Abstract
The ClC-2 chloride channel is a member of the voltage-gated chloride channel family. ClC-2 is involved in various physiological processes, including fluid transport and secretion, regulation of cell volume and pH, maintaining the membrane potential of the cell, cell-to-cell communication, and tissue homeostasis. Recently, our laboratory has accumulated evidence indicating a critical role of ClC-2 in the regulation of intestinal barrier function by altering inter-epithelial tight junction composition. This review will detail the role of ClC-2 in intestinal barrier function during intestinal disorders, including experimental ischemia/reperfusion injury and dextran sodium sulfate (DSS)-induced inflammatory bowel disease. Details of pharmacological manipulation of ClC-2 via prostone agonists will also be provided in an effort to show the potential therapeutic relevance of ClC-2 regulation, particularly during intestinal barrier disruption.
Keywords: ClC-2 chloride channel, gastrointestinal diseases, intestinal barrier function, lubiprostone, tight junctions
Abbreviations
- JAM
junctional adhesion molecules
- ZO
zonula occludens
- MDCK
Madin-Darby canine kidney
- TER
transepithelial electrical resistance
- CD
Crohn’s disease
- UC
ulcerative colitis
- IL
interleukin
- TNF
tumor necrosis factor
- INF
interferon
- LIGHT
lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- CFTR
cystic fibrosis transmembrane conductance regulator
- SGLT
sodium/glucose cotransporter
- NHE
Na/H exchanger
- PGE
prostaglandin E
- IBD
inflammatory bowel disease
- DSS
dextran sulfate sodium
- CIC
chronic idiopathic constipation
- IBS
irritable bowel syndrome
- EP4
prostaglandin E receptor 4
- TNBS
2,4,6-Trinitrobenzenesulfonic acid.
Introduction
The gastrointestinal epithelium forms the body's largest interface between biological compartments, namely the gut mucosa and its lumen. Epithelial cells allow for the absorption of nutrients while providing a physical barrier to the permeation of pro-inflammatory molecules, including pathogens, toxins, and antigens, from the luminal environment into the mucosal tissues and circulatory system. The intestinal barrier is composed of epithelial cells linked by tight junctions that, together with an adherent layer of mucus, form a physical barrier that separates the luminal contents from the lamina propria and associated circulatory elements. Tight junctions have a crucial role in maintaining the intestinal barrier, and can be altered acutely or chronically by physiological and pathological factors.1-3 Our research group has revealed that the ClC-2 chloride channel has a key role in regulating barrier function under various pathophysiological conditions.4-8 Our lab has also demonstrated that the prostone ClC-2 agonist, lubiprostone, induces barrier protective and barrier recovery processes in ischemic injury and experimental colitis models.9-12 Furthermore, knockout of ClC-2 has deleterious effects on the intestinal barrier under disease conditions. However, the function of ClC-2 and mechanisms of action of lubiprostone are still controversial. This review summarizes the structural and functional elements of the tight junction, and their regulation during gastrointestinal health and disease. Additionally, we review the role of ClC-2 in regulation of tight junction barrier function, and the role of ClC-2 prostone agonists in intestinal barrier function, suggesting potential therapeutic targeting of ClC-2 in diseases that compromise the intestinal barrier.
The Intestinal Mucosal Barrier
The intestinal barrier supports nutrient and water movement while preventing microbial penetration of the intestinal tissue.13 The mucosal barrier is composed of cellular as well as extracellular components including a layer of mucin.14 Mucins are secreted by intestinal goblet cells and create a barrier, limiting the exposure of intestinal epithelial cells to physical trauma from large particles within the lumen and also preventing direct contact of microorganisms with the epithelial cell layer.14-16 The cellular components of the intestinal barrier consist of a single-cell layer, of which the largest population is columnar enterocytes responsible for absorption and secretion, but which also includes goblet cells, Paneth cells, and enteroendocrine cells. Other cells include intraepithelial lymphocytes, which are far less numerous, and are not considered to contribute to barrier function. Columnar epithelial cells are polarized with an apical (luminal) and basolateral membrane, divided by the tight junction at the apical-lateral membrane.17
There are 2 major routes for ions and macromolecules to traverse the epithelial barrier: the transcellular (transepithelial) and paracellular pathways.18 The transcellular pathway is associated with active movement of solutes through transmembrane transport proteins in the plasma membrane.19-21 The paracellular pathway is associated with passive movement of water and solutes through the space between adjacent cells. The majority of transmucosal movement of solutes is via the paracellular pathway, particularly in the small intestine, where epithelia are considered to be ‘leaky’ as compared to colonic epithelium. This ‘leakiness’ in the small intestine is thought to enhance solute absorption.22 Paracellular permeability is regulated primarily by tight junctions,23-26 although the degree to which the lateral epithelial membranes are apposed is also thought to contribute to overall barrier function.27 The paracellular junctional pathway is composed of 2 functionally distinct tight junction pathways. The first of these pathways is the pore pathway, which is high capacity and charge-selective, and allows movement of small ions and uncharged molecules. The second of these pathways is a low-capacity leak pathway that allows flux of larger ions and molecules regardless of charge.26
Role of Tight Junctions in Intestinal Barrier Function
Tight junctions are the apical-most constituents of the intercellular junctional complex which also includes adherens junctions, desmosomes, and gap junctions.28 They have 2 functions: gate (barrier) function and fence function. Barrier function refers to regulation of passive diffusion of solutes and macromolecules through the interepithelial space, whereas fence function refers to the ability of tight junctions to restrict the movement of lipids and membrane proteins between apical and basolateral membranes.1 The anatomic structure of the tight junction was first visualized by electron microscopy, which identified regions where the outer leaflets of plasma membranes from adjacent cells appeared to fuse together and obliterate the intercellular space.1 However, freeze-fracture microscopy revealed that the tight junction is an intramembranous network of anastomosing strands lying within the apical-most aspect of the lateral membrane of epithelial cells. Several studies have shown that these strands consist of multiple protein complexes of transmembrane, cytoskeletal, and signaling proteins.29 At least 4 different types of transmembrane proteins have been identified at tight junctions: occludin,30 claudins,18 tricellulin,31 and junctional adhesion molecules (JAM).32 Also present within the tight junction are the scaffold PDZ domain-expressing zonula occludens (ZO) proteins, and peripheral membrane proteins. The latter adhere only temporarily to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer.33
Occludin is highly expressed at tight junctions and appears to be involved in barrier and fence functions. However, the precise role of occludin in tight junction regulation is controversial. Occludin homozygous null mice display intact morphology of tight junctions and barrier function despite post-natal growth retardation and infertility in the male mice.34 However, there is substantial evidence supporting a functional role for occludin. Firstly, the overexpression of occludin in cultured Madin-Darby canine kidney (MDCK) cells increases the number of tight junction strands and elevates the transepithelial electrical resistance (TER), as a measure of barrier permeability to ions.35,36 Secondly, the paracellular leakage of small molecules increases in MDCK cells or Xenopus embryo cells expressing C-terminal truncated occludin mutants.35,37 Lastly, stable occludin knockdown Caco-2BBe monolayers had markedly enhanced tight junction permeability by increased leak pathway.38
The Tsukita group first identified 2 22-kDa proteins from occludin-containing chicken liver junctional fractions: Claudin-1 and -2.39,40 To date, 24 claudins have been identified. Freeze-fracture electron microscopy revealed that the claudins constitute the tight junction strands formerly noted on freeze fracture electron microscopy.40 Cell-type-specific barrier properties in tight junctions appear to be determined by the combination and ratios of multiple claudin family members.41 Claudins have 2 different functional subcategories with regard to paracellular permeability. Some claudins, called “sealing claudins,” decrease paracellular permeability; the others, called “pore forming claudins,” enhance paracellular permeability in a charge-selective fashion.42,43 The “sealing claudins” include claudins-1, -3, -5, -9, and -11. Claudin-1 is crucial for barrier function, as shown in claudin-1 null mice, which die within hours after birth because of dehydration induced by an impaired epidermal barrier.44 The “pore forming claudins” include claudins-2, -7, -10, -15, and -16. Claudin-2 forms a paracellular channel, which is selective for small cations. Overexpression of claudin-2 in MDCK cells results in a decrease in TER and enhances the permeability to select small cations.45,46
Defect of the Intestinal Mucosal Barrier in Intestinal Disorders
The importance of an intact epithelial tight junction becomes evident in intestinal disorders. For example, the tight junction complex is structurally impaired, as revealed by electron microscopy, in tissues from patients suffering from Crohn's disease (CD),47 ulcerative colitis (UC)48 and ischemic injury.49 Dysregulation of tight junction proteins contributes to barrier loss in patients with intestinal diseases. For instance, claudin-2, a pore forming tight junction protein, was significantly upregulated in CD,47 UC,50 and in patients with collagenous colitis51 by a Th2 cell cytokine (IL-13)-dependent mechanism. Occludin and select sealing claudins (claudins-1, 3, and 4) were reduced in expression or redistributed in intestinal permeability disorders, including ischemic injury,8 CD,47 and UC.52 Reorganization of occludin and sealing claudins was mediated by cytokines (tumor necrosis factor-α [TNFα], interferon-γ [IFNγ], lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells [LIGHT], and IL-1β). These pro-inflammatory cytokines promote transcription of myosin light chain kinase (MLCK), which when activated, phosphorylates myosin II, inducing caveolae-mediated endocytosis of tight junction proteins via contraction of the perijunctional actinomyosin ring (Fig. 1).53-61 However, intestinal mucosal barrier dysfunction can also be caused by epithelial damage regardless of tight junction function, including apoptosis, erosion, and ulceration.62
Figure 1.
