Abstract
The NIH guidelines for survival bleeding of mice and rats note that using the retroorbital plexus has a greater potential for complications than do other methods of blood collection and that this procedure should be performed on anesthetized animals. Lateral saphenous vein puncture has a low potential for complications and can be performed without anesthesia. Mongolian gerbils (Meriones unguiculatus) are the preferred rodent model for filarial parasite research. To monitor microfilaria counts in the blood, blood sampling from the orbital plexus has been the standard. Our goal was to refine the blood collection technique. To determine whether blood collection from the lateral saphenous vein was a feasible alternative to retroorbital sampling, we compared microfilaria counts in blood samples collected by both methods from 21 gerbils infected with the filarial parasitic worm Brugia pahangi. Lateral saphenous vein counts were equivalent to retroorbital counts at relatively high counts (greater than 50 microfilariae per 20 µL) but were significantly lower than retroorbital counts when microfilarial concentrations were lower. Our results indicate that although retroorbital collection may be preferable when low concentrations of microfilariae need to be enumerated, the lateral saphenous vein is a suitable alternative site for blood sampling to determine microfilaremia and is a feasible refinement that can benefit the wellbeing of gerbils.
Abbreviations: FR3, Filariasis Research Reagent Resource Center
Lymphatic filariasis a major threat to human health worldwide. More than one billion people in more than 90 countries around the globe are at risk from lymphatic filariasis, and between 120 and 150 million people are infected.9,11,25 Infection with the filarioid parasitic worms Brugia malayi and Wuchereria bancrofti can result in severe sequelae, including elephantiasis and hydrocoele formation.3,11,15,25 In addition to the clinical manifestations of filariasis are the potential associated psychologic, social, and cultural effects in persons exhibiting visible signs of infection.9,23,34
The life cycle of filarioid nematodes requires an arthropod intermediate host and a definitive vertebrate host. Within the definitive host, dioecious adult filarial nematodes reproduce sexually. Inseminated adult female worms then release live, sheathed microfilariae into the lymph that circulate in the peripheral blood.21 In the case of B. malayi and W. bancrofti, the intermediate host is the mosquito.21 When an uninfected mosquito ingests a blood meal from an infected human, ingested microfilariae unsheathe to penetrate the midgut of the mosquito to reach the thoracic muscles and molt twice, to become the infectious third-stage larvae. The third-stage larvae then migrate to the mosquito's proboscis and can infect another human when the mosquito takes a blood meal.10,11 The third-stage larvae enter the new host's lymphatic system which is their final location, where they undergo 2 molts into adults.
Because of the complexity of filarioid life cycles, research involving these parasites can be logistically challenging. Although mice can be infected with W. bancrofti, they do not maintain the infection.35 Furthermore, there is no suitable nonhuman host that can maintain a patent infection, with the exception of the silvered leaf monkey (Trachypithecus cristatus).9 Because the closely related parasites B. malayi and B. pahangi have more extensive host ranges than does W. bancrofti, they are easier to maintain in a research setting. Domestic cats (Felis catus) can be experimentally infected with B. malayi and develop a patent infection, and both domestic cats and dogs (Canis familiaris) can be experimentally infected with B. pahangi13,29,37 and are suitable for obtaining microfilaremic blood for experimental feeding of mosquitoes. The Mongolian gerbil can be infected with B. pahangi. Because replacing a phylogenetically higher species with a lower species is preferable36 and because performing experiments involving dogs and cats can be logistically difficult and cost-prohibitive, many researchers prefer a rodent model, specifically gerbils.
The Filariasis Research Reagent Resource Center (FR3) is an NIH center whose mandate is to support filariasis research worldwide. The FR3 provides parasitic and molecular resources, as well as training in animal procedures, to researchers from many nations. The FR3 maintains both B. malayi and B. pahangi, and researchers occasionally require gerbils with patent infections. Because the required level of microfilaria counts varies among investigators, an accurate microfilaria count must be obtained prior to the shipment of gerbils. For example, some experiments require that live mosquitoes feed directly on infected gerbils, and when the microfilaria level is too low, the mosquitoes do not become infected. Conversely when the level is too high, the migration of microfilariae and the later larval stages can kill the mosquitoes. Historically, the FR3 has used retroorbital sampling under general anesthesia to obtain the blood for microfilaria counts.28 Although this method has been fairly successful, the FR3 has encountered occasional complications secondary to the procedure, including exophthalmia and, rarely, death under anesthesia. The NIH guidelines for survival bleeding of mice and rats notes that compared with other blood collection methods, retroorbital sampling has a greater potential for complications. The guidelines recommend a 10- to 14-d period between retroorbital blood collections and state that the procedure is “…best conducted under general anesthesia.”31 By comparison, collecting blood from the lateral saphenous vein is considered to have a low potential for complications or tissue damage, can be performed without general anesthesia,12,18,31 and can be performed repeatedly, even daily.31
In the current study, we proposed to refine the blood collection method being used by FR3 by developing sampling from the lateral saphenous vein as the new standard blood-collection method for monitoring microfilaremia. Our goal was to assess blood collection from the lateral saphenous vein as a feasible refinement technique to potentially replace retroorbital sampling by determining whether the microfilaria counts in blood collected from the lateral saphenous vein without anesthesia were sufficiently similar to those from retroorbital blood sampling with anesthesia to provide adequate information about the microfilaremia level.
Materials and Methods
Animals.
