a lower risk of UD in all of the publications that evaluated this dietary intervention. Caloric restriction has also been shown to increase the lifespan of B6 mice.3,20,26,27 Given that UD is a disease most commonly seen in aging mice, the protective effect of caloric restriction may be related to its associated effects on the aging phenotype and longevity. In addition, differences in gut microbiota associated with different diets might modulate inflammation or other aspects of the disease process. The mechanism by which caloric restriction reduces the incidence of UD has not been explored and remains a promising avenue for future research. One important caveat is that assessing the incidence or prevalence of UD was not a primary aim in any of the reviewed caloric-restriction studies. Accordingly, inclusion and exclusion criteria, case definitions, and confirmatory diagnostics were not described clearly.
Some evidence suggests that a diet high in fat may increase UD risk. Two studies19,31 reported an increased UD risk in mice fed a high-fat diet, although the difference was nonsignificant in one report. One of the studies31 reported proinflammatory changes in the skin of mice fed a high-fat diet; these changes involved macrophages and later were sustained by T cells and keratinocytes, thus perhaps providing a mechanism for promoting or initiating UD in mice on a high-fat diet. Other dietary interventions, including a serotonin-promoting diet and lithium supplementation, also significantly altered UD risk. This evidence was limited to a single study in each case, but these studies provide additional evidence that diet may be an important factor in the development of UD.
The evidence regarding the potential for an increased risk of UD in female mice is somewhat contradictory. Two studies that met the inclusion criteria for review revealed no significant differences in UD risk between female and male mice. In contrast, 4 studies showed a significantly increased risk for female mice. Of the 4 studies, 3 studies involving dietary interventions showed a significant effect on UD risk for both sex and diet group. Perhaps some risk factors, such as unrestricted feeding, may be more important in female mice. Sex-specific differences in response to diet, especially caloric restriction, may be relevant to the pathogenesis of UD and merits further investigation.
Age and seasonality were also evaluated as risk factors for UD. For the 3 studies that examined risk according to age, UD was most commonly seen between 13- to 24-mo-old mice, with some variation between the studies. Two of the studies1,12 were cohort studies with predetermined terminal collection intervals. This methodology may underestimate the average age of onset of UD, given that mice that would have developed UD after the predetermined time points are not represented in the data. A seasonal pattern in UD incidence has been reported,13,23 but only one of the publications that met the inclusion criteria of this review assessed this potential risk factor. In that report,13 the authors described an increased risk of UD in midsummer in association with changes in humidity, both increased and decreased. Seasonality is an important diagnostic feature in dermatology, and confirming the previously described seasonal pattern to UD might have important implications. Different seasons may be associated with different environmental risk factors, such as allergens and humidity. However, seasonality should be interpreted with caution in the laboratory setting, given that other annual cyclic events—such as funding cycles and the academic year—may alter the demographics of mouse populations in the vivarium, thus creating the appearance of a seasonal effect.
One limitation of the current literature review is the small number of publications analyzed. Although the search terms identified 280 unique articles, most mentioned UD only briefly as a cause of attrition among study animals. Few of the studies compared UD risk or treatments between groups of mice. The initial inclusion criteria limited the analysis to spontaneous UD in wild-type B6 mice, and descriptions of UD or UD-like lesions in mutant strains or GEM were excluded. This criterion subsequently was relaxed to collect studies that described B6 mice or GEM on a B6 background, because this population was more realistic in regard to an epidemiologic study of laboratory mouse disease. The initial exclusion of GEM was intended to omit reports of GEM that had lesions that were grossly similar to UD but had a different underlying pathogenesis than UD. Some GEM strains are known for developing open skin lesions that might be called ‘ulcerative dermatitis.’ For example, E- and P-selectin–deficient mice develop conjunctivitis and submandibular ulcerative skin lesions that appear to be T-cell–dependent.11 Another mutant strain, mice deficient in stearoyl CoA desaturase, also develops ulcerative skin lesions. This strain has been suggested as a model for UD, although the mutant phenotype also includes an absence of sebaceous glands, a condition that might interfere with evaluating a condition with dermatologic manifestations.12 It is entirely possible, if not likely, that at least one GEM that develops ulcerative skin lesions as part of its mutant phenotype has a similar underlying pathologic mechanism to the B6 UD syndrome and could be a valid model. An opportunity for future study is to compare mutant mouse strains that have a phenotype similar to UD with the condition in wild-type B6 mice to identify those that are highly similar. GEM with spontaneous ulcerative skin lesions that are similar in distribution and character to the B6 UD syndrome might provide insights into the genetic mechanisms that influence UD.