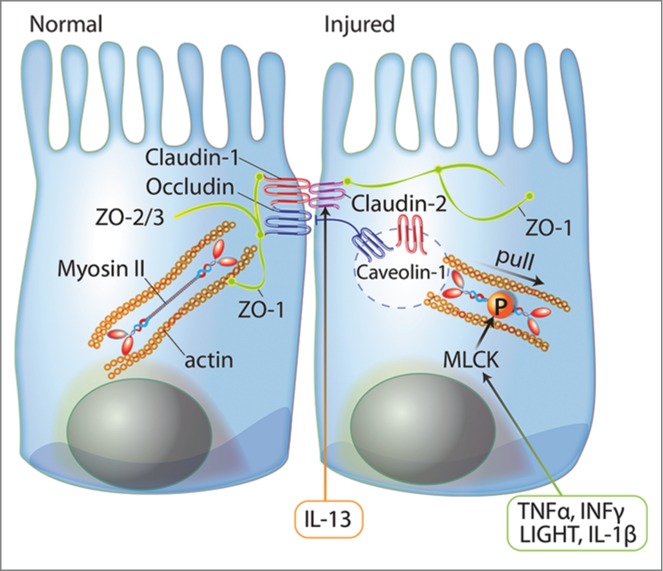
The role of tight junction proteins in signaling mechanisms affecting the intestinal mucosal barrier. Tight junctions consist of transmembrane proteins (e.g., claudins and occludin), cytoplasmic plaque proteins (e.g. ZO-1, -2, and -3), and signaling proteins (e.g., actin and myosin II). These proteins are dynamically regulated to maintain tight junction integrity. In intestinal disorders, proinflammatory cytokines (TNFα, INFγ, LIGHT, and IL-1β) stimulate MLCK expression and activity and induce caveolae-dependent endocytosis of tight junction proteins via contraction of perijunctional actinomyosin ring. Alternatively, IL-13 increases paracellular permeability via increased expression of pore forming claudin-2.
ClC-2 Chloride Channels
The ClC-0 chloride channel was originally discovered by expression cloning of the Torpedo marmorata electric organ.63 To date, 9 mammalian CLC family members have been discovered, and they can be divided into 3 homology groups: 1) ClC-1, -2, -Ka/K1, and –Kb/K2; 2) ClC-3, -4, and -5; and 3) ClC-6 and -7.64,65 The ClC-2 chloride channel has 18 helices that partially span the membrane. The two halves of the double-barreled structure form 2 identical, largely independent pores that have a binding site for chloride.66-68 ClC-2 is expressed in the plasma membranes of epithelial cells from many mammalian tissues, including the brain, pancreas, lung, intestine, kidney, liver, and heart.69 Activation of ClC-2 occurs under various physiological conditions including hypo-osmotic shock, membrane hyperpolarization, acidic extracellular pH, and cellular stress.70-77 ClC-2 is physiologically involved in several mammalian cell types, including Sertoli cells,78 sympathetic79 and hippocampal neurons,80,81 ocular rod bipolar cells,82 hepatocytes,83 erythrocytes,84 trabecular meshwork cells,85 colon epithelial cells,86 pancreatic acinar cells,87 as well as salivary acinar,88 and duct89 cells. Pathophysiologically, testicular and retinal degeneration,78 as well as leukodystrophy90 have been observed in ClC2−/− mice, suggesting a crucial role for the ClC-2 chloride channel in the control of the ionic environment in the germinal and retinal epithelia as well as central nervous system.
Role of ClC-2 in Intestinal Mucosal Homeostasis
Although ClC-2 is capable of secreting chloride in cultured intestinal cells as well as murine and pig intestinal epithelium,4,91,92 the physiological contribution of ClC-2 to chloride secretion remains unclear. There is some evidence suggesting that ClC-2 does not contribute to fluid secretion. In particular, ClC-2 is predominantly located in intestinal villus epithelia rather than in the epithelia of secretory crypts.93-95 Secondly, ClC-2−/− mice do not show any secretory functional change in gastric acid secretion96 and intestinal chloride secretion72 Finally, ClC-2-CFTR (cystic fibrosis transmembrane conductance regulator) double-knockout mice do not exhibit more severe pathogenic effects as compared to CFTR disruption alone.72 ClC-2 chloride channels are located in proximity to tight junctions on the lateral membrane of the murine villus enterocyte.91,97 Furthermore, our previous studies have shown that ClC-2 is located in close proximity to the tight junction region in porcine4 and murine.8 intestine. However, there is debate concerning the cellular and membrane location of ClC-2. The location of ClC-2 varies depending on species, tissue, methodology employed for localization, and ClC-2 antibodies used. Researchers have shown that ClC-2 may be located in the basolateral membrane, apical membrane, tight junction region, or cytosol of intestinal epithelia, although most studies indicate localization to the intercellular membranes (Table 1).4,5,7,8,91,97-102 For example, use of the anti-ClC-2 antibodies ACL−002, pAB-218, H-90, and YY9 has typically shown lateral membrane distribution in several tissues,97,99,100,102 whereas other antibodies such as ClC21-A, chicken anti-ClC-2 (in house, Dr. Blaisdell)92 and Rabbit anti-ClC-2 (in house, Dr. Bear)99 have shown apical membrane and tight junction distribution in several studies.4,8,91,98 The distribution of ClC-2 has also been shown to be species-dependent using the same antibody. For instance, the ACL−002 antibody showed basolateral distribution of ClC-2 in mouse colon, whereas ClC-2 was distributed in the cytosol in human colon.5 Thus, data on the localization of ClC-2 have to be interpreted cautiously depending upon the species being studied as well as the antibody being used. However, it is likely that different cellular fractions of ClC-2 exist within the membrane and cytosol, and when considering the membrane, a number of studies point to expression adjacent to or within the tight junction region.
Table 1.
Distribution of ClC-2 chloride channel in various species and intestinal tissues.