All animals were male Mongolian gerbils, stock Crl:MON(Tum), obtained from Charles River Laboratories (Wilmington, VA). The gerbils were housed socially, in suspended polysulfone cages (Ancare, Bellmore, NY) on an aluminum rack (Bryan Research Equipment, Bryan, TX). Cages were bedded with corncob bedding (The Anderson's, Maumee, OH), and cotton squares (Ancare, Bellmore, NY) were provided as environmental enrichment. Food (no. 5053, irradiated, Lab Diets, St Louis, MO) and filtered municipal water in water bottles were provided free-choice. The room temperature range was 18 to 24 °C and the light cycle was 12:12-h light:dark. Health surveillance for Clostridium piliforme has been performed 3 or 4 times annually, with negative results. The gerbils had been infected with B. pahangi for at least 300 d before the blood collection. All research procedures were approved by the University of Georgia's IACUC, followed the National Research Council Guide for the care and use of laboratory animals,20 and were part of an AAALAC-accredited program.
Parasites.
Microfilaremic blood was obtained from B. pahangi-infected dogs housed at the FR3 (College of Veterinary Medicine, University of Georgia, Athens, GA). This blood was then fed to Aedes aegypti (black-eyed Liverpool strain) mosquitoes. After 14 d, mosquitoes were mass dissected, and infectious third-stage larvae were harvested. Gerbils were infected through subcutaneous injection of 400 third-stage larvae. Counts were assessed at 300 d after infection or later.
Blood sample collection.
We collected blood samples from one lateral saphenous vein and one orbital sinus from 21 B. pahangi-infected gerbils on 2 occasions, 14 to 16 d apart, between 1300 and 1600. On both occasions, the sample from the lateral saphenous vein was collected without anesthesia and was followed immediately by collection of the retroorbital sample under isoflurane anesthesia. Both samples were collected at the same time to avoid variability in microfilaria counts due to parasite periodicity.
Lateral saphenous.
The gerbil was manually restrained (scruffed) by one person while a second person used clippers (Wahl Clipper Model 9860, Sterling, IL) to remove the fur between the lateral stifle and tarsus. The person restraining the gerbil applied pressure behind the stifle to occlude the lateral saphenous vein. The second person punctured the lateral saphenous vein by using a 20-gauge, 1 in. needle (no. 305175, BD Precision Glide Needle, Becton Dickinson, Franklin Lakes, NJ) and collected the blood with a 100-µL heparinized capillary tube (no. 15401-628, Micro-Hematocrit Capillary Tubes Red Band/Heparinized, VWR, Radnor, PA).
Anesthesia.
Cotton gauze (no. 9022, 2 in. x 2 in., Kendall Versalon All-Purpose Sponges, Covidien, Mansfield, MA) was placed in a 50-mL conical tube (no. 339653, Thermo Scientific, Rochester, NY), and 100 to 200 µL of liquid isoflurane (Patterson Veterinary Supply, Charlotte, NC) was applied to the gauze. The gerbil's nose was held in the opening of the tube until the gerbil was anesthetized (nonresponsive to stimulation, regular breathing rate). The gerbil then was removed from the tube and the blood sample collected immediately (within 10 s).
Retroorbital.
A 100-µL heparinized capillary tube was inserted into the medial canthus, behind the globe, and into the orbital sinus of the anesthetized gerbil.
Processing blood samples.
For each blood sample collected, 2 slides were made, with the exception of 3 samples for which the volume was sufficient for the lateral saphenous vein slide only. To make each slide, 20 µL of blood was withdrawn from the capillary tube by using a mechanical pipette (Fisher Scientific, Waltham, MA) and expelled onto a glass slide (Gold Seal RITE-ON, Portsmouth, NH), 70 µL of deionized water was dropped onto the blood to lyse the erythrocytes for better visualization of the microfilariae, and the diluted sample was spread on the slide by using a clean wooden, flat toothpick to create a thick blood smear, the ‘gold standard’ for counting microfilariae. The slides were allowed to air dry then were stained with Giemsa.
Analyzing blood samples.
Two personnel, blinded to the gerbil identification and sample collection site, analyzed the blood smears by counting all of the microfilariae on each entire smear. The microfilaria counts for each pair of slides were averaged to provide a single count for each collection site for each gerbil at each time point. The counts for each collection site for all gerbils at each time point then were averaged to provide the average count for each site at each time point. For the 3 samples for which only one lateral saphenous slide was made, the count for the single lateral saphenous slide was used as that gerbil's lateral saphenous average count.
Statistical analysis.
Microfilaria counts for each blood collection site were calculated as the mean count observed by 2 personnel on each of 2 separately prepared slides. Descriptive statistics for counts were reported as the median and interquartile range. Bland–Altman plots were constructed, and the corresponding 95% limits of agreement were calculated to evaluate the agreement between the paired blood samples collected from the lateral saphenous vein and orbital sinus in regard to microfilaria counts.7 Histograms were used to graphically assess the normality of differences between the saphenous and orbital counts. A paired t test was used to determine whether the mean difference in counts between the 2 blood-collection sites differed significantly from zero. All tests assumed a 2-sided alternative hypothesis, and P values less than 0.05 were considered statistically significant. Analyses were performed by using commercially available software (Stata version 12.1, StataCorp LP, College Station, TX).
