Abstract
Background
The motions causing non-contact ACL injury remain unclear. Tibiofemoral bone bruises are believed to be the result of joint impact near the time of ACL rupture. The locations and frequencies of these bone bruises have been reported, but there is limited data quantifying knee position and orientation near the time of injury based on these contusions.
Hypothesis
Knee position and orientation near the time of non-contact ACL injury include extension and anterior tibial translation.
Study Design
Descriptive Laboratory Study
Methods
Magnetic resonance (MR) images of eight subjects with non-contact ACL injuries were acquired within one month of injury and subsequently analyzed. All subjects exhibited bruises on both the femur and tibia in both medial and lateral compartments. The outer margins of bone and the bone bruise surfaces were outlined on each image to create a 3D model of each subject’s knee in its position during MR imaging (MRI position). Numerical optimization was used to maximize overlap of the bone bruises on the femur and tibia and predict the position of injury. Flexion angle, valgus orientation, internal tibial rotation, and anterior tibial translation were measured in both the MRI position and the predicted position of injury. Differences in kinematics between the MRI position, which served as an unloaded reference, and the predicted position of injury were compared using paired t-tests.
Results
Flexion angle was near full extension in both the MRI position and the predicted position of injury (8° versus 12°, p=0.2). Statistically significant increases in valgus orientation (5°, p=0.003), internal tibial rotation (15°, p=0.003), and anterior tibial translation (22mm, p<0.001) were observed in the predicted position of injury relative to the MRI position.
Conclusions
These results suggest that for the bone bruise pattern studied, landing on an extended knee is high risk for ACL injury. Extension was accompanied by increased anterior tibial translation (22mm), internal tibial rotation (15°), and valgus rotation (5°) in the predicted position of injury relative to the MRI position.
Clinical Relevance
This study provides novel data characterizing the motions associated with ACL injury, information critical to improving strategies aimed at injury prevention.
Key Terms: injury, mechanism, motion, valgus, contusion, MRI, anterior cruciate ligament, imaging
Introduction
Over 400,000 anterior cruciate ligament (ACL) injuries occur annually in the United States31. This devastating injury primarily affects a younger population, with a median age of 17 years at the time of injury20, 24. The high risk of developing early onset osteoarthritis (OA) after both injury and reconstruction19, 37 has prompted great interest in the mechanisms of ACL injury28, 40, 41, 50, 51, 57 and in the implementation of injury prevention programs21, 26, 49. Prevention programs have focused on training athletes to avoid non-contact ACL injuries, which account for more than 70% of ACL tears24. Some programs have shown significant reduction of ACL injuries in trained groups compared to untrained controls26, 38. However, other programs have been less successful4, 45, 49, 52, and ACL injury rates remain high2, 3. The efficacy of training programs is likely limited by unclear data describing the motions leading to ACL injury. Specifically, because there are conflicting theories and limited data on the precise motions causing non-contact ACL injuries7, 13, 14, 28, 40, prevention programs may target a wide range of exercises24, some of which may be ineffective. Furthermore, understanding which movement patterns lead to ACL rupture could aid in identifying individuals who are at high risk for injury and should be targeted for intervention22, 44. Thus, a clear understanding of the motions leading to ACL injury may allow for improved prevention strategies.
Several different approaches have been used to investigate motion patterns that are high risk for ACL injury. Based on video analysis of sports footage, some studies attribute ACL injury to valgus collapse8, 34, 47. Though video analysis provides valuable information, it has several limitations. For example, due to variability in the position and orientation of the camera relative to the injured athlete and challenges associated with identifying anatomic landmarks predictive of the motion of the underlying bones28, 33, it may be difficult to determine six degrees-of-freedom knee kinematics during ACL injury from videos alone. Furthermore, the exact time of injury is unknown8, 28, 32, making it difficult to determine which motions caused the ACL rupture. Another means of investigating ACL injury mechanisms is the use of cadaveric models. Such studies attempt to mimic the dynamic loading thought to cause ACL injury16, 25, 59. Although insightful, it is unclear how closely these models reproduce the complex, dynamic muscle and ground reaction forces experienced during in vivo loading.
Other studies have utilized bone bruise patterns observed on MR images of individuals with non-contact ACL rupture to elucidate the mechanisms of injury23, 29, 53, 57, 62. These bruises are visible as increased signal intensity on short Tau inversion recovery (STIR) or T2 weighted images, a result of increased water content due to trabecular microfracture36, 43, 53. These contusions are thought to be the result of impact between the femur and tibia near the time of ACL injury29, 48, 57, 62. Although previous studies have utilized MR images of individuals with non-contact ACL injuries to characterize the locations and frequencies of these bone bruises23, 29, 53, 57, 62, it may be difficult to determine the position and orientation of the knee at the time of injury from the examination of MR images alone. Thus, the objective of this study was to quantify the position and orientation of the knee near the time of non-contact ACL injury using MR imaging, together with 3D modeling and numerical optimization techniques, to maximize overlap of bone bruise surfaces on the femur and tibia. Based on previous studies observing ACL loading and bone bruise patterns, we hypothesized that the position and orientation of the knee at the time of injury involve knee extension6, 53, 54, 56 and anterior tibial translation10, 16, 57.