| Research Groups | Species | Cells/Tissues | Methods | Anti-ClC-2 Antibodies | Api | BL | TJ | Cyt | Refferences |
|---|---|---|---|---|---|---|---|---|---|
| Cuppoletti, J. | Human | T84 | Immunoconfocal microscopy | Chicken anti-ClC-2 (in house, Dr. Blaisdell) | +++ | + | Cuppoletti et al., 2004. | ||
| Melvin, J.E. | Mice | Early distal colon | Immunohistochemistry | Rabbit anti-ClC-2 (ACL−002, Alomone labs) | +++ | + | Catalan et al., 2012 | ||
| Late distal colon | + | +++ | |||||||
| Sepulveda, F.V. | Human | Caco-2 | Immunoconfocal microscopy | Transfected cells (fused with GFP) | ++ | Pena-Munzenmayer et al., 2005 | |||
| Mice | Duodenum | Immunohistochemistry | Rabbit anti-ClC-2 (ACL−002, Alomone labs) | +++ | |||||
| Colon | +++ | ||||||||
| Bear, C.E. | Human | Caco-2 | Immunoconfocal microscopy | Rabbit anti-ClC-2 (ACL−002, Alomone labs) | + | + | Mohammad-Panah et al., 2001 | ||
| Mice | Small intestine | Immunofluorescense microscopy | Rabbit anti-ClC-2 (in house, Dr. Bear) | + | |||||
| Immunogold electron microscopy | + | Gyomorey et al., 2000 | |||||||
| Fritsch, J. | Rat | Small intestine | Immunoconfocal microscopy | Rabbit anti-ClC-2 (pAb-218, in house, AgroBio) | +++ | + | Lipecka et al., 2002 | ||
| Colon enterocytes | +++ | + | |||||||
| Human | Colon enterocytes | + | +++ | ||||||
| Blikslager, A.T. | Pig | ileum | Immunogold electron microscopy | Rabbit anti-ClC-2 (ClC21-A, Alpha Diagnostic International) | + | + | Moeser et al., 2004 | ||
| Immunofluorescense microscopy | +++ | ||||||||
| Mice | Jejunum | Immunoconfocal microscopy | Rabbit anti-ClC-2 (ClC21-A, Alpha Diagnostic International) | ++ | +++ | Nighot et al., 2009 | |||
| Mice | Colon | Immunohistochemistry | Rabbit anti-ClC-2 (ACL−002, Alomone labs) | +++ | + | Nighot et al., 2013 | |||
| Human | Colon | +++ | |||||||
| Ammen, N.A. | Rat | Proximal colon | Immunoconfocal microscopy | Rabbit anti-ClC-2 (ACL−002, Alomone labs) | +++ | + | Jakab et al., 2012 | ||
| Human | Colon | Immunoconfocal microscopy | Rabbit anti-ClC-2 (ACL−002, Alomone labs) | +++ | + | ||||
| Rabbit anti-ClC-2 (H-90, Santa Cruz Biotechnology) | +++ | + | |||||||
| Rabbit anti-ClC-2 (YY9, Santa Cruz Biotechnology) | +++ | + |
Api: apical membrane, BL: basolateral membrane, TJ: tight junction, Cyt: cytosol
The expression of ClC-2 within the tight junction region presented questions regarding its role in the regulation of these structures. Recent studies have shown that other ion channels and transporters (e.g. Na+-K+-ATPase, SGLT-1, NHE3, and CFTR) are also involved in the regulation of tight junction structure and functions.3 One common theme among these studies is that transport proteins are linked indirectly to the tight junction complex by the cytoskeleton, which provides a functional link to increase paracellular permeability during active transport. Our lab has examined the role of ClC-2 chloride channels in regulating intestinal barrier function using a ClC-2−/− mouse model.6 and ClC-2 knockdown in human intestinal Caco-2BBe epithelial cells.7 For instance, functional and morphological alterations of the tight junction barrier were observed in the intestinal mucosa of ClC-2−/− mice. Our lab group has shown that the ClC-2−/− mice have tapering, rounded apical villus tips and dilated lateral paracellular spaces. The reason the lateral paracellular spaces are dilated in ClC-2−/− mice is unknown, but may relate to breakdown of alternate junctional structures such as the adherens junction (unpublished observations). Interestingly, the ClC-2−/− mouse jejunal mucosa also has increased baseline TER, reduced paracellular permeability, and altered tight junction morphology in terms of a less well-defined, poorly apposed, and narrowed tight junction structure on electron microscopy.6 The ClC-2−/− mouse colon also has increased baseline TER and reduced paracellular permeability.5,99 Additionally, the ClC-2−/− intestinal mucosa had reduced expression of phospho-myosin light chain (MLC) and displayed comparatively small increases in TER and reductions in mannitol fluxes in response to MLCK inhibition. MLCK-mediated phosphorylation of MLC is known to increase tight junction permeability via contraction of the perijunctional actinomyosin ring.6 Although the relationship between ClC-2 and phosphorylation of MLC is not clear, the reduction of phospho-MLC may be associated with increased baseline barrier function in ClC-2−/− intestinal mucosa. The increase in baseline barrier function in ClC-2−/− mice was in direct contrast to studies on damaged epithelia in mice with experimental ischemic injury and DSS-induced colitis, in which ClC-2 played a role in repair or re-sealing of tight junctions.5,8 Taken together, these findings suggest that ClC-2 reduces barrier function of the tight junction in normal epithelium, possibly by virtue of forming a pore as a Cl− channel within the tight junction, whereas ClC-2 is involved in recovery of injured epithelia, apparently by contributing to re-structuring of the tight junction. Additional studies on ClC-2 knockdown human intestinal epithelial Caco-2BBe cells provide further support for this apparent dual role of ClC-2. Specifically, cells with ClC-2 knockdown cells showed a significant delay in the development of TER and disruption of occludin distribution during early monolayer formation similar to the absence of ClC-2 causing a delay in epithelial repair in ClC-2−/− mice.7 Alternatively, fully differentiated ClC-2 knockdown Caco-2BBe cells showed increased TER and reduced paracellular permeability of FITC-dextran compared to control shRNA cells, similar to normal intestinal mucosa in ClC-2−/− mice.5 Using proteomic LC/MS/MS studies in Caco-2BBe cells we demonstrated that ClC-2 was closely associated with caveolin-1 and the small GTPase Rab5, both crucial molecules in caveolar transport (Fig. 2). The association of ClC-2 with caveolin-1 and Rab5 was confirmed by co-immunoprecipitation and confocal immunofluorescence. These results suggest that the role of ClC-2 in regulation of tight junction permeability is associated with endocytic recycling of tight junction proteins.
Figure 2.
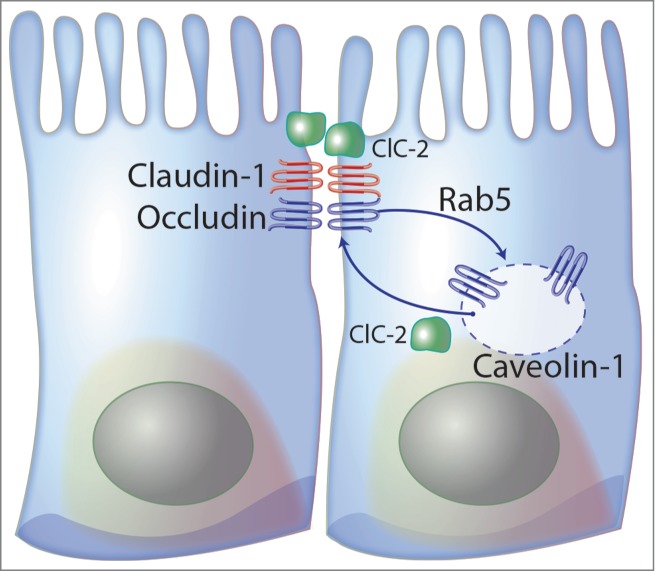
ClC-2 has a key role in re-formation of the tight junction. ClC-2 regulates endocytosis and recycling of tight junction proteins associated with caveolin-1 and the small GTPase Rab5, both crucial molecules in caveolar transport.
ClC-2 as a Key Factor in Restoring the Intestinal Barrier
Ischemia-injured intestinal disease model
We first reported that barrier function recovery in ischemia-injured porcine ileum was associated with chloride secretion via ClC-2 chloride channels. Application of prostaglandin E2 (PGE2) to ischemic-injured ileal mucosa stimulated increases in short-circuit current (Isc, an indicator of Cl− secretion) that was followed by marked increases in TER, an indicator of barrier function recovery. Ex vivo studies revealed that recovery of barrier function was initiated by ClC-2 chloride channels co-expressed with occludin and localized to tight junctions within restituting epithelium.4 The requirement for chloride secretion is difficult to understand in the context of barrier repair, but has been proven in 2 ways: removal of chloride from tissue bathing solutions prevents epithelial repair in response to ClC-2 agonists, and pre-treatment of tissues with the loop diuretic bumetanide has a similar effect as a result of blocking entry of chloride into epithelium.103 In further experiments, ClC-2−/− mice had increased paracellular permeability in jejunal mucosa following ischemic injury compared to wild type mice. Electronmicroscopic examination of recovering tissue revealed tight junction dilation in ClC-2−/− mice, whereas wild type epithelium had tightly opposed tight junctions. Using western analyses of cell fractions, occludin and claudin-1 showed increased expression in the cytosol fractions and reduced expression in the membrane fractions of ClC-2−/− mice following ischemia as compared to wild type mice. In a confocal immunofluorescence study, the tight junction protein, occludin, was co-localized with ClC-2 in the tight junction region. Occludin was internalized during post-ischemic recovery, but regained its membrane localization after 3-hours post-ischemic recovery. In ClC-2 deficient mouse intestine, however, the occludin remained diffusely present within the subapical region even after 3-hours post-ischemic recovery.8 Collectively, these findings indicated that ClC-2 plays a key role in restoration of the intestinal epithelium barrier by anchoring assembly of tight junctions following ischemic injury. The interaction between chloride secretion and the apparent ability of ClC-2 to orchestrate re-structuring of tight junctions is not fully understood. We speculate that ClC-2 undergoes a conformational change during active secretion that may initiate its ability to recruit select tight junction proteins to the apical-lateral membrane of cells.