Results
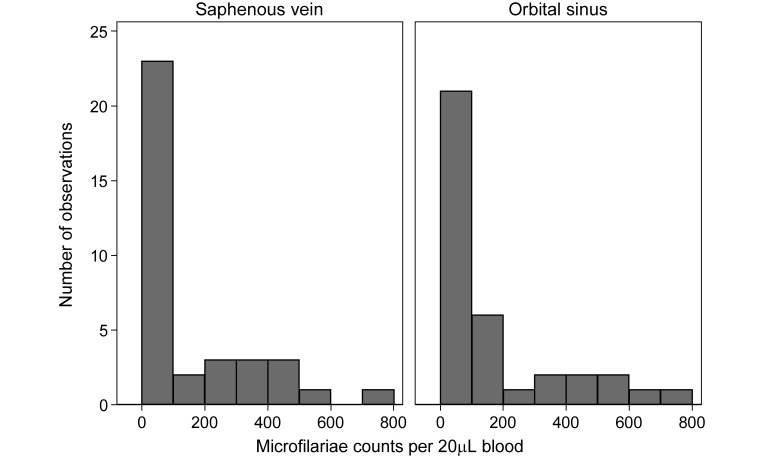
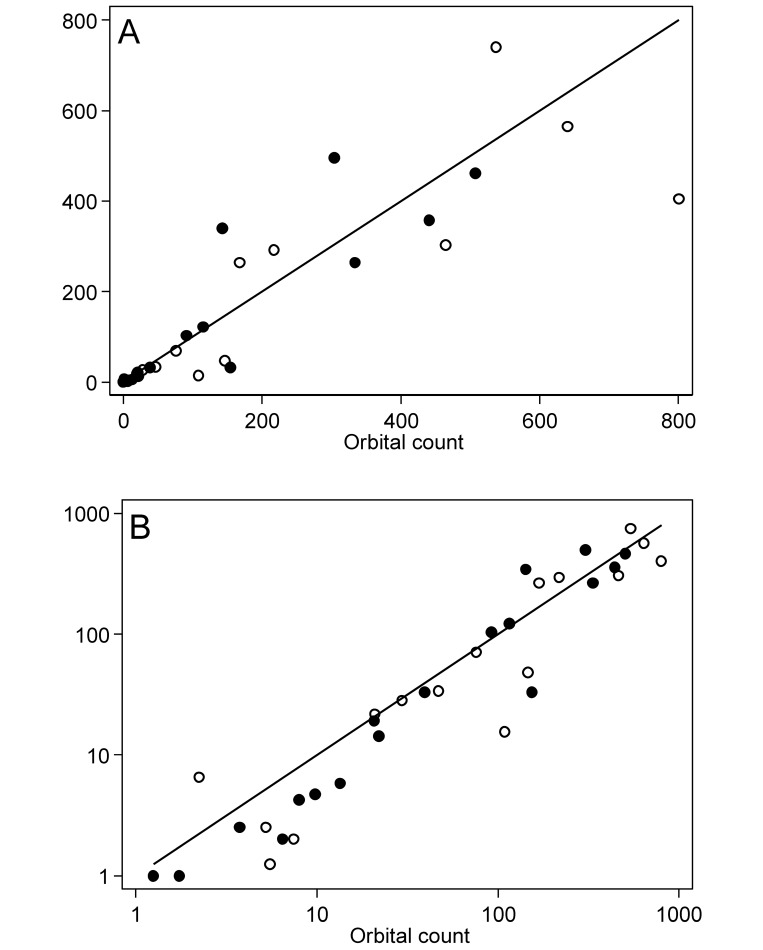
Three of the 21 gerbils sampled did not have any microfilariae in their blood and were excluded from subsequent analysis. This infection rate is typical with gerbils in our experience and did not indicate a lack of detection sensitivity. Microfilaria counts for the remaining 18 gerbils are presented in Table 1, and the distributions of counts are illustrated in (Figure 1). The median counts in the lateral saphenous vein samples and the orbital sinus samples were 29.5 (I interquartile range, 2.4 to 277) and 42.3 (interquartile range, 6.0 to 193) microfilariae per 20 µL of blood, respectively. Scatter plots compared the lateral saphenous counts with the orbital counts (Figure 2). Because the variability between counts increased with the mean, differences in both the raw (Figure 2 A) and log-transformed (Figure 2 B) counts were evaluated.
Table 1.
Mean microfilaria counts (per 20 µL blood) in samples obtained from the saphenous vein and orbital sinus of 18 gerbils on each of 2 occasions, 14 to 16 d apart
| First sample collection |
Second sample collection |
|||
| Gerbil | Saphenous vein | Orbital sinus | Saphenous vein | Orbital sinus |
| 1 | 292 | 218 | 121 | 115 |
| 2 | 0.0 | 0.3 | 0.0 | 0.8 |
| 3 | 5.5 | 1.3 | 3.3 | 7.0 |
| 4 | 741 | 538 | 495 | 304 |
| 5 | 404 | 801 | 263 | 334 |
| 6 | 27.0 | 28.8 | 4.8 | 12.5 |
| 7 | 1.5 | 4.3 | 3.8 | 8.8 |
| 8 | 20.8 | 20.0 | 13.3 | 21.0 |
| 9 | 33.0 | 46.0 | 18.0 | 19.8 |
| 10 | 564 | 640 | 358 | 442 |
| 11 | 14.5 | 109 | 32.3 | 154 |
| 12 | 47.5 | 146 | 32.0 | 38.5 |
| 13 | 263 | 167 | 340 | 143 |
| 14 | 69.3 | 75.5 | 103 | 91.0 |
| 15 | 0.0 | 0.8 | 0.0 | 0.3 |
| 16 | 1.0 | 6.5 | 1.0 | 5.5 |
| 17 | 303 | 465 | 462 | 507 |
| 18 | 0.3 | 4.5 | 1.5 | 2.8 |
Figure 1.
