Abstract
Three small molecules were identified in high throughput screens that 1) block renal inward rectifier potassium (Kir) channels of Aedes aegypti expressed in HEK cells and Xenopus oocytes, 2) inhibit the secretion of KCl but not NaCl in isolated Malpighian tubules, and after injection into the hemolymph, 3) inhibit KCl excretion in vivo, and 4) render mosquitoes flightless or dead within 24h. Some mosquitoes had swollen abdomens at death consistent with renal failure. VU625, the most potent and promising small molecule for development as mosquitocide, inhibits AeKir1-mediated currents with an IC50 less than 100 nM. It is highly selective for AeKir1 over mammalian Kir channels, and it affects only 3 of 68 mammalian membrane proteins. These results document 1) renal failure as a new mode-of-action for mosquitocide development, 2) renal Kir channels as molecular target for inducing renal failure, and 3) the promise of the discovery and development of new species-specific insecticides.
Keywords: drug discovery, high throughput screen, insecticide, Kir channels, Malpighian tubules, mosquitocide, renal failure, small molecules
Introduction
Most insecticides currently in use are derived from plants. Carbamates insecticides mimic the effects of physostigmine from the Calabar bean of Africa. They kill by inhibiting cholinesterase, the enzyme that inactivates acetylcholine at synaptic clefts.1 Pyrethroids, the most widely used insecticides in the United States, are derived from pyrethrin in the flower of chrysanthemums. They delay the closing of voltage-gated Na+ channels in nerve cells, leading to the loss of motor coordination, paralysis and death.2 Neonicotinoids mimic the effect of nicotine from the nightshade family of plants that includes the tobacco plant. Nicotine is the natural ligand of nicotinic acetylcholine receptors at synaptic and neuromuscular junctions of animals. Accordingly, neonicotinoids kill insects by overstimulating acetylcholine receptors.3,4
What the aforementioned compounds have in common are 1) the nervous system as target of insecticides, and 2) toxic effects on virtually every insect. Other insecticides target mechanisms fundamental to the life of all insect cells. Ryanodines, derived from the South American plant Ryanio speciosa, kill insects by depleting intracellular Ca2+ stores, thereby causing paralysis of muscles including the heart.5 Rotenone, derived from the jicama vine plant or the roots of the plant family Fabaceae, kills by interfering in the electron transport chain of mitochondria.6
Though the benefit of the above insecticides to agriculture, public health, animal health and the economy is undisputed, they also possess undesirable effects such as the killing of beneficial insects (e.g. honey bees) and in some cases vertebrate animals, and polluting the environment. Though some insecticides have been banned, the use of other insecticides has increased as a result. Moreover, new insecticides for public health purposes have not been developed in the past 40 years,7 and the long term reliance on a few insecticides has selected for insects that are insensitive to them.8 In parallel, global warming enhances the northward migration of tropical diseases. For these reasons, the need of new insecticides is urgent.
New insecticides should aim at greater selectivity than those currently in use. It should now be possible to develop a mosquitocide that specifically kills mosquito vectors of malaria and other diseases while leaving other species unharmed. Toward this end we are attempting to find new agents that kill the mosquito Aedes aegypti, the vector of yellow, dengue, and chikungunya fevers. Instead of interfering in neural functions we have focused on agents that disrupt renal functions. So far, we have identified several small molecules that disrupt renal functions and kills mosquitoes by disabling epithelial inward rectifier potassium (Kir) channels expressed in Malpighian (renal) tubules. As will be discussed below, one of these small molecules is 80 times more selective for mosquito than mammalian Kir channels.
We begin this review with the central role of K+ in renal functions of the yellow fever mosquito, we then describe the K+ channels we have identified in renal Malpighian tubules of Aedes aeypti, and recapitulate how 3 small molecules, VU573, VU590 and VU625 1) disable K+ channels expressed in heterologous expression systems, 2) inhibit specifically K+ secretion in isolated Malpighian tubules, and 3) cause renal failure and death when injected into the hemolymph of mosquitoes. Thus we have proven the disruption of salt and water balance in insects as a promising new strategy for controlling insect populations. Our next ambition is to fine tune existing small molecules (or identify new ones) that kill disease vectors but spare beneficial insects.