Inflammatory bowel disease
A defect in intestinal barrier function is known to contribute to the progression of inflammatory bowel disease (IBD).47,48,50 Thus, we hypothesized that the ClC-2 chloride channel also has a critical role in the regulation of colonic barrier function under inflammatory conditions. Our recent study found that the severity of experimental colitis was significantly increased in the ClC-2−/− mice as compared with WT mice.5 This was in contrast to previous studies on unaffected ClC-2−/− mice in which knockout animals had heightened barrier function,6 suggesting that the role of ClC-2 in tight junction re-organization becomes more critical under injurious conditions. ClC-2−/− mice had a higher disease activity index, higher histological scores, and increased paracellular permeability compared with wild-type mice when treated with DSS, associated with marked disruption of tight junctions. More specifically, DSS-treated ClC-2 deficient mice had increased claudin-2 (pore-forming claudin) expression, and greater loss of occludin in the apical membrane of colonic mucosa. Thus, the absence of ClC-2 appears to make tissues susceptible to destabilization of tight junction proteins when subjected to injurious conditions. Similarly, ClC-2 knockdown in Caco-2BBe cells resulted in a significant loss of TER in the presence of DSS compared to wild type cells. In addition, the protein and mRNA expression of ClC-2 was dramatically reduced in colonic biopsies from UC patients.5 We concluded that ClC-2 plays a key role in regulation of tight junction barrier function in the development of DSS-induced murine colitis.5 Thus, we considered the possibility that ClC-2 could be a molecular target for enhanced therapeutic efforts in intestinal diseases characterized by a defect in barrier function, including CD, UC, and ischemic injury.
Pharmaceutical Targeting of ClC-2
Prostones
Lubiprostone (Amitiza®, RU-0211), a purported ClC-2 agonist, is a bicyclic fatty acid compound derived from a prostone metabolite of prostaglandin E1 (PGE1).104 Lubiprostone results in efflux of chloride into the lumen of the gastrointestinal tract and promotes intestinal fluid secretion.105,106 The drug is used as a treatment for chronic idiopathic constipation (CIC) and irritable bowel syndrome (IBS) with constipation.107-109 The originally proposed mechanism of action of lubiprostone in the intestine was that it directly activates ClC-2 chloride channels without affecting the CFTR on the apical membrane of human colonic T84 cells.98,110 However, mechanisms of lubiprostone-induced ClC-2-mediated chloride secretion remain controversial. Several recently published papers suggest that lubiprostone results in opening of the CFTR chloride channel via prostaglandin E receptor 4 (EP4) initiated cAMP signaling, without affecting ClC-2.111-113 These studies typically used CFTRihn172 as a selective CFTR inhibitor in order to differentiate the role of CFTR and ClC-2.111-113 However, a recent study has shown that CFTRihn172 also inhibits ClC-2 Cl− currents.114 Other laboratories have detected dual activation of CFTR and ClC-2 in a dose-dependent manner. However, this may relate to dose-dependent effects of lubiprostone, which when used at dosages ˜10-fold higher that those required to activate ClC-2 can stimulate CFTR Cl− currents.115 In further studies, use of the CFTR inhibitor N-(4-methylphenylsulfonyl)-N’-(4-trifluoromethylphenyl) urea (DASU-02), which does not inhibit ClC-2, had no effect on lubiprostone-stimulated ∆Isc in T84 cells. In addition, ClC-2 knockdown T84 cells did not respond to lubiprostone whereas CFTR knockdown T84 cells had significantly increased Cl− current in response to lubiprostone (Table 2).9,98,102,111,112,114-126 Collectively, these findings indicate that lubiprostone selectively stimulates ClC-2 Cl− currents in intestinal epithelial cells at low doses. However, there are several alternate mechanisms of action of lubiprostone revealed by recent studies including ion transporter trafficking, mucus release, and smooth muscle contraction.9,11,98,102,106,110-113,116,117,119-131
Table 2.
Mechanisms of actions of lubiprostone.
| Mechanisms of action | Targets of action | Signalings | Models | Results | References |
|---|---|---|---|---|---|
| Cl− secretion | ClC-2 | independent of PKA | T84 cells | ↑ Cl− currents (Isc) | Cuppoletti et al., 2004 |
| ClC-2 of CFTR expressing HEK-293 cells | |||||
| - | ischemic porcine ileum | ↑ Cl− currents (Isc) | Moeser et al., 2007 | ||
| ↑ Barrier (TER/Flux) | |||||
| - | nasal epithelium in mice | ↑ Cl− secrestion (NPD) | MacDonald et al., 2008 | ||
| - | ischemic porcine jejunum | ↑ Cl− currents (Isc) | Cuppoletti et al., 2012 | ||
| Cytokines-induced damaged T84 cells | ↑ Barrier (TER/Flux) | ||||
| independent of enteric nerve | Guinea pig small intestine and colon | ↑ Cl− currents (Isc) | Fei et al., 2009 | ||
| independent of PGs | |||||
| - | ClC-2 or CFTR KD T84 cells | ↑ Cl− currents (Isc) | Cuppoletti et al., 2014 | ||
| ClC-2 (low dose)/CFTR (high dose) | - | A6 cell | ↑ Cl− currents (Isc) | Boa et al., 2008 | |
| ↑ Voltage | |||||
| CFTR | EP4 receptor/cAMP/PKA | T84 cells | ↑ Cl− currents (Isc) | Bijvelds et al., 2009 | |
| Intestinal epithelium of WT and CF mice | |||||
| Intestinal epithelium of CF patient and controls | |||||
| cAMP/PKA | T84 cells | ↑ Cl− currents (Isc) | Ao et al., 2011 | ||
| HCO3− secretion | CFTR | EP4 receptor | rat proximal duodenal loops | ↑ HCO3− secretion | Mizumori et al., 2009 |
| smooth muscle contraction | EP1 receptor | - | rat and human stomach longitudinal mucsle | ↑ contraction | Bassil et al., 2008 |
| EP4 receptor | rat and human colon circular mucsle | ↓ neuronally mediated contraction | |||
| independent of EP receptor | - | human uterine smooth muscle cells | ↓ [Ca2+] | Cuppoletti et al., 2008 | |
| ↑ membrane potential | |||||
| EP1 receptor | - | murine small intestine (longitudinal) | No effect on contraction | Chan and Mahimo, 2013 | |
| murine small intestine (circular) | ↑ EFS-induced contraction | ||||
| murine pyloric tissue | ↑ basal tone | ||||
| independent of CFTR | - | mice small intestine | ↑ small intestine transit | De Lisle et al., 2010 | |
| Mucosal contractility | Serotonergic, EP4/PKA and EP1 | - | human and rat colon | ↑ contraction of villi proximal colonic plicae | Jakab et al., 2012 |
| Mucus seretion | ClC-2, CaCC, and CFTR | - | human airway epithelia | ↑ gland secretion | Joo et al., 2009 |
| independent of CFTR | - | CF mice | ↑ mucus accumulation in the crypts | De Lisle et al., 2010 | |
| EP4 receptor ? | - | mouse proximal-mid small intestine | ↑ mucin release | De Lisle, 2012 | |
| independent of CFTR | - | mouse proximal and distal colon | ↓ inner mucus layer thickness | Musch et al., 2013 |
PKA: Protein kinase A, PG: Prostaglandin, EP: prostaglandin E, CaCC: Calcium-activated chloride channel, CF: cystic fibrosis, EFS: electrical field stimulation, Isc:
Cobiprostone, another synthetic member of the prostone family, also serves as a ClC-2 agonist and is an investigational prostone as a potential treatment for gastrointestinal, liver and respiratory diseases. In previous research, cobiprostone dose-dependently activated ClC-2 in a protein kinase A-independent manner in vitro and protected against formation of gastric ulcers induced by NSAIDs and stress in in vivo.132,133,134
Prostones in intestinal barrier dysfunction
Previous studies showed that lubiprostone promoted repair of barrier properties in a ClC-2-dependent manner in ischemic-injured intestine.9-11 Treatment of ischemia-injured mucosa with lubiprostone increased TER and significantly reduced mucosal-to-serosal fluxes of 3H-labeled mannitol. During peak recovery of TER in ischemic tissue, occludin was localized exclusively to the tight junction in lubiprostone-treated tissues, as compared to diffuse occludin staining in untreated tissues.9 Lubiprostone also showed protective and reparative properties in T84 cells injured by exposure to IFN-γ and TNF-α. The barrier protective and reparative properties were diminished by a ClC-2 inhibitor (methadone), indicating that the barrier protective effect of lubiprostone was dependent on ClC-2.11 In recent experimental work in our laboratory, lubiprostone was shown to protect against colonic injury in DSS- and 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced murine colitis models, as well as to therapeutically enhance repair of damaged colonic mucosa.12 Application of lubiprostone in these chemically-induced IBD models ameliorated body weight loss, disease activity index, colon shortening, histological score, and intestinal permeability. Using immunofluorescence confocal microscopy analysis of the tight junction proteins, application of lubiprostone resulted in recovery of tight junction distribution of occludin, claudin-1, and claudin-2 in the apical membrane of DSS colitis mice colon. However, this drug showed very limited protective effects in the ClC-2−/− mouse subjected to DSS administration.12 These results indicated that the protective effect of the lubiprostone was attributable to reconstitution of tight junction structure and maintenance of intestinal barrier function in a ClC-2-dependent manner.135 In our previous paper, pretreatment of porcine gastric mucosa with cobiprostone protected against acid-induced injury by protecting the tight junction barrier.134 However, additional investigation is required in order to determine the detailed mechanisms of action of prostones in diseases characterized by intestinal barrier dysfunction such as ischemia/reperfusion injury and IBD.