Methods
Selection Criteria and MR Imaging
Institutional Review Board approval was obtained for this study. Eight subjects (5 males and 3 females, average age: 23 years, age range: 16–30 years) with non-contact ACL injury were included in this retrospective study. The subjects reported that the injury occurred during the following activities: soccer (4), football (1), tennis (1), basketball (1), and horseplay (1). Six of the eight subjects had signal abnormality suggestive of a Grade 1 medial collateral ligament (MCL) tear, while the remaining two had no evidence of MCL injury. With regard to meniscal injuries, four subjects had no evidence of tears or abnormal MR signals in their menisci, and the remaining four subjects had small tears to their medial or lateral menisci.
All subjects underwent MR imaging within one month of their non-contact ACL rupture with an average of 14 days (range: 4–22 days) after injury. MR images acquired beyond one month of injury were excluded. Five studies were performed on a 1.5T Signa (GE), two on a 1.5T Avanto (Siemens), and one on a 3T Trio Tim (Siemens). Fast Spin Echo Imaging parameter ranges were as follows: repetition time (TR) of 3310–4083ms, echo time (TE) of 50–75ms, matrix of 256×192 or 384×384 interpolated to 512×512 for display, number of excitations (NEX) of 1–2, slice thickness of 3–4mm, and interslice gap of 0.3–0.4mm. Fat saturation was used on all sequences, and all images were obtained in a dedicated receive-only coil appropriate for the scanner. As part of the selection criteria, bone bruises were visible in both medial and lateral compartments of the femur and tibia for all subjects. This particular bone bruise pattern was selected because it allows for a unique solution for the numerical optimization. Furthermore, recent studies have suggested that more than 80% of patients with non-contact ACL injury have evidence of bone bruises in both medial and lateral compartments60.
Model Creation and Analysis
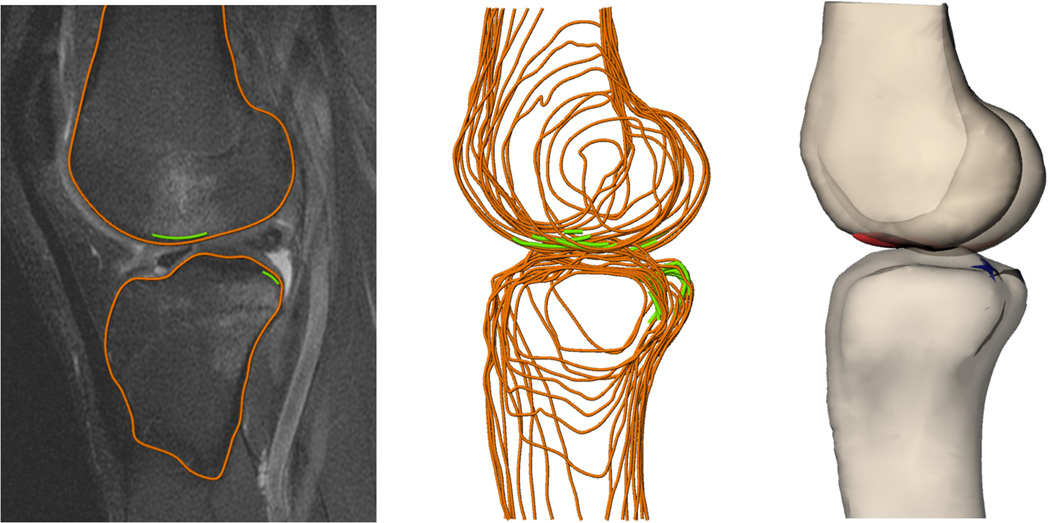
These MR images were used to create 3D models of the knee1, 11, 46 (Figure 1). First, the outer margins of the femur and tibia were outlined on all MR images in which they were visible using 3D modeling software (Rhinoceros 4.0, Robert McNeel and Associates, Seattle, WA). Next, the bone bruises were outlined along the outer surfaces of the femur and tibia. All slices were outlined by a single investigator and reviewed by a board-certified musculoskeletal radiologist with over 25 years of experience. The outlines were combined to form a 3D model of the knee and bone bruise surfaces.
Figure 1.
Bone (orange) and bone bruise surfaces (green) were outlined on each MR slice to create 3D models of the knee.
Numerical optimization was then used to maximize overlap of bone bruise surfaces on both the femur and tibia with the assumption that the bruises were the result of impact between the femur and tibia near the time of ACL injury. Specifically, the femur was rigidly translated and rotated in 3D space such that the distance between points evenly distributed across the surface of the femoral bone bruises and points evenly distributed across the surface of the tibial bone bruises was minimized (Figures 2 and 3). The optimization was constrained to minimize penetration of the bony surfaces.