Transepithelial K+ secretion
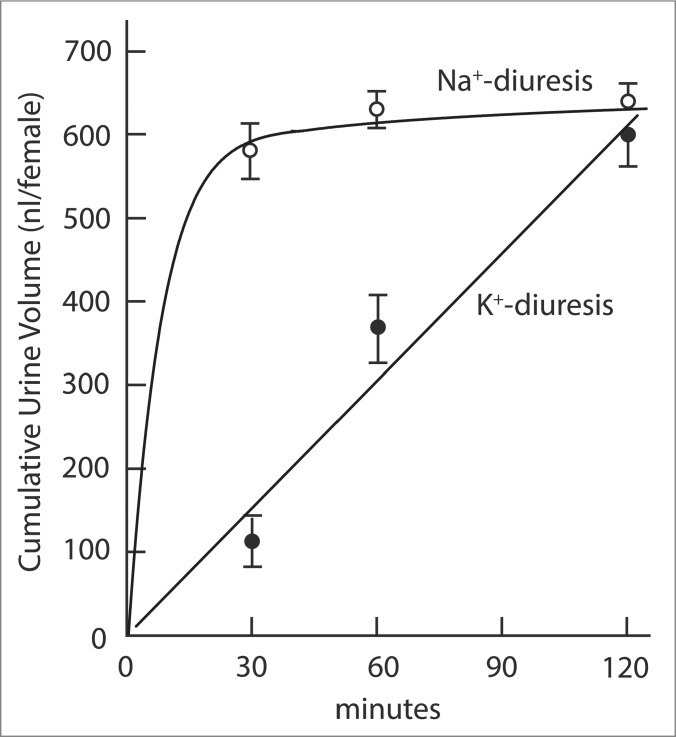
In view of the rich K+ content of pants and animals, Malpighian tubules of most insects secrete KCl and water as the first step in urine formation. The tubules respond to changing K+ concentrations by accelerating the secretion of K+ as the hemolymph K+ concentration rises and by slowing down secretion as the hemolymph K+ concentration falls.9-11 A hormone that regulates specifically the secretion of K+ has not yet been identified in any insect. Indeed, we find no obvious evidence for the role of a specifically kaliuretic hormone after injecting a K+ load into the hemolymph of the yellow fever mosquito Aedes aegypti. The hemolymph injection of 68 nanomoles K+, which is expected to increase the hemolymph K+ concentration more than tenfold, is excreted by the mosquito within 2 hours.9,10 Significantly, the rate of K+ excretion is constant during this time (Fig. 1). A hyperbolic time course of K+ excretion would be expected in response to the release of a specific kaliuretic hormone. Such a hyperbolic time course is observed for Na+ excretion after the hemolymph injection of Na+ load (Fig. 1), reflecting the known release of the natriuretic hormone.9,12-14
Figure 1.
Cumulative urine excretion as a function of time in yellow fever mosquitoes injected with of Na+ and K+ loads. Hyperbolic and linear slopes are urine excretion rates in response to respectively the injection of Na+ and K+ loads. The hemolymph of mosquitoes was injected with 900 nl containing 146 mM Na+ or 68 mM K+. The mosquito excreted the Na+ load in less than 1 h and the K+ load in 2 h. The excretion of the Na+ load is hyperbolic with time, consistent with the role of the natriuretic hormone. The excretion of the K+ load is linear with time which does not suggest the role of a specifically kaliuretic hormone. Adapted from Hine et al.9
Isolated Malpighian tubules spontaneously respond to elevated K+ concentrations in the peritubular bath. Bathed in Na+-Ringer solution containing 3.4 mM K+, the tubules secrete a fluid containing 30 mM K+ (Fig. 2). Raising the peritubular [K+] to 34 mM promptly and significantly increases [K+] in the secreted fluid to 127 mM (Fig. 2). In parallel, the basolateral membrane voltage of principal cells significantly depolarizes from −65.6 mV in the presence of 3.4 mM K+ to −34.5 mV in the presence of 34 mM K+ together with the respective increase in the cell input conductance from 2.82 µS to 3.44 µS.15 These electrophysiological changes reflect increased rates of K+ entry from the peritubular medium into the cytoplasm of epithelial cells. Together with increased rates of K+ extrusion into the tubule lumen, the balance of basolateral K+ entry and apical K+ exit brings about a new functional steady state characterized by 1) increased rates of transepithelial K+ and fluid secretion, and 2) decreased rates of transepithelial Na+ secretion (Fig. 2). Accordingly, increased K+ concentrations in the peritubular medium of isolated Malpighian tubules have kaliuretic and anti-natriuretic effects in the certain absence of endocrine agents. However, autocrine or paracrine kaliuretic agents operating at the level of the tubule cannot be excluded.
Figure 2.