Conclusions
The intestinal tight junction barrier is dynamically regulated by physiological and pathological factors, including growth factors, cytokines, drugs, hormones, and ion channels.1-3 Recent studies have shown that although ClC-2 is known to be involved in chloride secretion, the importance of this secretion to homeostasis is uncertain. However, we have shown that absence of ClC-2 in genetically modified mice results in altered tight junctions, dilated lateral paracellular space, and changes of the shape of the villi in small intestine. Furthermore, ClC-2 appears to play a role in development of barrier function in immature intestinal epithelial cells. Additionally, ClC-2 is intimately associated with re-structuring of tight junctions within injured epithelium. In ClC-2−/− mice, ischemic injury and chemically induced colitis models have shown a greater level of tight junction protein disruption as compared to WT mice (Fig. 3). We have also found that the ClC-2 chloride channel has a critical role in regulation of tight junctions during recovery of the tight junction barrier, and we have also shown that prostones capable of activating ClC-2 enhance barrier recovery as well as having a barrier protective role in porcine and murine models of intestinal dysfunction. We continue to have questions as to precisely how ClC-2 regulates the tight junction barrier. For instance, does ClC-2 activation orchestrate tight junction assembly, or is it mechanistically associated with fundamental mechanisms of tight junction formation? These findings may lead to the full realization of ClC-2 pharmacological agonists for the treatment of intestinal diseases associated with intestinal barrier dysfunctions.
Figure 3.
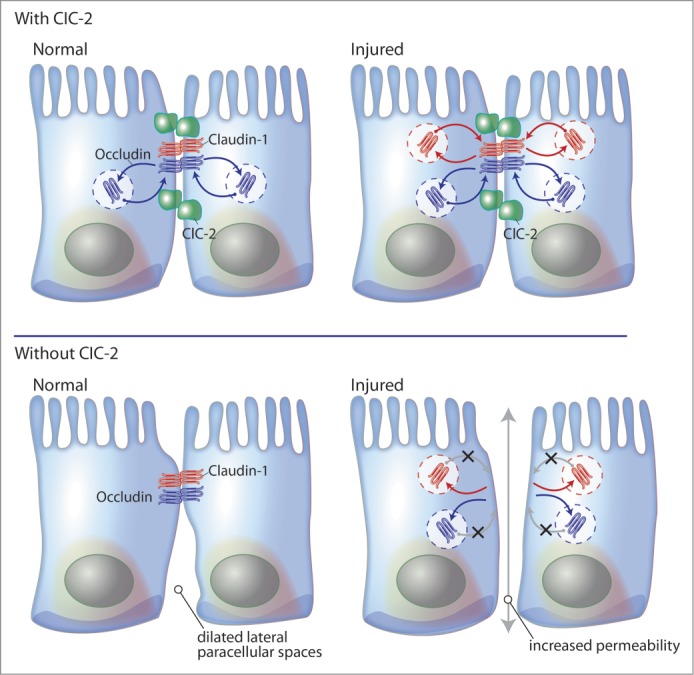
Model summary for the role of ClC-2 in repair of the intestinal epithelial barrier. In normal intestinal mucosa, ClC-2 is associated with dynamic trafficking of tight junction proteins to maintain tight junction integrity. In the absence of ClC-2, intestinal epithelial cells show altered tight junction morphology and dilated lateral paracellular spaces. In injured intestinal mucosa, ClC-2 has a critical role in reconstitution of tight junction proteins. Intestinal mucosa without ClC-2 has greater loss of barrier functions than epithelia with ClC-2 and resulting in development of digestive disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell B 2004; 36:1206-37; http://dx.doi.org/ 10.1016/j.biocel.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013; 70:631-59; PMID:22782113; http://dx.doi.org/ 10.1007/s00018-012-1070-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajasekaran SA, Beyenbach KW, Rajasekaran AK. Interactions of tight junctions with membrane channels and transporters. Biochim Biophys Acta 2008; 1778:757-69; PMID:18086552; http://dx.doi.org/ 10.1016/j.bbamem.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 4.Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterol 2004; 127:802-15; PMID:15362036; http://dx.doi.org/ 10.1053/j.gastro.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 5.Nighot P, Young K, Nighot M, Rawat M, Sung EJ, Maharshak N, Plevy SE, Ma T, Blikslager A. Chloride Channel ClC-2 is a Key Factor in the Development of DSS-induced Murine Colitis. Inflamm Bowel Dis 2013; 19(13):2867-77; PMID:24030525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nighot PK, Blikslager AT. ClC-2 regulates mucosal barrier function associated with structural changes to the villus and epithelial tight junction. Am J Physiol Gastrointest Liver Physiol 2010; 299:G449-56; PMID:20489043; http://dx.doi.org/ 10.1152/ajpgi.00520.2009 [DOI] [PubMed] [Google Scholar]
- 7.Nighot PK, Blikslager AT. Chloride channel ClC-2 modulates tight junction barrier function via intracellular trafficking of occludin. Am J Physiol Cell Physiol 2012; 302:C178-87; PMID:21956164; http://dx.doi.org/ 10.1152/ajpcell.00072.2011 [DOI] [PubMed] [Google Scholar]
- 8.Nighot PK, Moeser AJ, Ryan KA, Ghashghaei T, Blikslager AT. ClC-2 is required for rapid restoration of epithelial tight junctions in ischemic-injured murine jejunum. Exp Cell Res 2009; 315:110-8; PMID:18976652; http://dx.doi.org/ 10.1016/j.yexcr.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 9.Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointestinal Liver Physiol 2007; 292:G647-56; PMID:17053162; http://dx.doi.org/ 10.1152/ajpgi.00183.2006 [DOI] [PubMed] [Google Scholar]
- 10.Moeser AJ, Nighot PK, Roerig B, Ueno R, Blikslager AT. Comparison of the chloride channel activator lubiprostone and the oral laxative Polyethylene Glycol 3350 on mucosal barrier repair in ischemic-injured porcine intestine. World J Gastroenterol 2008; 14:6012-7; http://dx.doi.org/ 10.3748/wjg.14.6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuppoletti J, Blikslager AT, Chakrabarti J, Nighot PK, Malinowska DH. Contrasting effects of linaclotide and lubiprostone on restitution of epithelial cell barrier properties and cellular homeostasis after exposure to cell stressors. BMC Pharmacol 2012; 12:3; PMID:22553939; http://dx.doi.org/ 10.1186/1471-2210-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Y, Pridgen TA, Blikslager AT. Pharmaceutical Activation or Genetic Absence of ClC-2 Alters Tight Junctions During Experimental Colitis. Inflamm Bowel Dis 2015; PMID:26332307 [DOI] [PubMed] [Google Scholar]
- 13.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Ann Rev Pathol 2010; 5:119-44; PMID:20078218; http://dx.doi.org/ 10.1146/annurev.pathol.4.110807.092135 [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Digest Dis 2009; 27:443-9; PMID:19897958; http://dx.doi.org/ 10.1159/000233282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. P Natl Acad Sci USA 2008; 105:15064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9:799-809; PMID:19855405; http://dx.doi.org/ 10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- 17.Groschwitz KR, Hogan SP. Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allergy Clin Immun 2009; 124:3-20; PMID:19560575; http://dx.doi.org/ 10.1016/j.jaci.2009.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Bio 2001; 2:285-93; http://dx.doi.org/ 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- 19.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: Mechanisms and implications for disease. Physiol Rev 2002; 82:245-89; PMID:11773614; http://dx.doi.org/ 10.1152/physrev.00026.2001 [DOI] [PubMed] [Google Scholar]
- 20.Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev 1997; 77:257-302; PMID:9016304 [DOI] [PubMed] [Google Scholar]
- 21.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008; 88:249-86; PMID:18195088; http://dx.doi.org/ 10.1152/physrev.00018.2006 [DOI] [PubMed] [Google Scholar]
- 22.Fasano A, Troncone R, Branski D. Frontiers in celiac disease Basel; New York: Karger, 2008 [Google Scholar]
- 23.Goodenou Da, Revel JP. A Fine Structural Analysis of Intercellular Junctions in Mouse Liver. J Cell Biol 1970; 45:272-&; PMID:4105112; http://dx.doi.org/ 10.1083/jcb.45.2.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machen TE, Wooding FBP, Erlij D. Permeable Junctional Complexes - Movement of Lanthanum across Rabbit Gallbladder and Intestine. J Cell Biol 1972; 54:302-12; PMID:5040861; http://dx.doi.org/ 10.1083/jcb.54.2.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond JM, Bossert WH. Standing-Gradient Osmotic Flow - a Mechanism for Coupling of Water and Solute Transport in Epithelia. J Gen Physiol 1967; 50:2061-83; PMID:6066064; http://dx.doi.org/ 10.1085/jgp.50.8.2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 2011; 73:283-309; PMID:20936941; http://dx.doi.org/ 10.1146/annurev-physiol-012110-142150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gawenis LR, Boyle KT, Palmer BA, Walker NM, Clarke LL. Lateral intercellular space volume as a determinant of CFTR-mediated anion secretion across small intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 2004; 286:G1015-23; PMID:14764448; http://dx.doi.org/ 10.1152/ajpgi.00468.2003 [DOI] [PubMed] [Google Scholar]