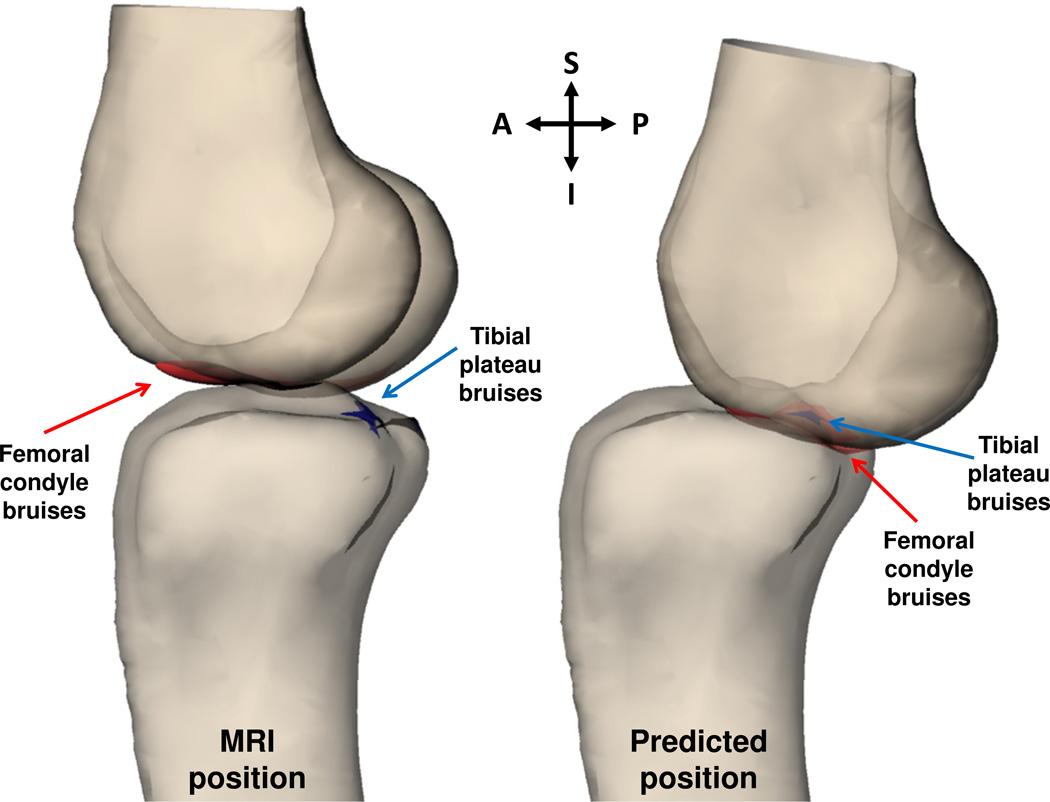
Figure 2.
Numerical optimization was used to maximize overlap of bone bruises on the femoral condyle (red) and tibial plateau (blue) and predict the position of injury. A sagittal view of a 3D model of one subject’s knee is shown in the MRI position (left) and in the predicted position of injury (right). (A=anterior, P=posterior, S=superior, I=inferior)
Figure 3.
Numerical optimization was used to maximize overlap of bone bruises on the femoral condyle (red) and tibial plateau (blue) and predict the position of injury. A coronal view of a 3D model of one subject’s knee is shown in the MRI position (left) and in the predicted position of injury (right). (M=medial, L=lateral, S=superior, I=inferior)
The relative positions of the femur and tibia in six degrees of freedom were measured before and after the numerical optimization using an anatomic coordinate system previously described in the literature56. The long axis of the tibia was defined by a cylinder fit to the tibial shaft. A mediolateral axis was created perpendicular to the long axis of the tibia and tangent to the posterior edges of the tibial plateau. An anteroposterior axis was drawn orthogonal to the long and mediolateral axes of the tibia. The long axis of the femur was defined by a cylinder fit to the femoral shaft. The transepicondylar line of the femur defined the flexion axis. Valgus was measured using the angle between the long axis of the tibia and the transepicondylar line of the femur. An angle of 90° between these axes corresponded to 0° valgus56. Internal rotation of the tibia was measured as the angle between the mediolateral axis of the tibia and the transepicondylar line of the femur projected onto the tibial plateau. Anterior tibial translation was measured using the position of the transepicondylar line of the femur relative to the tibial coordinate system. For each subject, the extended position of the knee during MR imaging (MRI position) served as an unloaded reference9, 30, 55, 58. Differences in kinematics between the MRI position and the predicted position of the knee at the time of injury (as determined from numerical optimization) were compared using paired t-tests. Differences were considered statistically significant where p<0.05.