Histograms of microfilaria counts in paired blood samples collected from the saphenous vein and orbital sinus of 18 gerbils on each of 2 occasions.
Figure 2.
Scatter plots of microfilaria counts in paired blood samples from the saphenous vein compared with the orbital sinus on (A) linear and (B) logarithmic scales with lines of equality. Paired samples were collected from 18 gerbils on each of 2 occasions; markers in each panel differentiate between the first (hollow circles) and second (solid circles) sampling occasions. Markers for the first and second sampling occasions overlapped for 2 gerbils.
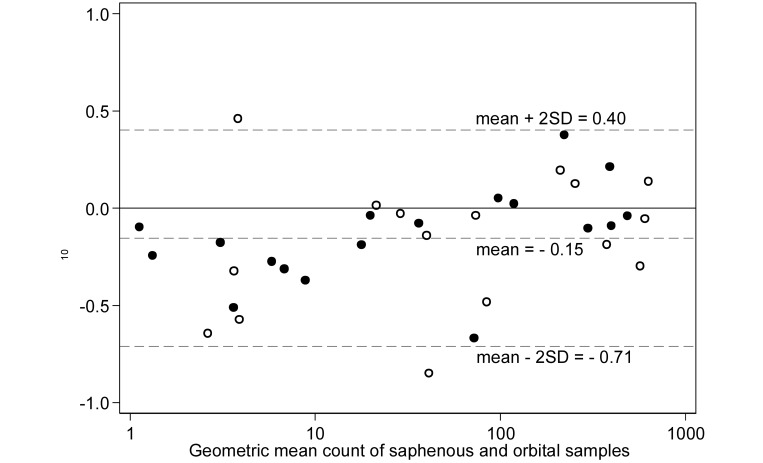
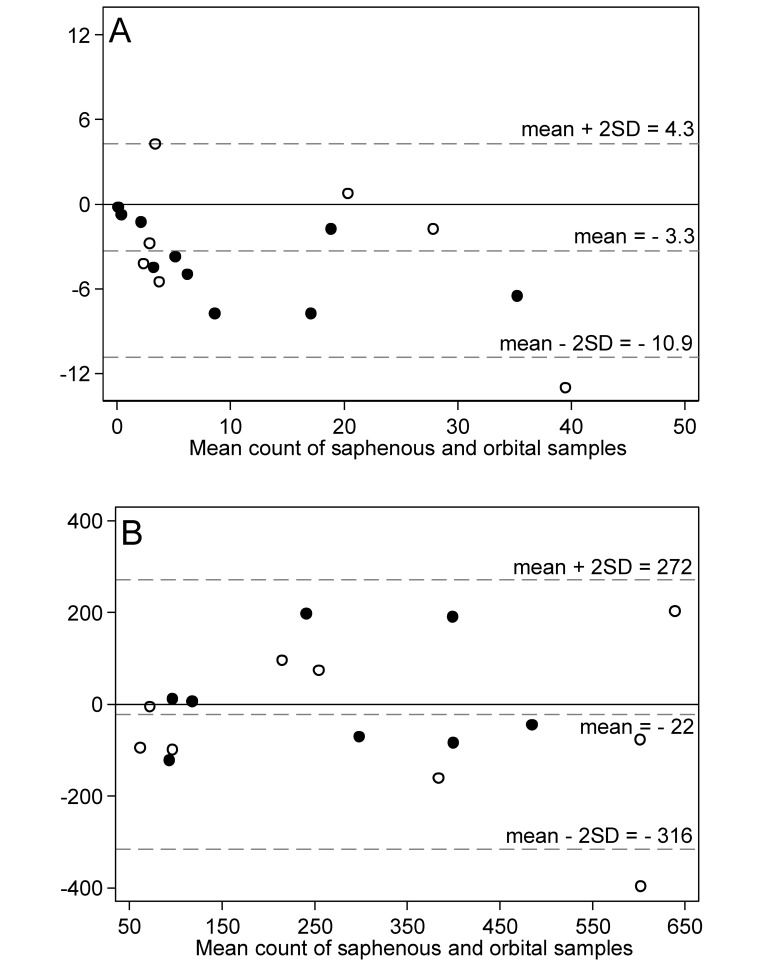
To illustrate the agreement between methods, a Bland–Altman plot compared the difference in the log-transformed microfilaria counts between the 2 blood collection sites with the geometric mean of the 2 sites (Figure 3). The mean difference in log-transformed counts between the lateral saphenous vein and orbital sinus was –0.15 log10, with 95% limits of agreement of –0.71 log10 and 0.40 log10. The mean difference in the log-transformed counts was significantly less than 0 (paired t test, P = 0.003), indicating an overall bias toward observing lower counts from the lateral saphenous vein compared with the orbital sinus. However, an examination of the mean difference plot suggested that the bias was most pronounced for sample pairs with low geometric mean counts. To further illustrate the effect of counts, separate Bland–Altman plots of the raw microfilaria counts (Figure 4) were generated for sample pairs with a mean count of 50 or fewer microfilariae (Figure 4 A) and greater than 50 microfilariae (Figure 4 B) per 20 μL. For pairs with a mean count of 50 or fewer (Figure 4 A), the mean difference between lateral saphenous and orbital counts was –3.3, with 95% limits of agreement of –10.9 and 4.3 (paired t test, P = 0.002); that is, counts differed significantly between collection methods. For pairs with a mean count greater than 50 (Figure 4 B), the mean difference between lateral saphenous and orbital counts was –22, with 95% limits of agreement of –316 and 272 (paired t test, P = 0.552); this difference was not significant. Consistent with the visual assessment of the log-transformed mean difference plot in (Figure 3), lateral saphenous vein counts were significantly lower than orbital sinus counts at low, but not at high, microfilaria concentrations.