- 28.Farquhar MG, Palade GE. Junctional Complexes in Various Epithelia. J Cell Biol 1963; 17:375-412; PMID:13944428; http://dx.doi.org/ 10.1083/jcb.17.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 2006; 22:207-35; PMID:16771626; http://dx.doi.org/ 10.1146/annurev.cellbio.22.010305.104219 [DOI] [PubMed] [Google Scholar]
- 30.La Staeheli.. Further Observations on Fine-Structure of Freeze-Cleaved Tight Junctions. J Cell Science 1973; 13:763-86; PMID:4203962 [DOI] [PubMed] [Google Scholar]
- 31.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 2005; 171:939-45; PMID:16365161; http://dx.doi.org/ 10.1083/jcb.200510043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al.. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998; 142:117-27; PMID:9660867; http://dx.doi.org/ 10.1083/jcb.142.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima T, Murata M, Yamamoto T, Lan M, Imamura M, Son S, Takano K, Yamaguchi H, Ito T, Tanaka S, et al.. Tight junction proteins and signal transduction pathways in hepatocytes. Histol Histopathol 2009; 24:1463-72; PMID:19760595 [DOI] [PubMed] [Google Scholar]
- 34.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11:4131-42; PMID:11102513; http://dx.doi.org/ 10.1091/mbc.11.12.4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. Mol Biol Cell 1996; 7:3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci 1996; 109:2287-98; PMID:8886979 [DOI] [PubMed] [Google Scholar]
- 37.Chen YH, Merzdorf C, Paul DL, Goodenough DA. C-terminus of occludin is required for tight junction barrier function in early Xenopus embryos. Mol Biol Cell 1997; 8:1182; http://dx.doi.org/ 10.1091/mbc.8.7.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, et al.. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell 2013; 24:3056-68; PMID:23924897; http://dx.doi.org/ 10.1091/mbc.E12-09-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141:1539-50; PMID:9647647; http://dx.doi.org/ 10.1083/jcb.141.7.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 2001; 153:263-72; PMID:11309408; http://dx.doi.org/ 10.1083/jcb.153.2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 1999; 147:891-903; PMID:10562289; http://dx.doi.org/ 10.1083/jcb.147.4.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanItallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Science 1997; 110:1113-21; PMID:9175707 [DOI] [PubMed] [Google Scholar]
- 43.Overgaard CE, Daugherty BL, Mitchell LA, Koval M. Claudins: control of barrier function and regulation in response to oxidant stress. Antioxid Redox Signal 2011; 15:1179-93; PMID:21275791; http://dx.doi.org/ 10.1089/ars.2011.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weng XH, Beyenbach KW, Quaroni A. Cultured monolayers of the dog jejunum with the structural and functional properties resembling the normal epithelium. Am J Physiol-Gastr L 2005; 288:G705-G17 [DOI] [PubMed] [Google Scholar]
- 45.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of Zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 2001; 153:263-72; PMID:11309408; http://dx.doi.org/ 10.1083/jcb.153.2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol 2006; 16:181-8; PMID:16537104; http://dx.doi.org/ 10.1016/j.tcb.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 47.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 2007; 56:61-72; PMID:16822808; http://dx.doi.org/ 10.1136/gut.2006.094375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, Panda SK, Gupta SD, Makharia GK. Comparative tight junction protein expressions in colonic Crohn's disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch 2012; 460:261-70; PMID:22297703; http://dx.doi.org/ 10.1007/s00428-012-1195-1 [DOI] [PubMed] [Google Scholar]
- 49.Little D, Dean RA, Young KM, McKane SA, Martin LD, Jones SL, Blikslager AT. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am J Physiol-Gastr L 2003; 284:G46-G56 [DOI] [PubMed] [Google Scholar]
- 50.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, et al.. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol 2005; 129:550-64; PMID:16083712; http://dx.doi.org/ 10.1016/j.gastro.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 51.Burgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterol 2002; 123:433-43; PMID:12145796; http://dx.doi.org/ 10.1053/gast.2002.34784 [DOI] [PubMed] [Google Scholar]
- 52.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterol 1999; 116:301-9; PMID:9922310; http://dx.doi.org/ 10.1016/S0016-5085(99)70126-5 [DOI] [PubMed] [Google Scholar]
- 53.Wang FJ, Graham WV, Wang YM, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 2005; 166:409-19; PMID:15681825; http://dx.doi.org/ 10.1016/S0002-9440(10)62264-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham WV, Wang FJ, Clayburgh DR, Cheng JX, Yoon B, Wang YM, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events - Characterization of the human long myosin light chain kinase promoter. J Biol Chem 2006; 281:26205-15; PMID:16835238; http://dx.doi.org/ 10.1074/jbc.M602164200 [DOI] [PubMed] [Google Scholar]
- 55.Ma TY, Boivin MA, Ye DM, Pedram A, Said HM. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol-Gastr L 2005; 288:G422-G30 [DOI] [PubMed] [Google Scholar]
- 56.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. (vol 123, pg 163, 2002). Gastroenterology 2002; 123:1412; http://dx.doi.org/ 10.1053/gast.2002.34235 [DOI] [PubMed] [Google Scholar]
- 57.Clayburgh DR, Barrett TA, Tang YM, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest 2005; 115:2702-15; PMID:16184195; http://dx.doi.org/ 10.1172/JCI24970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 2006; 86:191-201; PMID:16402035; http://dx.doi.org/ 10.1038/labinvest.3700373 [DOI] [PubMed] [Google Scholar]
- 59.Schwarz BT, Wang FJ, Shen L, Clayburgh DR, Su LP, Wang YM, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterol 2007; 132:2383-94; PMID:17570213; http://dx.doi.org/ 10.1053/j.gastro.2007.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Sadi R, Ma TY. Myosin light chain kinase (MLCK) mediates the IL-1 β-incluced increase in intestinal epithelial Tight Junction permeability. Gastroenterol 2007; 132:A8-A; http://dx.doi.org/ 10.1053/j.gastro.2006.11.038 [DOI] [Google Scholar]
- 61.Al-Sadi RM, Ma TY. IL-1 β causes an increase in intestinal epithelial tight junction permeability. J Immunol 2007; 178:4641-9; http://dx.doi.org/ 10.4049/jimmunol.178.7.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: Characterization and quantification by electrophysiological imaging. Gastroenterol 2001; 121:1320-8; PMID:11729111; http://dx.doi.org/ 10.1053/gast.2001.29694 [DOI] [PubMed] [Google Scholar]
- 63.Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature 1990; 348:510-4; PMID:2174129; http://dx.doi.org/ 10.1038/348510a0 [DOI] [PubMed] [Google Scholar]
- 64.Stauber T, Weinert S, Jentsch TJ. Cell biology and physiology of CLC chloride channels and transporters. Comprehensive Physiol 2012; 2:1701-44; PMID:23723021 [DOI] [PubMed] [Google Scholar]
- 65.Strange K. Of mice and worms: novel insights into ClC-2 anion channel physiology. News Physiol Sci 2002; 17:11-6; PMID:11821530 [DOI] [PubMed] [Google Scholar]
- 66.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 2002; 415:287-94; PMID:11796999; http://dx.doi.org/ 10.1038/415287a [DOI] [PubMed] [Google Scholar]
- 67.Ramjeesingh M, Li C, She YM, Bear CE. Evaluation of the membrane-spanning domain of ClC-2. Biochem J 2006; 396:449-60; PMID:16526942; http://dx.doi.org/ 10.1042/BJ20060043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramjeesingh M, Li C, Huan LJ, Garami E, Wang Y, Bear CE. Quaternary structure of the chloride channel ClC-2. Biochem 2000; 39:13838-47; PMID:11076524; http://dx.doi.org/ 10.1021/bi001282i [DOI] [PubMed] [Google Scholar]