Repeatability of Kinematics Measurements
Although previous studies have reported on the location, volume, intensity, depth, and frequency at which bone bruising occurs with ACL injury36, 43, 48, 53, 57, little data is available quantifying the position and orientation of the knee at the time of injury. Thus, in order to assess the repeatability of our measurements, three independent trials of bone bruise outlining were performed on a single knee using the same procedure as described above. The standard deviations of the femoral and tibial bone bruise surface areas were both within 1% of the total area. Additionally, numerical optimization was performed separately for each trial, and a relatively small standard deviation was measured for each degree of freedom (flexion: 0.9°, valgus: 0.8°, internal tibial rotation: 0.1°, anterior tibial translation: 0.1mm), indicating that this methodology has a high degree of repeatability in quantifying the predicted position of injury.
Results
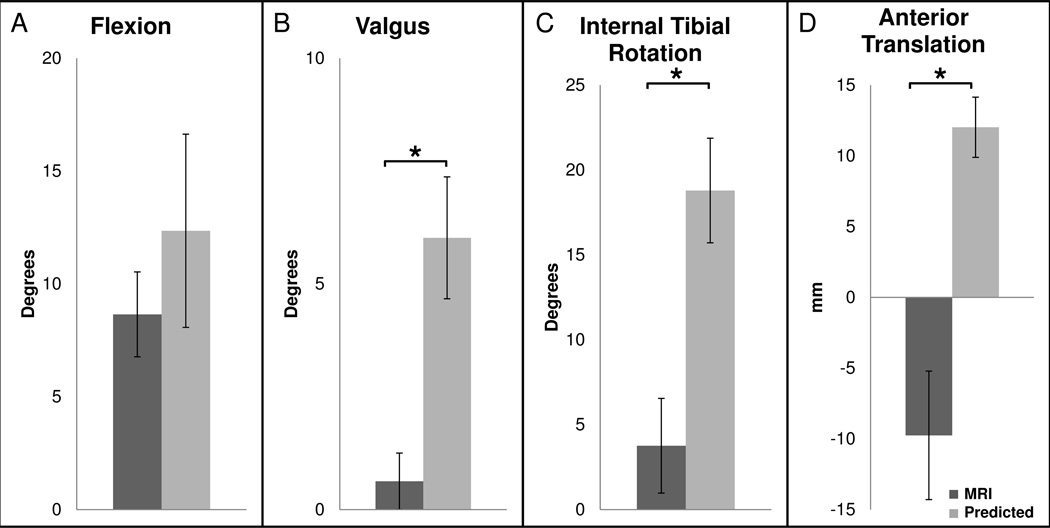
In the MRI position, the knee flexion angle was 8 ± 2° (mean ± standard error). In the predicted position of injury, the flexion angle was 12 ± 4° (Figure 4A). Flexion did not change significantly between the MRI position and the predicted position of injury (p=0.2). The valgus orientation of the knee in the MRI position was 1 ± 1° as compared to 6 ± 1° in the predicted position of injury, a significant increase of 5° (p=0.003, Figure 4B). In the MRI position, internal tibial rotation was 4 ± 3° as compared to 19 ± 3° in the predicted position of injury, a significant increase of 15° (p=0.003, Figure 4C). Lastly, anterior tibial translation was −10 ± 5mm in the MRI position and 12 ± 2mm in the predicted position, a significant increase of 22mm (p<0.001, Figure 4D).
Figure 4.
Average (A) flexion angle, (B) valgus orientation, (C) internal tibial rotation, and (D) anterior tibial translation are shown both when the knee is in the MRI position and in the predicted position at the time of injury. Valgus orientation, internal tibial rotation, and anterior tibial translation increased significantly in the predicted position at the time of injury relative to the MRI position (*p<0.05).
Discussion
Despite the implementation of ACL injury prevention programs, ACL injury rates remain high2, 3. The efficacy of these programs is likely hindered by an unclear understanding of the motions causing non-contact ACL injury8, 24, 27, 28, 32, 34, 50, 56. In order to address this important clinical problem, this study estimated the position and orientation of the knee during ACL rupture using MR imaging, 3D modeling, and numerical optimization. Specifically, with the assumption that the femoral and tibial bone bruises in these subjects resulted from joint impact near the time of non-contact ACL injury, numerical optimization was used to position 3D models of the femur and tibia such that overlap of bone bruise surfaces was maximized. Our results suggest that ACL injuries with medial and lateral compartment bruising occur with the knee near extension and are accompanied by a large anterior tibial translation and a large internal tibial rotation. A slight valgus rotation was also observed.