Figure 3.
Bland–Altman plot of the difference in log10 microfilaria counts compared with the geometric mean count for paired blood samples collected from the saphenous vein and orbital sinus of 18 gerbils on each of 2 occasions. Markers differentiate between the first (hollow circles) and second (solid circles) sampling occasions. Dashed lines represent the mean difference between the methods and the upper and lower 95% limits of agreement (mean ± 2 SD).
Figure 4.
Bland–Altman plots of the difference in raw microfilaria counts compared with the mean count for paired blood samples with a mean count (A) 50 or fewer and (B) more than 50 microfilariae per 20 µL. Paired blood samples were collected from the saphenous vein and orbital sinus of 18 gerbils on each of 2 occasions. Markers differentiate between the first (hollow circles) and second (solid circles) sampling occasions. Dashed lines represent the mean difference between the methods and the upper and lower 95% limits of agreement (mean ± 2 SD).
Overall, we found that microfilaria counts from blood from the lateral saphenous vein did not differ significantly from retroorbital counts when counts were than 50 microfilariae per 20 µL or greater. However, at lower counts, those from the lateral saphenous vein were significantly lower than those from retroorbital blood.
Discussion
In this study, we assessed the potential for the lateral saphenous vein to replace the retroorbital sinus as a site for blood collection to determine microfilaria counts in gerbils. Collection from the lateral saphenous vein is a feasible standard for assessing levels of microfilaremia. This method involves less training time and skill than does the retroorbital technique, does not require anesthesia, and typically yields sufficient blood to create slides for counting microfilariae. Our results suggest that the lateral saphenous vein is a suitable site for blood collection when microfilaria counts are relatively high. For accurate assessment of lower numbers of microfilariae, the retroorbital sinus potentially should be used.
We needed to compare the microfilaria counts obtained from the traditional retroorbital method with those from the lateral saphenous method to ensure that the methods themselves were comparable. The method of collection is known to influence some blood parameters,1,14,41 and we had to consider the parasite biology as a possible variable. Blood collection from a tail clip of unanesthetized mice had higher levels of total leukocytes, neutrophils, and lymphocytes than did lateral saphenous samples.1 In another study, submandibular vein blood collection yielded higher AST, ALT, protein, albumin, triglycerides, total cholesterol, and creatinine levels than did retroorbital blood collection under anesthesia; the authors attributed the differences to differences in handling stress, anesthesia, tissue damage, and hemolysis between methods.14 Furthermore, blood samples collected from rats by retroorbital puncture under ether anesthesia contained higher concentrations of pCO2 and Na+ whereas lateral saphenous samples collected without anesthesia had higher pH, RBC, Hgb, and Hct values.41
At low microfilaria counts (that is, fewer than 50 microfilariae per 20 µL), the counts from blood obtained from the retroorbital sinus differed from those from the lateral saphenous vein. This discrepancy might be due to anatomic differences: an increased microfilaria count might reflect greater pooling of blood in the retroorbital sinus compared with a peripheral vein. An alternative explanation involves the possible effect of restraint and lateral saphenous vein puncture on an unanesthetized gerbil immediately before retroorbital sampling: handling stress during the first (lateral saphenous) sample collection might have affected the microfilaria levels in the second (retroorbital) sample collected. Another possible reason could be anesthesia: the gerbils were not anesthetized during the lateral saphenous bleeding, whereas they were anesthetized during retroorbital bleeding. For ethical reasons, we could not perform retroorbital bleeds on unanesthetized animals. Furthermore, we did not anesthetize gerbils for lateral saphenous bleeds, because rodents are not normally anesthetized for this procedure,12,18,31 and our goal was to compare the FR3′s current method with the intended replacement—lateral saphenous blood collection without anesthesia. Because anesthesia brings additional risks, including gerbil death during procedures and the hazards of personnel exposure to waste inhalant anesthetic, our overall goal was to replace retroorbital collection under anesthesia with lateral saphenous vein collection without anesthesia. A previous study found that the microfilaria counts in animals that were anesthetized during retroorbital bleeding were significantly higher than those that were not anesthetized, depending on the anesthetic used.5 This result corresponds with our results in the unanesthetized gerbils with the relatively low microfilaria counts (that is, fewer than 50 microfilariae per 20 µL). Additional research in this area could investigate the effect of anesthesia on microfilaria counts in gerbils, if the use of anesthesia were desired.