- 69.Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 1992; 356:57-60; PMID:1311421; http://dx.doi.org/ 10.1038/356057a0 [DOI] [PubMed] [Google Scholar]
- 70.Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J 1997; 16:1582-92; PMID:9130703; http://dx.doi.org/ 10.1093/emboj/16.7.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grunder S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature 1992; 360:759-62; PMID:1334533; http://dx.doi.org/ 10.1038/360759a0 [DOI] [PubMed] [Google Scholar]
- 72.Zdebik AA, Cuffe JE, Bertog M, Korbmacher C, Jentsch TJ. Additional disruption of the ClC-2 Cl(-) channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J Biol Chem 2004; 279:22276-83; PMID:15007059; http://dx.doi.org/ 10.1074/jbc.M309899200 [DOI] [PubMed] [Google Scholar]
- 73.Hinzpeter A, Lipecka J, Brouillard F, Baudoin-Legros M, Dadlez M, Edelman A, Fritsch J. Association between Hsp90 and the ClC-2 chloride channel upregulates channel function. Am J Physiol-Cell Ph 2006; 290:C45-C56; http://dx.doi.org/ 10.1152/ajpcell.00209.2005 [DOI] [PubMed] [Google Scholar]
- 74.Roman RM, Smith RL, Feranchak AP, Clayton GH, Doctor RB, Fitz JG. ClC-2 chloride channels contribute to HTC cell volume homeostasis. Am J Physiol-Gastr L 2001; 280:G344-G53 [DOI] [PubMed] [Google Scholar]
- 75.Bali M, Lipecka J, Edelman A, Fritsch J. Regulation of ClC-2 chloride channels in T84 cells by TGF-α. Am J Physiol Cell Physiol 2001; 280:C1588-98; PMID:11350754 [DOI] [PubMed] [Google Scholar]
- 76.Tewari KP, Malinowska DH, Sherry AM, Cuppoletti J. PKA and arachidonic acid activation of human recombinant ClC-2 chloride channels. Am J Physiol-Cell Ph 2000; 279:C40-C50 [DOI] [PubMed] [Google Scholar]
- 77.Ahmed N, Ramjeesingh M, Wong S, Varga A, Garami E, Bear CE. Chloride channel activity of ClC-2 is modified by the actin cytoskeleton. Biochem J 2000; 352 Pt 3:789-94; PMID:11104687; http://dx.doi.org/ 10.1042/bj3520789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bosl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2Cl(-) channel disruption. Embo J 2001; 20:1289-99; PMID:11250895; http://dx.doi.org/ 10.1093/emboj/20.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clark S, Jordt SE, Jentsch TJ, Mathie A. Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons. J Physiol-London 1998; 506:665-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rinke I, Artmann J, Stein V. ClC-2 Voltage-Gated Channels Constitute Part of the Background Conductance and Assist Chloride Extrusion. J Neurosci 2010; 30:4776-86; PMID:20357128; http://dx.doi.org/ 10.1523/JNEUROSCI.6299-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Staley K. The Role of an Inwardly Rectifying Chloride Conductance in Postsynaptic Inhibition. J Neurophysiol 1994; 72:273-84; PMID:7965011 [DOI] [PubMed] [Google Scholar]
- 82.Enz R, Ross BJ, Cutting GR. Expression of the voltage-gated chloride channel CIC-2 in rod bipolar cells of the rat retina. J Neurosci 1999; 19:9841-7; PMID:10559393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lan WZ, Abbas H, Lam HD, Lemay AM, Hill CE. Contribution of a time-dependent and hyperpolarization-activated chloride conductance to currents of resting and hypotonically shocked rat hepatocytes. Am J Physiol-Gastr L 2005; 288:G221-G9 [DOI] [PubMed] [Google Scholar]
- 84.Bouyer G, Egee S, Thomas SLY. Toward a unifying model of malaria-induced channel activity. P Natl Acad Sci USA 2007; 104:11044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Comes N, Borras T, Morales M, Gual A, Gasull X. Identification and electrophysiological characterization of CLC-2 chloride channels in trabecular meshwork cells: Modulation by pH and cell swelling. Exper Eye Res 2006; 83:877-89 [DOI] [PubMed] [Google Scholar]
- 86.Inagaki A, Yamaguchi S, Takahashi-Iwanaga H, Iwanaga T, Ishikawa T. Functional Characterization of a ClC-2-Like Cl− Conductance in Surface Epithelial Cells of Rat Rectal Colon. J Membrane Biol 2010; 235:27-41; http://dx.doi.org/ 10.1007/s00232-010-9253-6 [DOI] [PubMed] [Google Scholar]
- 87.Carew MA, Thorn P. Identification of CIC-2-like chloride currents in pig pancreatic acinar cells. Pflug Arch Eur J Phy 1996; 433:84-90; http://dx.doi.org/ 10.1007/s004240050252 [DOI] [PubMed] [Google Scholar]
- 88.Nehrke K, Arreola J, Nguyen HV, Pilato J, Richardson L, Okunade G, Baggs R, Shull GE, Melvin JE. Loss of hyperpolarization-activated Cl− current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem 2002; 277:23604-11; PMID:11976342; http://dx.doi.org/ 10.1074/jbc.M202900200 [DOI] [PubMed] [Google Scholar]
- 89.Romanenko VG, Nakamoto T, Catalan MA, Gonzalez-Begne M, Schwartz GJ, Jaramillo Y, Sepúlveda FV, Figueroa CD, Melvin JE. Clcn2 encodes the hyperpolarization-activated chloride channel in the ducts of mouse salivary glands. Am J Physiol-Gastr L 2008; 295:G1058-G67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hubner CA, Jentsch TJ. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci 2007; 27:6581-9; PMID:17567819; http://dx.doi.org/ 10.1523/JNEUROSCI.0338-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gyomorey K, Yeger H, Ackerley C, Garami E, Bear CE. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol 2000; 279:C1787-94; PMID:11078693 [DOI] [PubMed] [Google Scholar]
- 92.Fritsch J, Edelman A. Modulation of the hyperpolarization-activated Cl− current in human intestinal T84 epithelial cells by phosphorylation. J Physiol 1996; 490 ( Pt 1):115-28; PMID:8745282; http://dx.doi.org/ 10.1113/jphysiol.1996.sp021130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Catalan M, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterol 2004; 126:1104-14; PMID:15057749; http://dx.doi.org/ 10.1053/j.gastro.2004.01.010 [DOI] [PubMed] [Google Scholar]
- 94.Catalan M, Cornejo I, Figueroa CD, Niemeyer MI, Sepulveda FV, Cid LP. ClC-2 in guinea pig colon: mRNA, immunolabeling, and functional evidence for surface epithelium localization. Am J Physiol Gastrointest Liver Physiol 2002; 283:G1004-13; PMID:12223361; http://dx.doi.org/ 10.1152/ajpgi.00158.2002 [DOI] [PubMed] [Google Scholar]
- 95.Inagaki A, Yamaguchi S, Takahashi-Iwanaga H, Iwanaga T, Ishikawa T. Functional characterization of a ClC-2-like Cl(-) conductance in surface epithelial cells of rat rectal colon. J Membr Biol 2010; 235:27-41; PMID:20411246; http://dx.doi.org/ 10.1007/s00232-010-9253-6 [DOI] [PubMed] [Google Scholar]
- 96.Bosl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl(-) channel disruption. EMBO J 2001; 20:1289-99; PMID:11250895; http://dx.doi.org/ 10.1093/emboj/20.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 2002; 282:C805-16; PMID:11880269; http://dx.doi.org/ 10.1152/ajpcell.00291.2001 [DOI] [PubMed] [Google Scholar]
- 98.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 2004; 287:C1173-83; PMID:15213059; http://dx.doi.org/ 10.1152/ajpcell.00528.2003 [DOI] [PubMed] [Google Scholar]
- 99.Catalan MA, Flores CA, Gonzalez-Begne M, Zhang Y, Sepulveda FV, Melvin JE. Severe defects in absorptive ion transport in distal colons of mice that lack ClC-2 channels. Gastroenterology 2012; 142:346-54; PMID:22079595; http://dx.doi.org/ 10.1053/j.gastro.2011.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepúlveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci 2005; 118:4243-52; PMID:16155254; http://dx.doi.org/ 10.1242/jcs.02525 [DOI] [PubMed] [Google Scholar]
- 101.Mohammad-Panah R, Gyomorey K, Rommens J, Choudhury M, Li C, Wang Y, Bear CE. ClC-2 contributes to native chloride secretion by a human intestinal cell line, Caco-2. J Biol Chem 2001; 276:8306-13; PMID:11096079; http://dx.doi.org/ 10.1074/jbc.M006764200 [DOI] [PubMed] [Google Scholar]
- 102.Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Digest Dis Sci 2012; 57:2826-45; PMID:22923315; http://dx.doi.org/ 10.1007/s10620-012-2352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am J Physiol 1999; 276:G28-36; PMID:9886975 [DOI] [PubMed] [Google Scholar]