The small flexion angle predicted at the time of ACL injury by this study is consistent with previous findings in the literature. For example, videographic analyses suggest that non-contact ACL injury is frequently associated with landing with the knee near full extension8, 34, 47. Furthermore, previous in vivo studies measuring ACL function under various loading conditions have shown that ACL length and strain are maximal at low flexion angles6, 12, 54–56, 61. For example, during dynamic activities such as jumping and walking, maximum ACL strain occurs with the knee near full extension54, 55, 61. These findings suggest that the ACL may be at an increased risk for injury when the knee is extended and ACL strain is elevated. The increased strain in low flexion angles may be explained by the orientation of the patellar tendon during extension and flexion15. When the knee is extended, the patellar tendon is oriented anteriorly relative to the anterior tibial cortex, resulting in an anteriorly directed force on the tibia and increased ACL load15. However, as the knee is flexed, the patellar tendon is oriented such that a posteriorly directed force is applied to the tibia, resulting in decreased ACL load15.
Additionally, the ACL has been shown to be an important restraint to anterior tibial translation5, 10, 18, 39, which is consistent with the large anterior translation near the time of injury suggested by the present study. For example, Butler et al. showed that in cadaveric knees, the ACL provides 90% of the resistance to anteriorly directed loads applied to the tibia10. In another cadaveric study inducing ACL injury with the knee fixed at 20° of flexion, 20mm of anterior tibial displacement was reported in response to quadriceps loads of up to 4500 N16. This amount of anterior tibial translation is comparable to the 22mm of anterior tibial translation found in the present study. These findings suggest that a large anterior tibial translation is likely associated with ACL injury.
Previous video analysis studies have observed both internal and external rotations during ACL injury32, 34, 47. Other studies have reported that internal tibial rotation increases ACL loading while external tibial rotation does not dramatically elevate ACL loading18, 39. For example, a cadaveric study indicated that a combination of anterior shear force and internal tibial torque dramatically increased ACL load, particularly with the knee near full extension39. The addition of an external tibial torque generally decreased ACL load39. Similarly, an in vivo study utilizing strain transducers implanted on the ACL found that internal tibial rotation increased ACL strain, particularly in low knee flexion18. The similar results observed in the present study suggest that internal tibial rotation may indeed be a contributing factor to ACL tears.
Our findings differ from previous studies analyzing video footage of ACL tears that have reported a large valgus collapse motion during injury8, 28, 32. For example, one video analysis study reported no difference in valgus orientation between ACL injured subjects and controls performing similar athletic activities at the time of contact with the ground8. However, soon after impact, differences in valgus between injured subjects and controls reached nearly 30°8. Another study used a model-based image matching technique to approximate the position of the knee during ACL injury in videos of ten female handball and basketball players32. At the estimated time of injury, a significant increase of 12° was reported in the valgus orientation of the knee relative to the neutral valgus position at initial ground contact. In contrast, our results suggest that, for the ACL injured subjects in this study, there is less valgus present at the time of injury. However, our findings of a small amount of valgus are consistent with the results of other studies in the literature. Specifically, the increase of 5° in valgus orientation observed in the present study is comparable to the increase of 2.3° in valgus orientation reported by DeMorat et al. when loading the quadriceps of cadaveric knees to induce ACL injury16. Furthermore, a previous in vivo study found that in a simulated valgus collapse position, ACL length was shorter compared to when in a neutral valgus position at the same flexion angle, suggesting that valgus does not increase ACL strain56. Another study employing strain transducers to quantify in vivo ACL strains has also reported minimal changes with varus and valgus loading of the knee18. These findings support our results that a large valgus collapse motion does not occur at the time of ACL injury in subjects with this bone bruise pattern. Since it is difficult to identify the precise time of injury from videographic analyses32, 34, the large valgus collapse motion reported in these studies could be the result of buckling of the knee after ACL rupture42, 47, 56.
Previous studies reporting on the location, volume, intensity, depth, and frequency at which bone bruising occurs with ACL injury have not come to a consensus regarding the mechanisms of ACL injury23, 48, 53, 57, 60. Some studies have suggested that the high incidence of bruising near the terminal sulcus and the posterolateral tibial plateau may be due to a pivot shift event in which anterior subluxation occurs concurrently with an ACL tear17, 23, 53. Due to the high incidence of bone bruising in the lateral compartment in individuals with ACL injury53, 57, 62, some studies have emphasized the importance of valgus rotation48, 50. Medial compartment bruising in non-contact injuries has been attributed to anterior tibial translation and internal tibial rotation rather than valgus stress due to the location of femoral condyle bruising relative to tibial plateau bruising57, 60. These different conclusions in the literature may be due in part to difficulties associated with inferring the position and orientation of the knee at the time of injury by observation of the location of bone bruising on MR images alone.
While previous studies have largely focused on lateral compartment bruises, recent studies have reported a relatively high incidence of medial compartment bone bruises57, 60. Perhaps one reason that medial compartment bruises are reported less frequently is that the intensity and depth of lateral compartment bruising may be significantly greater than medial compartment bruising on both the femoral condyles and the tibial plateau57. However, in a study reporting bone bruise frequency in non-contact and contact ACL injuries, bone bruises on the medial femoral condyle and medial tibial plateau were present in 38% and 60%, respectively, of subjects with a non-contact injury mechanism57. Similarly, a recent study analyzing 73 subjects with non-contact ACL injury reported that 84% of males and 89% of females had MR evidence of bone bruises in both medial and lateral compartments with no differences observed between the sexes60. These studies57, 60 suggest that the present study focused on a relatively common pattern of bone bruising. Future studies may investigate knee positions associated with other patterns of bone bruising.