The retroorbital method has significant disadvantages compared with the lateral saphenous method. Retroorbital collection has a greater potential for complications when compared with other methods of blood collection, and it should be performed under general anesthesia, according to the NIH guidelines for survival bleeding of mice and rats.31 The Removal of Blood from Laboratory Mammals and Birds8 contains more explicit recommendations than those from the NIH, stating “…we do not recommend retroorbital bleeding for use with recovery other than in exceptional circumstances when there is no other method available.”8 The document states that the method should always be performed under general anesthesia, only one eye should be sampled, and only competent persons should perform the procedure. The conclusion is that, under most circumstances, retroorbital bleeding is only acceptable as a terminal procedure performed under general anesthesia.8 Others recommend 2 wk between bleeds, to allow damaged tissue to heal.12,30 A topical ophthalmic anesthetic administered to the eye before puncture has been recommended.31 However, contamination of the blood sample with topical anesthetic is a potential complication for some research.31
Retroorbital puncture to collect blood can cause tissue damage and severe adverse effects.8 The procedure has a moderate to high potential for tissue damage12 and thus an increased risk of complications,31 at a reported rate of 1% to 2%.22,30 The rate may be even higher when performed by inexperienced personnel;12 complications are less likely when the personnel are experienced in performing the procedure. However, complications including ocular discharge, corneal lesions, enophthalmia, exophthalmia, and intraocular alterations have been reported subsequent to retroorbital collection performed on rats by technicians with over 10 y of experience.40 Adverse consequences from retroorbital sampling have been well documented. In particular, hemorrhage can lead to a retrobulbar hematoma, putting pressure on the globe.12,8,30 If great enough, this pressure is probably painful and can lead to proptosis.19 If the proptosis hinders blinking, corneal ulceration can result.8 Corneal ulceration can be induced inadvertently due to applying pressure to the eye to stop the bleeding after sample collection.12 Ulceration from either cause can lead to keratitis, pannus formation, or rupture of the globe.12,8,30 The optic nerve and other intraorbital structures can be damaged, resulting in visual deficits, including blindness.4,12,19,8,30 Fracture of the orbital bone, leading to neural damage, can occur.12,8,30 The globe itself can be penetrated inadvertently and ruptured.12,8,30 Another potential complication of retroorbital puncture is infection of the orbital structures,4,19,8 which can result in abscess formation,4 and swelling and inflammation of the area can lead to degeneration of the eye.4,8 Necrotic dacryoadenitis of the Harderian gland has been reported.26,30 Some of these adverse consequences, such as degeneration and proptosis, can induce microphthalmia or enophthalmia.4,8,30
In one study, retroorbital blood collection was performed on rats in one eye; the other (unmanipulated) eye was used as a comparative control. After blood collection, the eyes were scored for “clinically visible alterations.”40 Significantly more abnormalities were present in the punctured eyes compared with the control eyes. The abnormalities noted were enophthalmia, exophthalmia, ocular discharge, and corneal alterations.40Another study also compared eyes, one sampled retroorbitally and one not, and found that enophthalmia was more frequent in the sampled eye.6 One long-term study demonstrated the ocular damage induced by repeated retroorbital blood collection.24 For 15 mo, rats had blood collected through retroorbital sampling of the right eye by an experienced operator every 6 wk. The left eye was never sampled, and served as the comparison control. After 15 mo, the eyes were examined. Macroscopically, there was atrophy and discoloration (gray) of the right optic nerves, which correlated with microscopic Wallerian degeneration of the optic nerve. There were similar lesions in the left cerebral orbital tracts. Variable thinning of the outer retina layers that could not be distinguished from age- and light-exposure–dependent retinal atrophy affected the right and left retinas. However, the atrophy was more severe in the right retinas. In addition, the right retinas suffered loss of approximately 40% of their ganglion cells.24
In addition, there are indications that retroorbital access causes discomfort or pain, although the research in this area is less clear.39,42 Investigators have studied the endocrine and behavioral effects of retroorbital blood collection in an attempt to assess potential pain and distress. One challenge is that routine procedures, such as moving cages or handling animals, are associated with stress,4 and common, nonretroorbital methods of collecting blood lead to increased cortisol levels in rats and mice.4 In addition, anesthesia can induce stress,16,17,27,42 although some anesthetics cause more stress than do others, and manual restraint without anesthesia can induce stress.27 Therefore, separating the effects of retroorbital blood collection alone can be difficult. Although results have been limited or contradictory, some studies demonstrate14,40 behavioral effects that do indicate at least transient pain or distress after retroorbital sampling.
Although we found no published assessments of complications of retroorbital blood collection specifically in gerbils, retroorbital blood collection in gerbils is likely more similar to the procedure in mice than that in rats because the orbital venous anatomy of gerbils is more similar to that of mice than rats. The venous structures around the orbit in laboratory rodents are described as an orbital venous sinus, which is a dilation of the vein, or an orbital venous plexus, which is a network of veins.38 Gerbils, hamsters, and mice have a venous sinus, whereas rats have a venous plexus.32,38 Retroorbital sampling is described as being difficult in rats because the plexus is deep in the orbit.38
Many of the disadvantages of the retroorbital sampling method can be avoided by collecting blood from the lateral saphenous vein instead. The lateral saphenous method has a low potential for complications or tissue damage.12,31 Reported potential complications are temporary lameness31 and persistent, minor bleeding.12 The procedure can be performed without general anesthesia12,18,31 and can be performed repeatedly, even daily.31 After the first puncture, the scab or clot can be removed to provide additional samples, so puncture is not required at each sample time point.18,31 In addition, because the procedure is fairly easy to learn, extensive technical skill or training is not required to perform it successfully. Blood collection from the lateral saphenous vein is used in multiple species, including rats, mice, hamsters, gerbils, guinea pigs, ferrets, mink, and larger animals.12 The technique is very similar across multiple rodent species, given that the vein runs across the lateral tarsal joint12,33 similarly among species. Specifically, the lateral saphenous vein is a “superficial vein of the lower hind limb that wraps proximally from the cranial aspect of the tarsus across the lateral surface of the leg to the caudal aspect of the stifle.”2 A small to moderate volume of blood can be collected from the lateral saphenous vein of rodents. Because,8,18 when compared with the lateral saphenous vein, the retroorbital method allows larger volumes of blood to be collected, the retroorbital method may be the most appropriate method in research situations that require a larger volume of blood. However, the lateral saphenous provides sufficient blood volume for microfilaria counts.