- 104.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol 2007; 41:345-51; PMID:17413599; http://dx.doi.org/ 10.1097/01.mcg.0000225665.68920.df [DOI] [PubMed] [Google Scholar]
- 105.Ueno R, Osama H, Habe T, Engelke K, Patchen M. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels. Gastroenterol 2004; 126:A298-A [Google Scholar]
- 106.Fei G, Raehal K, Liu S, Qu MH, Sun X, Wang GD, Xia Y, Schmid CL, Bohn LM, Wood JD. Lubiprostone reverses the inhibitory action of morphine on intestinal secretion in guinea pig and mouse. J Pharmacol Exp Therapeutics 2010; 334:333-40; PMID:20406855; http://dx.doi.org/ 10.1124/jpet.110.166116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and Safety of Lubiprostone in Patients with Chronic Constipation. Digest Dis Sci 2010; 55:1090-7; PMID:20012484; http://dx.doi.org/ 10.1007/s10620-009-1068-x [DOI] [PubMed] [Google Scholar]
- 108.Drossman DA, Chey WD, Johanson JF, Fass R, Scott C, Panas R, Ueno R. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome - results of two randomized, placebo-controlled studies. Alimentary Pharmacol Therapeutics 2009; 29:329-41; PMID:19006537; http://dx.doi.org/ 10.1111/j.1365-2036.2008.03881.x [DOI] [PubMed] [Google Scholar]
- 109.Schey R, Rao SSC. Lubiprostone for the Treatment of Adults with Constipation and Irritable Bowel Syndrome. Digest Dis Sci 2011; 56:1619-25; PMID:21523369; http://dx.doi.org/ 10.1007/s10620-011-1702-2 [DOI] [PubMed] [Google Scholar]
- 110.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 2008; 295:G234-51; PMID:18511742; http://dx.doi.org/ 10.1152/ajpgi.00366.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bijvelds MJ, Bot AG, Escher JC, De Jonge HR. Activation of intestinal Cl− secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterol 2009; 137:976-85; PMID:19454284; http://dx.doi.org/ 10.1053/j.gastro.2009.05.037 [DOI] [PubMed] [Google Scholar]
- 112.Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Digest Dis Sci 2011; 56:339-51; PMID:21140215; http://dx.doi.org/ 10.1007/s10620-010-1495-8 [DOI] [PubMed] [Google Scholar]
- 113.Norimatsu Y, Moran AR, MacDonald KD. Lubiprostone activates CFTR, but not ClC-2, via the prostaglandin receptor (EP(4)). Biochem Biophys Res Commun 2012; 426:374-9; PMID:22960173; http://dx.doi.org/ 10.1016/j.bbrc.2012.08.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cuppoletti J, Chakrabarti J, Tewari KP, Malinowska DH. Differentiation between human ClC-2 and CFTR Cl− channels with pharmacological agents. Am J Physiol Cell Physiol 2014; 307:C479-92; PMID:25009109; http://dx.doi.org/ 10.1152/ajpcell.00077.2014 [DOI] [PubMed] [Google Scholar]
- 115.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol-Gastr L 2008; 295:G234-G51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 2008; 154:126-35; PMID:18332851; http://dx.doi.org/ 10.1038/bjp.2008.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan WW, Mashimo H. Lubiprostone Increases Small Intestinal Smooth Muscle Contractions Through a Prostaglandin E Receptor 1 (EP1)-mediated Pathway. J Neurogastroenterol Motility 2013; 19:312-8; PMID:23875097; http://dx.doi.org/ 10.5056/jnm.2013.19.3.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cuppoletti J, Chakrabarti J, Tewari K, Malinowska DH. Methadone but not Morphine Inhibits Lubiprostone-Stimulated Cl(-) Currents in T84 Intestinal Cells and Recombinant Human ClC-2, but not CFTR Cl(-) Currents. Cell Biochem Biophys 2013; 66(1):53-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cuppoletti J, Malinowska DH, Chakrabarti J, Ueno R. Effects of lubiprostone on human uterine smooth muscle cells. Prostaglandins Other Lipid Mediat 2008; 86:56-60; PMID:18440264; http://dx.doi.org/ 10.1016/j.prostaglandins.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 120.De Lisle RC. Lubiprostone stimulates small intestinal mucin release. BMC Gastroenterol 2012; 12:156; PMID:23130661; http://dx.doi.org/ 10.1186/1471-230X-12-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Lisle RC, Mueller R, Roach E. Lubiprostone ameliorates the cystic fibrosis mouse intestinal phenotype. BMC Gastroenterol 2010; 10:107; PMID:20843337; http://dx.doi.org/ 10.1186/1471-230X-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fei G, Wang YZ, Liu S, Hu HZ, Wang GD, Qu MH, et al.. Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol 2009; 296:G823-32; PMID:19179625; http://dx.doi.org/ 10.1152/ajpgi.90447.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joo NS, Wine JJ, Cuthbert AW. Lubiprostone stimulates secretion from tracheal submucosal glands of sheep, pigs, and humans. Am J Physiol Lung Cell Mol Physiol 2009; 296:L811-24; PMID:19233902; http://dx.doi.org/ 10.1152/ajplung.90636.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacDonald KD, McKenzie KR, Henderson MJ, Hawkins CE, Vij N, Zeitlin PL. Lubiprostone activates non-CFTR-dependent respiratory epithelial chloride secretion in cystic fibrosis mice. Am J Physiol Lung Cell Mol Physiol 2008; 295:L933-40; PMID:18805957; http://dx.doi.org/ 10.1152/ajplung.90221.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mizumori M, Akiba Y, Kaunitz JD. Lubiprostone stimulates duodenal bicarbonate secretion in rats. Digest Dis Sci 2009; 54:2063-9; PMID:19657734; http://dx.doi.org/ 10.1007/s10620-009-0907-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Musch MW, Wang Y, Claud EC, Chang EB. Lubiprostone decreases mouse colonic inner mucus layer thickness and alters intestinal microbiota. Digest Dis Sci 2013; 58:668-77; PMID:23329012; http://dx.doi.org/ 10.1007/s10620-012-2509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sweetser S, Busciglio IA, Camilleri M, Bharucha AE, Szarka LA, Papathanasopoulos A, Burton DD, Eckert DJ, Zinsmeister AR. Effect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2009; 296:G295-301; PMID:19033530; http://dx.doi.org/ 10.1152/ajpgi.90558.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun X, Wang X, Wang GD, Xia Y, Liu S, Qu M, Needleman BJ, Mikami DJ, Melvin WS, Bohn LM, et al.. Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in human small intestine. Digest Dis Sci 2011; 56:330-8; PMID:21181441; http://dx.doi.org/ 10.1007/s10620-010-1515-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.MacVinish LJ, Cope G, Ropenga A, Cuthbert AW. Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol 2007; 150:1055-65; PMID:17339840; http://dx.doi.org/ 10.1038/sj.bjp.0707175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cuthbert AW. Lubiprostone targets prostanoid EP(4) receptors in ovine airways. Br J Pharmacol 2011; 162:508-20; PMID:20883477; http://dx.doi.org/ 10.1111/j.1476-5381.2010.01058.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schiffhauer ES, Vij N, Kovbasnjuk O, Kang PW, Walker D, Lee S, Zeitlin P. Dual activation of CFTR and CLCN2 by lubiprostone in murine nasal epithelia. Am J Physiol Lung Cell Mol Physiol 2013; 304:L324-31; PMID:23316067; http://dx.doi.org/ 10.1152/ajplung.00277.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santis K. Cobiprostone Preclinical Data from NSAID-Induced Gastric Mucosal Injury Models Reported at Digestive Disease Week 2012. Sucampo Pharmaceuticals, Inc.: Sucampo Pharmaceuticals, Inc., 2012 [Google Scholar]
- 133.Santis K. Phase 2 Cobiprostone Data Presented at DDW. Sucampo Pharmaceuticals, Inc.: Sucampo Pharmaceuticals, Inc., 2010 [Google Scholar]
- 134.Nighot M, Moeser A, Ueno R, Blikslager A. Gastro protective properties of the novel prostone SPI-8811 against acid-injured porcine mucosa. World J Gastroenterol 2012; 18:4684-92; http://dx.doi.org/ 10.3748/wjg.v18.i34.4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jin Y, Pridgen TA, Blikslager AT. Tu2028 Lubiprostone reduces murine colitis principally in a ClC-2-dependent manner. Gastroenterol 2014; 146:S-901 [Google Scholar]