Assuming that the bone bruises analyzed in this study resulted from impact between the femur and tibia near the time of ACL rupture, the results of the present study provide important information regarding the mechanisms of ACL injury. For the pattern of bone bruising considered in this study, our results suggest that landing on an extended knee may put the knee at high risk for injury. As indicated above, these findings are consistent with recent studies in the literature indicating that for a variety of different in vivo activities (including walking and landing from a jump), ACL lengths and strains generally increase with decreasing flexion35, 54–56, 61. These findings therefore suggest that ACL injury prevention programs should focus on training athletes to avoid stiff landings with knees near full extension. However, more research is needed to determine which motion patterns place the ACL at high risk for injury. In this regard, in vivo, dynamic measurements of ACL strain12, 54, 55, 61 may provide important insights into which motions elevate ACL loading and potentially increase injury risk. Furthermore, such measurements could also be useful in identifying which interventions are most effective at decreasing ACL injury risk.
In conclusion, this paper provides a novel methodology to characterize the position and orientation of the knee near the time of ACL rupture using bone bruises found on MR images of subjects with non-contact ACL injuries. Numerical optimization maximizing the overlap of bone bruises on the femur and tibia suggested that landing on an extended knee may be high risk for ACL injury. Extension was accompanied by increases in anterior tibial translation (22mm), internal tibial rotation (15°), and valgus rotation (5°) in the predicted position of injury relative to the MRI position. This data provides critical insights into the mechanisms leading to ACL injury. Ultimately, understanding these mechanisms is critical to improving strategies aimed at injury prevention.
References
- 1.Abebe ES, Kim JP, Utturkar GM, Taylor DC, Spritzer CE, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effect of femoral tunnel placement on ACL graft orientation and length during in vivo knee flexion. J Biomech. 2011;44(10):1914–1920. doi: 10.1016/j.jbiomech.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 3.Agel J, Klossner D. Epidemiologic review of collegiate acl injury rates across 14 sports: national collegiate athletic association injury surveillance system data 2004–05 through 2011–12. British journal of sports medicine. 2014;48(7):560. [Google Scholar]
- 4.Barber-Westin SD, Noyes FR, Smith ST, Campbell TM. Reducing the risk of noncontact anterior cruciate ligament injuries in the female athlete. Phys Sports med. 2009;37(3):49–61. doi: 10.3810/psm.2009.10.1729. [DOI] [PubMed] [Google Scholar]
- 5.Berns GS, Hull ML, Patterson HA. Strain in the anteromedial bundle of the anterior cruciate ligament under combination loading. J Orthop Res. 1992;10(2):167–176. doi: 10.1002/jor.1100100203. [DOI] [PubMed] [Google Scholar]
- 6.Beynnon BD, Johnson RJ, Fleming BC, Stankewich CJ, Renstrom PA, Nichols CE. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension. A comparison of an open and a closed kinetic chain exercise. Am J Sports Med. 1997;25(6):823–829. doi: 10.1177/036354659702500616. [DOI] [PubMed] [Google Scholar]
- 7.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 8.Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37(2):252–259. doi: 10.1177/0363546508328107. [DOI] [PubMed] [Google Scholar]
- 9.Brandsson S, Karlsson J, Eriksson BI, Karrholm J. Kinematics after tear in the anterior cruciate ligament: dynamic bilateral radiostereometric studies in 11 patients. Acta Orthop Scand. 2001;72(4):372–378. doi: 10.1080/000164701753542032. [DOI] [PubMed] [Google Scholar]
- 10.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 11.Caputo AM, Lee JY, Spritzer CE, Easley ME, DeOrio JK, Nunley JA, 2nd, DeFrate LE. In vivo kinematics of the tibiotalar joint after lateral ankle instability. Am J Sports Med. 2009;37(11):2241–2248. doi: 10.1177/0363546509337578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerulli G, Benoit DL, Lamontagne M, Caraffa A, Liti A. In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: case report. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):307–311. doi: 10.1007/s00167-003-0403-6. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhari AM, Andriacchi TP. The mechanical consequences of dynamic frontal plane limb alignment for non-contact ACL injury. J Biomech. 2006;39(2):330–338. doi: 10.1016/j.jbiomech.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Dai B, Herman D, Liu H, Garrett WE, Yu B. Prevention of ACL injury, part I: injury characteristics, risk factors, and loading mechanism. Res Sports Med. 2012;20(3–4):180–197. doi: 10.1080/15438627.2012.680990. [DOI] [PubMed] [Google Scholar]