In summary, using the lateral saphenous vein to collect blood to assess microfilaria counts has advantages with important animal welfare implications compared with the retroorbital method, and should be preferentially used when it is suitable. For gerbils infected with B. pahangi, the lateral saphenous vein is suitable when microfilaria counts are expected to exceed 50 microfilariae per 20 µL.
Acknowledgment
We acknowledge the Filariasis Research Reagent Resource Center (FR3) for use of their gerbils during this study, and we acknowledge the gerbils, for their contributions to research.
References
- 1.Abatan OI, Welch KB, Nemzek JA. 2008. Evaluation of saphenous venipuncture and modified tail-clip blood collection in mice. J Am Assoc Lab Anim Sci 47:8–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Alworth LC, Kelly LM, Cooper TL, Salyards GW. 2012. Unassisted blood collection from unanesthetized rats and gerbils. Lab Anim (NY) 41:155–156. [DOI] [PubMed] [Google Scholar]
- 3.Ash LR, Schacher JF. 1971. Early life cycle and larval morphogenesis of Wuchereria bancrofti in the jird Meriones unguiculatus. J Parasitol 57:1043–1051. [PubMed] [Google Scholar]
- 4.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 5.Beerntsen BT, Lowenberger CA, Klinkhammer JA, Christensen LA, Christensen BM. 1996. Influence of anesthetics on the peripheral blood microfilaremia of Brugia malayi in the Mongolian jird, Meriones unguiculatus. J Parasitol 82:327–330. [PubMed] [Google Scholar]
- 6.Beynen AC, Baumans V, Haas JWM, Van Hellemond KK, Stafleu FR, Van Tintelen G. 1988. Assessment of discomfort induced by orbital puncture in rats, p 431–436. In: Beynen AC, Solleveld HA. New developments in biosciences: their implications for laboratory animal science. Proceedings of the Third Symposium of the Federation of European laboratory Animal Science Associations, Amsterdam, The Netherlands, 1–5 June 1987. Dordrecht (the Netherlands): Nijhoff Publishers. [Google Scholar]
- 7.Bland JM, Altman DG. 1986. Statistical methods for assessing agreement between 2 methods of clinical measurement. Lancet 327:307–310. [PubMed] [Google Scholar]
- 8.BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. 1993. Removal of blood from laboratory mammals and birds. Lab Anim 27:1–22. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JR, Marwoto HA, Tirtokusumo S, Masbar S, Rusch JT, Purnomo , Trenggono B. 1987. The silvered leaf monkey (Presbytis cristata) as a model for human bancroftian filariasis. Lab Anim Sci 37:502–504. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. [Internet]. 2013. Parasites: lymphatic filariasis. [Cited 6 July 2014]. Available at: http://www.cdc.gov/parasites/lymphaticfilariasis/index.html.
- 11.Choi YJ, Ghedin E, Berriman M, McQuillan J, Holroyd N, Mayhew GF, Christensen BM, Michalski ML. 2011. A deep-sequencing approach to comparatively analyze the transcriptome of lifecycle stages of the filarial worm Brugia malayi. PLoS Negl Trop Dis 5:e1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C, European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods 2001. A good-practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23. [DOI] [PubMed] [Google Scholar]
- 13.Ewert A, el-Bihari S. 1971. Rapid recovery of Brugia malayi larvae following experimental infection of cats. Trans R Soc Trop Med Hyg 65:364–368. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez I, Pena A, Del Teso N, Perez V, Rodriguez-Cuesta J. 2010. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci 49:202–206. [PMC free article] [PubMed] [Google Scholar]
- 15.Foster J, Ganatra M, Kamal I, Ware J, Makarova K, Ivanova N, Bhattacharyya A, Kapatral V, Kumar S, Posfai J, Vincze T, Ingram J, Moran L, Lapidus A, Omelchenko M, Kyrpides N, Ghedin E, Wang S, Goltsman E, Joukov V, Ostrovskaya O, Tsukerman K, Mazur M, Comb D, Koonin E, Slatko B. 2005. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol 3:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartner K, Buttner D, Dohler K, Friedel R, Lindena J, Trautschold I. 1980. Stress response of rats to handling and experimental procedures. Lab Anim 14:267–274. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K, Murakami K, Takao T, Makino S, Sugawara M, Ota Z. 1989. Effect of acute ether or restraint stress on plasma corticotropin-releasing hormone, vasopressin, and oxytocin levels in the rat. Acta Med Okayama 43:161–167. [DOI] [PubMed] [Google Scholar]
- 18.Hem A, Smith AJ, Solberg P. 1998. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret, and mink. Lab Anim 32:364–368. [DOI] [PubMed] [Google Scholar]