The publications that analyzed risk factors used several different substrains of B6 mice including C57BL/6J,2,7 C57BL/6NNia,1,21,26, C57BL/6Crl,12, 20 and B6 mice obtained from Harlan (possibly substrain C57BL/6OlaHsd).15 The retrospective study13 included B6 from various commercial vendors and outside institutions, thus representing a variety of substrains. In addition, 3 papers3,19,31 did not report the substrain used. Substrains of B6 have limited genetic differences, but if these differences modify the risk of UD or how UD manifests, then the combining of multiple substrains for analysis is another limitation of the current review.
Various treatments for UD have been reported, including Caladryl lotion, cyclosporine, nail trimming, and bandaging.1,5,9,18,22 Unfortunately few treatments have been evaluated in a rigorous or controlled fashion. Many commonly cited treatments, such as nail trimming, can be traced to conference posters, abstracts, and anecdotal reports. The current review identified very few peer-reviewed studies that evaluated treatments. Furthermore, specifically in regard to the quality of the evidence provided, several studies lacked a control group for comparison. Given the variability in UD incidence and recovery rates reported in the literature, the absence of controls prompts serious concerns in regard to determining the efficacy of a treatment. Several reports cited humane reasons for the lack of an untreated control group—a reasonable concern given the distress that UD lesions can cause. Many institutions treat UD with a topical antimicrobial or analgesic (or both) and nail trimming and achieve a fair response. Consequently including a ‘standard of care’ treatment group might be a reasonable option that addresses concerns about both scientific validity and animal wellbeing. Well-designed, controlled, peer-reviewed studies of UD treatments are needed urgently.
UD is a devastating disease that affects one of the most commonly used strains of mice in biomedical research. The effects of UD on the research community—confounding research endpoints, compromising animal welfare, and consuming animal care resources—are compounded by the disease's rapidly progressive nature and poor response to treatment. Although systemic reviews are uncommon in veterinary medicine, they are useful approaches to summarizing existing literature and can indicate areas needing additional research. The results of this systematic review clearly show that more work is necessary to elucidate epidemiologic aspects of UD and to develop standardized diagnostic and clinical criteria to facilitate future comparisons. Furthermore, this review has revealed the paucity of controlled clinical trials that compare treatment options; such trials are crucial for developing an evidence-based approach to treating this common and refractory disease.
References
- 1.Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson KJ. 1994. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol 31:293–300. [DOI] [PubMed] [Google Scholar]
- 2.Bender A, Beckers J, Schneider I, Holter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, Quintanilla-Martinez L, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Schmidt J, Klopstock T. 2008. Creatinine improves health and survival of mice. Neurobiol Aging 29:1404–1411. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell BN, Bucci TJ, Hart RW, Turturro A. 1995. Longevity, body weight, and neoplasia in ad-libitum-fed and diet-restricted C57BL6 mice fed NIH31 open-formula diet. Toxicol Pathol 23:570–582. [DOI] [PubMed] [Google Scholar]
- 4.Bryant CD. 2011. The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann N Y Acad Sci 1245:31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley ME, Delano ML, Kirchain SM. 2008. Successful treatment of C57BL/6 ulcerative dermatitis with Caladryl lotion. Abstracts presented at The American Association for Laboratory Animal Science 59th National Meeting, Indianapolis, Indiana, 9–13 November 2008. J Am Assoc Lab Anim Sci 47:109–110. [Google Scholar]
- 6.Duarte-Vogel SM, Lawson GW. 2011. Association between hair-induced oronasal inflammation and ulcerative dermatitis in C57BL/6 mice. Comp Med 61:13–19. [PMC free article] [PubMed] [Google Scholar]
- 7.Dufour BD, Adeola O, Cheng HW, Donkin SS, Klein JD, Pajor EA, Garner JP. 2010. Nutritional upregulation of serotonin paradoxically induces compulsive behavior. Nutr Neurosci 13:256–264. [DOI] [PubMed] [Google Scholar]
- 8.Ezell PC, Papa L, Lawson GW. 2012. Palatability and treatment efficacy of various ibuprofen formulations in C57BL/6 mice with ulcerative dermatitis. J Am Assoc Lab Anim Sci 51:609–615. [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman SH, McVay L, Kessler MJ. 2006. Resolution of ulcerative dermatitis of mice by treatment with topical 0.2% cyclosporine. Abstracts presented at The American Association for Laboratory Animal Science 57th National Meeting, Salt Lake City, Utah, 15–19 October 2006. J Am Assoc Lab Anim Sci 45:92–93. [Google Scholar]