- 15.Defrate LE, Nha KW, Papannagari R, Moses JM, Gill TJ, Li G. The biomechanical function of the patellar tendon during in-vivo weight-bearing flexion. J Biomech. 2007;40(8):1716–1722. doi: 10.1016/j.jbiomech.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32(2):477–483. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- 17.Fayad LM, Parellada JA, Parker L, Schweitzer ME. MR imaging of anterior cruciate ligament tears: is there a gender gap? Skeletal Radiol. 2003;32(11):639–646. doi: 10.1007/s00256-003-0694-1. [DOI] [PubMed] [Google Scholar]
- 18.Fleming BC, Renstrom PA, Beynnon BD, Engstrom B, Peura GD, Badger GJ, Johnson RJ. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 19.Frobell RB, Roos HP, Roos EM, Roemer FW, Ranstam J, Lohmander LS. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ. 2013;346:f232. doi: 10.1136/bmj.f232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett WE, Jr, Swiontkowski MF, Weinstein JN, Callaghan J, Rosier RN, Berry DJ, Harrast J, Derosa GP. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660–667. doi: 10.2106/JBJS.E.01208. [DOI] [PubMed] [Google Scholar]
- 21.Gilchrist J, Mandelbaum BR, Melancon H, Ryan GW, Silvers HJ, Griffin LY, Watanabe DS, Dick RW, Dvorak J. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008;36(8):1476–1483. doi: 10.1177/0363546508318188. [DOI] [PubMed] [Google Scholar]
- 22.Goetschius J, Smith HC, Vacek PM, Holterman LA, Shultz SJ, Tourville TW, Slauterbeck J, Johnson RJ, Beynnon BD. Application of a clinic-based algorithm as a tool to identify female athletes at risk for anterior cruciate ligament injury: a prospective cohort study with a nested, matched case-control analysis. Am J Sports Med. 2012;40(9):1978–1984. doi: 10.1177/0363546512456972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graf BK, Cook DA, De Smet AA, Keene JS. "Bone bruises" on magnetic resonance imaging evaluation of anterior cruciate ligament injuries. Am J Sports Med. 1993;21(2):220–223. doi: 10.1177/036354659302100210. [DOI] [PubMed] [Google Scholar]
- 24.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 25.Hashemi J, Breighner R, Jang TH, Chandrashekar N, Ekwaro-Osire S, Slauterbeck JR. Increasing pre-activation of the quadriceps muscle protects the anterior cruciate ligament during the landing phase of a jump: an in vitro simulation. Knee. 2010;17(3):235–241. doi: 10.1016/j.knee.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 27.Hewett TE, Lynch TR, Myer GD, Ford KR, Gwin RC, Heidt RS., Jr Multiple risk factors related to familial predisposition to anterior cruciate ligament injury: fraternal twin sisters with anterior cruciate ligament ruptures. Br J Sports Med. 2010;44(12):848–855. doi: 10.1136/bjsm.2008.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417–422. doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan PA, Gehl RH, Dussault RG, Anderson MW, Diduch DR. Bone contusions of the posterior lip of the medial tibial plateau (contrecoup injury) and associated internal derangements of the knee at MR imaging. Radiology. 1999;211(3):747–753. doi: 10.1148/radiology.211.3.r99jn30747. [DOI] [PubMed] [Google Scholar]
- 30.Karrholm J, Brandsson S, Freeman MA. Tibiofemoral movement 4: changes of axial tibial rotation caused by forced rotation at the weight-bearing knee studied by RSA. J Bone Joint Surg Br. 2000;82(8):1201–1203. doi: 10.1302/0301-620x.82b8.10715. [DOI] [PubMed] [Google Scholar]
- 31.Kibler WB. Orthopaedic knowledge update. Sports medicine 4. 4th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. American Orthopaedic Society for Sports Medicine. [Google Scholar]
- 32.Koga H, Nakamae A, Shima Y, Iwasa J, Myklebust G, Engebretsen L, Bahr R, Krosshaug T. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–2225. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- 33.Krosshaug T, Bahr R. A model-based image-matching technique for three-dimensional reconstruction of human motion from uncalibrated video sequences. J Biomech. 2005;38(4):919–929. doi: 10.1016/j.jbiomech.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, Hewett TE, Bahr R. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 35.Li G, Defrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23(2):340–344. doi: 10.1016/j.orthres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Ma BC, Bolbos RI, Stahl R, Lozano J, Zuo J, Lin K, Link TM, Safran M, Majumdar S. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging. 2008;28(2):453–461. doi: 10.1002/jmri.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 38.Mandelbaum BR, Silvers HJ, Watanabe DS, Knarr JF, Thomas SD, Griffin LY, Kirkendall DT, Garrett W., Jr Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 39.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 40.McLean SG. The ACL injury enigma: we can't prevent what we don't understand. J Athl Train. 2008;43(5):538–540. doi: 10.4085/1062-6050-43.5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLean SG, Huang X, van den Bogert AJ. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: implications for ACL injury. Clin Biomech (Bristol, Avon) 2005;20(8):863–870. doi: 10.1016/j.clinbiomech.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Meyer EG, Haut RC. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008;41(16):3377–3383. doi: 10.1016/j.jbiomech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 43.Mink JH, Deutsch AL. Occult cartilage and bone injuries of the knee: detection, classification, and assessment with MR imaging. Radiology. 1989;170(3 Pt 1):823–829. doi: 10.1148/radiology.170.3.2916038. [DOI] [PubMed] [Google Scholar]