- 19.Hoff J. 2000. Methods of blood collection in the mouse. Lab Anim (NY) 29:47–53. [Google Scholar]
- 20.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 21.Kazura JW. 2002Lymphatic filarial infections: an introduction to the filariae, p 1–8. In: Klei TR, Rajan TV. World-class parasites: the filaria, vol 5 New York (NY): Springer. [Google Scholar]
- 22.Krinke A, Kobel W, Krinke G. 1988. Does the repeated orbital sinus puncture alter the occurrence of changes with age in the retina, the lens, or the Harderian gland of laboratory rats? Z Versuchstierkd 31:111–119. [PubMed] [Google Scholar]
- 23.Krishna Kumari A, Harichandrakumar KT, Das LK, Krishnamoorthy K. 2005. Physical and psychosocial burden due to lymphatic filariasis as perceived by patients and medical experts. Trop Med Int Health 10:567–573. [DOI] [PubMed] [Google Scholar]
- 24.Le Net J-L, Abbott DP, Mompon RP, Leblanc B. 1994. Repeated orbital sinus puncture in rats induces damage to optic nerve and retina. Abstract presented at the American College of Veterinary Pathologists’ 45th Annual Meeting, Montreal, Quebec, Canada, 30 October–4 November 1994. Vet Pathol 31:621. [Google Scholar]
- 25.McCarthy JS, Nutman TB. 1996. Perspective: prospects for development of vaccines against human helminth infections. J Infect Dis 174:1384–1390. [DOI] [PubMed] [Google Scholar]
- 26.McGee MA, Maronpot RR. 1979. Harderian gland dacryoadenitis in rats resulting from orbital bleeding. Lab Anim Sci 29:639–641. [PubMed] [Google Scholar]
- 27.McGuill MW, Rowan AN. 1989. Biological effects of blood loss: implications for sampling volumes and techniques. ILAR J 31:5–20. [Google Scholar]
- 28.Michalski ML, Griffiths KG, Williams SA, Kaplan RM, Moorhead AR. 2011. The NIH–NIAID Filariasis Research Reagent Resource Center. PLoS Negl Trop Dis 5:e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller JF. 1965. Helminth life cycles. Am Zool 5:131–139. [DOI] [PubMed] [Google Scholar]
- 30.National Centre for the Replacement Refinement and Reduction of Animals in Research. [Internet]. 2009. Blood sampling: rat: retroorbital (nonsurgical/terminal). [Cited 6 July 2014]. Available at: http://www.nc3rs.org.uk/bloodsamplingmicrosite/page.asp?id=388.
- 31.National Institutes of Health–Animal Research Advisory Committee. [Internet]. 2012. Guidelines for survival bleeding of mice and rats. [Cited July 6 2014]. Available at: http://oacu.od.nih.gov/ARAC/documents/Rodent_Bleeding.pdf.
- 32.Ninomiya H, Inomata T. 2006. Microvasculature of the mouse eye: scanning electron microscopy of vascular corrosion casts. J Exp Anim Sci 43:149–159. [Google Scholar]
- 33.Oruganti M, Gaidhani S. 2011. Routine bleeding techniques in laboratory rodents. Int J Pharm Sci Res 2:516–524. [Google Scholar]
- 34.Partono F. 1987. The spectrum of disease in lymphatic filariasis. Ciba Found Symp 127:15–31. [DOI] [PubMed] [Google Scholar]
- 35.Rao CK, Dey KP, Venkatanarayana M, Ravindranathan TC, Sahai R, Menon PK, Subrahmanyam D. 1980. Attempts to establish Wuchereria bancrofti in laboratory animals. J Commun Dis 12:219–221. [PubMed] [Google Scholar]
- 36.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (UK): Methuen. [Google Scholar]
- 37.Schacher JF, Sahyoun PF. 1967. A chronological study of the histopathology of filarial disease in cats and dogs caused by Brugia pahangi (Buckley and Edeson, 1956). Trans R Soc Trop Med Hyg 61:234–243. [DOI] [PubMed] [Google Scholar]
- 38.Timm KI. 1989. Orbital venous anatomy of the Mongolian gerbil with comparison to the mouse, hamster, and rat. Lab Anim Sci 39:262–264. [PubMed] [Google Scholar]
- 39.van Herck H, Baumans V, Boere HAG, Hesp APM, van Lith HA, Beynen AC. 2000. Orbital sinus blood sampling in rats: effects upon selected behavioural variables. Lab Anim 34:10–19. [DOI] [PubMed] [Google Scholar]
- 40.van Herck H, Baumans V, Brandt CJWM, Hesp APM, Sturkenboom JH, van Lith HA, van Tintelen G, Beynen AC. 1998. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab Anim 32:377–386. [DOI] [PubMed] [Google Scholar]
- 41.van Herck H, Baumans V, Brandt CJWM, Boere HAG, Hesp APM, van Lith HA, Schurink M, Beynen AC. 2001. Blood sampling from the retroorbital plexus, the saphenous vein, and the tail vein in rats: comparative effects on selected behavioural and blood variables. Lab Anim 35:131–139. [DOI] [PubMed] [Google Scholar]
- 42.van Herck H, Baumans V, de Boer SF, van der Gugten J, van Woerkom AB, Beynen AC. 1991. Endocrine stress response in rats subjected to singular orbital puncture while under diethyl-ether anaesthesia. Lab Anim 25:325–329. [DOI] [PubMed] [Google Scholar]