- 10.Flurkey K, Currer J, Leiter EH, Witham B. 2009. The Jackson Laboratory handbook on genetically standardized mice, 6th ed. Bar Harbor (ME): Jackson Laboratory. [Google Scholar]
- 11.Forlow SB, White EJ, Thomas KL, Bagby GJ, Foley PL, Ley K. 2002. T-cell requirement for development of chronic ulcerative dermatitis in E- and P-selectin–deficient mice. J Immunol 169:4797–4804. [DOI] [PubMed] [Google Scholar]
- 12.Hampton AL, Hish GA, Aslam MN, Rothman ED, Bergin IL, Patterson KA, Naik M, Paruchuri T, Varani J, Rush HG. 2012. Progression of ulcerative dermatitis lesions in C57BL/6Crl mice and the development of a scoring system for dermatitis lesions. J Am Assoc Lab Anim Sci 51:586–593. [PMC free article] [PubMed] [Google Scholar]
- 13.Kastenmayer RJ, Fain MA, Perdue KA. 2006. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45:8–12. [PubMed] [Google Scholar]
- 14.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2012. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage 20:256–260. [DOI] [PubMed] [Google Scholar]
- 15.Krugner-Higby L, Brown R, Rassette M, Behr M, Okwumabua O, Cook M, Bell C, Flowers MT, Ntambi J, Gendron A. 2012. Ulcerative dermatitis in C57BL/6 mice lacking stearoyl CoA desaturase 1. Comp Med 62:257–263. [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson GW, Sato A, Fairbanks LA, Lawson PT. 2005. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci 44:18–21. [PubMed] [Google Scholar]
- 17.Mader JR, Mason MA, Bale LK, Gades NM, Conover CA. 2010. The association of early dietary supplementation with vitamin E with the incidence of ulcerative dermatitis in mice on a C57BL/6 background: diet and ulcerative dermatitis in mice. Scand J Lab Anim Sci 37:253–259. [PMC free article] [PubMed] [Google Scholar]
- 18.Mufford T, Richardson L. 2009. Nail trims versus the previous standard of care for treatment of mice with ulcerative dermatitis. Abstracts presented at The American Association for Laboratory Animal Science 60th National Meeting, Denver, Colorado, 8–12 November 2009. J Am Assoc Lab Anim Sci 48:546. [Google Scholar]
- 19.Neuhaus B, Niessen CM, Mesaros A, Withers DJ, Krieg T, Partridge L. 2012. Experimental analysis of risk factors for ulcerative dermatitis in mice. Exp Dermatol 21:712–713. [DOI] [PubMed] [Google Scholar]
- 20.Pugh TD, Oberley TD, Weindruch R. 1999. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res 59:1642–1648. [PubMed] [Google Scholar]
- 21.Sell DR, Kleinman NR, Monnier VM. 2000. Longitudinal determination of skin collagen glycation and glycoxidation rates predicts early death in C57BL/6NNIA mice. FASEB J 14:145–156. [DOI] [PubMed] [Google Scholar]
- 22.Seta S. 2009. A simplified method for the treatment of mouse dermatitis. Abstracts presented at The American Association for Laboratory Animal Science 60th National Meeting, Denver, Colorado, 8–12 November 2009. J Am Assoc Lab Anim Sci 48:548. [Google Scholar]
- 23.Sundberg JP, Brown KS, McMahon WM. 1994. Chronic ulcerative dermatitis in black mice, p 485–492. In: Sundberg JP. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. Boca Raton (FL): CRC Press. [Google Scholar]
- 24.Sundberg JP, Taylor D, Lorch G, Miller J, Silva KA, Sundberg BA, Roopenian D, Sperling L, Ong D, King LE, Everts H. 2011. Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet Pathol 48:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swann JW, Skelly BJ. 2013. Systematic review of evidence relating to the treatment of immune-mediated hemolytic anemia in dogs. J Vet Intern Med 27:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Turturro A, Duffy P, Hass B, Kodell R, Hart R. 2002. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci 57:B379–389. [DOI] [PubMed] [Google Scholar]
- 27.Weindruch R, Walford RL. 1988. The retardation of aging and disease by dietary restriction. Springfield (IL): Charles C Thomas. [Google Scholar]
- 28.Williams LK, Csaki LS, Cantor RM, Reue K, Lawson GW. 2012. Ulcerative dermatitis in C57BL/6 mice exhibits an oxidative stress response consistent with normal wound healing. Comp Med 62:166–171. [PMC free article] [PubMed] [Google Scholar]
- 29.Williams-Fritze MJ, Scholz JAC, Zeiss C, Deng Y, Wilson SR, Franklin R, Smith PC. 2011. Maropitant citrate for treatment of ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 50:221–226. [PMC free article] [PubMed] [Google Scholar]
- 30.Wylie CE, Collins SN, Verheyen KLP, Newton JR. 2012. Risk factors for equine laminitis: a systematic review with quality appraisal of published evidence. Vet J 193:58–66. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Li Q, Rao E, Sun Y, Grossmann ME, Morris RJ, Cleary MP, Li B. 2015. Epidermal fatty acid binding protein promotes skin inflammation induced by high-fat diet. Immunity 42:953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]