- 44.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Development and validation of a clinic-based prediction tool to identify female athletes at high risk for anterior cruciate ligament injury. Am J Sports Med. 2010;38(10):2025–2033. doi: 10.1177/0363546510370933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245–255. doi: 10.1016/j.arthro.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Okafor EC, Utturkar GM, Widmyer MR, Abebe ES, Collins AT, Taylor DC, Spritzer CE, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J Biomech. 2014;47(1):96–101. doi: 10.1016/j.jbiomech.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury Mechanisms for Anterior Cruciate Ligament Injuries in Team Handball: A Systematic Video Analysis. American Journal of Sports Medicine. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 48.Patel SA, Hageman J, Quatman CE, Wordeman SC, Hewett TE. Prevalence and location of bone bruises associated with anterior cruciate ligament injury and implications for mechanism of injury: a systematic review. Sports Med. 2014;44(2):281–293. doi: 10.1007/s40279-013-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeiffer RP, Shea KG, Roberts D, Grandstrand S, Bond L. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. J Bone Joint Surg Am. 2006;88(8):1769–1774. doi: 10.2106/JBJS.E.00616. [DOI] [PubMed] [Google Scholar]
- 50.Quatman CE, Hewett TE. The anterior cruciate ligament injury controversy: is "valgus collapse" a sex-specific mechanism? Br J Sports Med. 2009;43(5):328–335. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quatman CE, Quatman CC, Hewett TE. Prediction and prevention of musculoskeletal injury: a paradigm shift in methodology. Br J Sports Med. 2009;43(14):1100–1107. doi: 10.1136/bjsm.2009.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soderman K, Werner S, Pietila T, Engstrom B, Alfredson H. Balance board training: prevention of traumatic injuries of the lower extremities in female soccer players? A prospective randomized intervention study. Knee Surg Sports Traumatol Arthrosc. 2000;8(6):356–363. doi: 10.1007/s001670000147. [DOI] [PubMed] [Google Scholar]
- 53.Speer KP, Spritzer CE, Bassett FH, 3rd, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992;20(4):382–389. doi: 10.1177/036354659202000403. [DOI] [PubMed] [Google Scholar]
- 54.Taylor KA, Cutcliffe HC, Queen RM, Utturkar GM, Spritzer CE, Garrett WE, DeFrate LE. In vivo measurement of ACL length and relative strain during walking. J Biomech. 2013;46(3):478–483. doi: 10.1016/j.jbiomech.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor KA, Terry ME, Utturkar GM, Spritzer CE, Queen RM, Irribarra LA, Garrett WE, DeFrate LE. Measurement of in vivo anterior cruciate ligament strain during dynamic jump landing. J Biomech. 2011;44(3):365–371. doi: 10.1016/j.jbiomech.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Utturkar GM, Irribarra LA, Taylor KA, Spritzer CE, Taylor DC, Garrett WE, Defrate LE. The effects of a valgus collapse knee position on in vivo ACL elongation. Ann Biomed Eng. 2013;41(1):123–130. doi: 10.1007/s10439-012-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viskontas DG, Giuffre BM, Duggal N, Graham D, Parker D, Coolican M. Bone bruises associated with ACL rupture: correlation with injury mechanism. Am J Sports Med. 2008;36(5):927–933. doi: 10.1177/0363546508314791. [DOI] [PubMed] [Google Scholar]
- 58.Wilson DR, Feikes JD, O'Connor JJ. Ligaments and articular contact guide passive knee flexion. J Biomech. 1998;31(12):1127–1136. doi: 10.1016/s0021-9290(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 59.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon) 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Wittstein J, Vinson E, Garrett W. Comparison Between Sexes of Bone Contusions and Meniscal Tear Patterns in Noncontact Anterior Cruciate Ligament Injuries. Am J Sports Med. 2014;42(6):1401–1407. doi: 10.1177/0363546514527415. [DOI] [PubMed] [Google Scholar]
- 61.Wu JL, Hosseini A, Kozanek M, Gadikota HR, Gill TJ, Li G. Kinematics of the anterior cruciate ligament during gait. Am J Sports Med. 2010;38(7):1475–1482. doi: 10.1177/0363546510364240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon KH, Yoo JH, Kim KI. Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. J Bone Joint Surg Am. 2011;93(16):1510–1518. doi: 10.2106/JBJS.J.01320. [DOI] [PubMed] [Google Scholar]