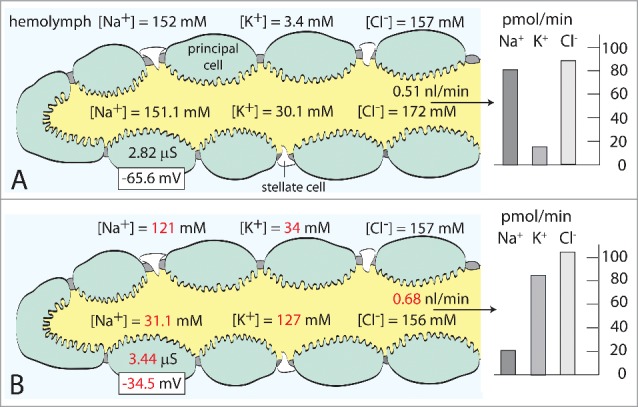
The spontaneous response of isolated Malpighian tubules of the yellow fever mosquito to the increase in the peritubular K+ concentration from 3.4 mM (A) to 34 mM (B). Numbers in red indicate statistical significant difference. Next to increasing the transepithelial secretion of K+, the increase in peritubular [K+] depolarizes the basolateral membrane voltage and increases the input conductance of principal cells as shown. For numerical detail see Hine et al9,15 The concentrations of K+, Na+ and Cl− in the hemolymph of the mosquito are respectively 6.5 mM, 96 mM and 135 mM.10
Isolated Malpighian tubules tolerate peritubular [K+] of 34 mM without ill effect; they secrete K+ at elevated rates for hours.9,15 Since a single tubule (bathed in 34 mM K+ Ringer solution) secretes K+ at a rate of 85.2 pmol/min in vitro (Fig. 2), 5 Malpighian tubules operating in vivo16 can account for the excretion of a hemolymph K+ load of 67.5 nanomoles within 2 hours as observed in Fig. 1. Thus, the spontaneous response of tubules to elevated [K+] in the peritubular medium is sufficient to deal with a similar K+ increase in vivo. A specific endocrine, kaliuretic hormone is apparently not needed.
The hypothesis that Malpighian tubules autoregulate the secretion of K+ has been stated several times.11,17-20 In support of this hypothesis, as much as 64 % of the basolateral membrane conductance of the epithelial cells in Malpighian tubules of Aedes aegypti stem from K+ channels.18 In addition to K+ channels, the Na/K ATPase and NKCC mediate the influx of K+ from the peritubular medium or hemolymph into the cytoplasm of epithelial cells (see also Fig. 5). At the apical membrane, NHA1, NHA2, and KCC mediate the efflux of K+ into the tubule lumen (see also Fig. 5). Thus, the tubules appear well equipped to autoregulate the [K+] in the hemolymph of the mosquito.
Figure 5.
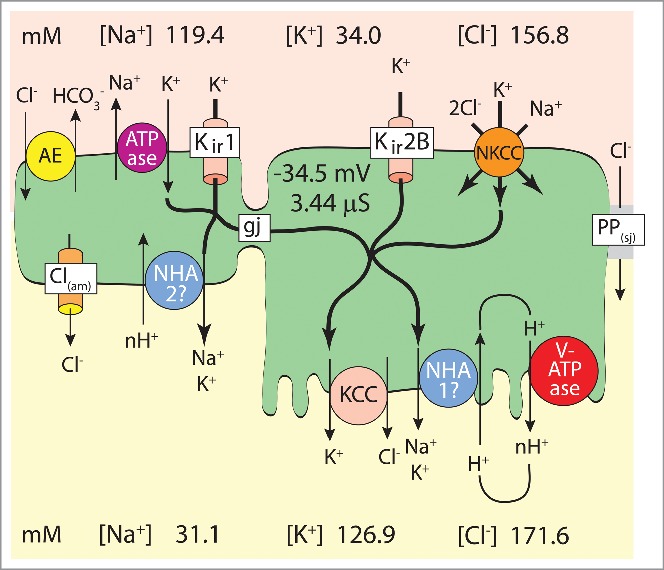
Model of transepithelial K+ secretion mediated in distal (upper) segments of Malpighian tubules of the yellow fever mosquito (Aedes aegypti) challenged to secrete K+ at high rates.9,15 Reabsorptive transport is generally attributed to proximal (lower) segments of insect Malpighian tubules that enter the gut.17,70,71 AE, Cl/HCO3 anion exchanger72; Na/K ATPase, ouabain-sensitive Na/K pump9; Kir1, AeKir1 channel15; gj, gap junction48; Kir2B, AeKir2B channel15; NKCC, Na/K/Cl cotransporter49,50; PP(sj), septate junctional paracellular pathway permeable to Cl39, 41; Cl(am), apical membrane Cl− channel of stellate cell73; NHA1, NHA2, Na/proton antiporters74; KCC, K/Cl cotransporter75; V-ATPase, electrogenic proton pump.34,76,77
Our understanding of the mechanism of transepithelial K+ secretion in Aedes Malpighian tubules has taken several steps forward by 1) the cloning and functional characterization of 2 Kir channel cDNAs expressed in Aedes Malpighian tubules, 2) the localization of these Kir channels to specific cells of the tubule, and 3) the discovery of VU small molecules that modulate the activity of these Kir channels.
Cloning and functional characterization of K+ channels in Aedes Malpighian tubules
The Aedes genome contains 5 genes that encode putative subunits of Kir channels.21 Qualitative RT-PCR and the cloning of full-length cDNAs show that Malpighian tubules of Aedes aegypti express 3 genes: AeKir1, AeKir2B and AeKir3.22 The heterologous expression of AeKir1 in Xenopus oocytes yields inward K+ currents as does the heterologous expression of AeKir2B; but the expression of AeKir3 does not. Expression of AeKir3 may require the co-expression of 1) an accessory protein such as the sulphonylurea receptor, or 2) subunits of AeKir1 or AeKir2B.
The three Kir channel subunits consist of 2 transmembrane segments (TM1, TM2) with N- and C-termini located in the cytoplasm.23 The symmetrical association of 4 subunits brings certain subunit motifs in proximity to form functional domains such as the channel gate and the selectivity filter (pore). For example, the signature motif GYG flanked by TM1 and TM2 forms the selectivity filter in AeKir1 and AeKir2B as well as all other known K+ channels.24
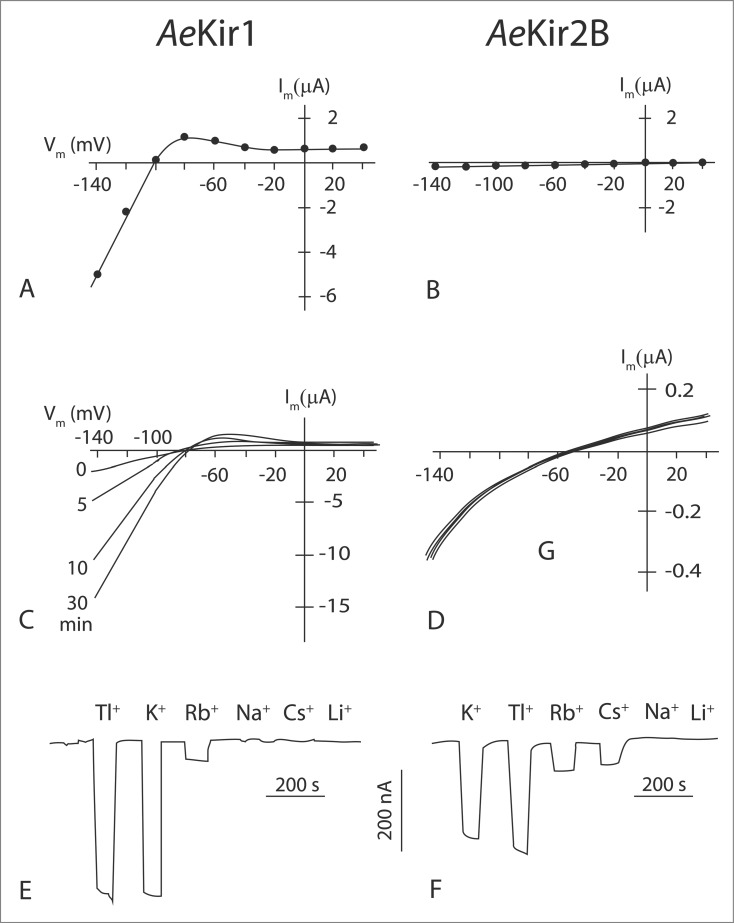
Homomeric channels formed by AeKir1 or AeKir2B subunits are functionally similar in Xenopus oocytes in that they 1) exhibit spontaneous channel activity, 2) are inhibited by extracellular barium, 3) mediate inward-rectifying currents at hyperpolarizing membrane voltages that are strongly selective for K+ over Na+, 4) pass small outward currents at depolarizing membrane voltages, and 5) allow the passage of Tl+.22 On the assumption that the proteins AeKir1 and AeKir2B express equally well in Xenopus oocytes, AeKir1 passes far higher inward K+ currents than AeKi2B at cell-negative voltages (Figs. 3A, B). At a holding membrane voltage of −120 mV (cell negative) and an extracellular [K+] of 2 mM, the inward conductance of AeKir1 (95 µS) is nearly 100 times greater than that (0.92 µS) of AeKir2B (Fig. 3A, B). The high inward conductance of AeKir1 may reflect the presence of the fully conserved motif (R265 PKK) on the cytoplasmic side of TM2. The motif is known to bind to phosphatidylinositol 4.5-biphosphate (PIP2), the well-known intracellular activator of some mammalian Kir channels.23,25 Significantly, this motif is not perfectly conserved in AeKir2B where a neutral Ser replaces the second positively charged Lys (R232PKS). The loss of a positively-charged residue in this motif is known to reduce the activity of mammalian Kir channels.26
Figure 3.
Functional differences between AeKir1 and AeKir2B expressed in Xenopus oocytes. A,B, current-voltage (I-V) plots of oocytes bathed in Ringer solution containing 2 mM K+ and 96 mM Na+; C,D, I-V plots of oocytes bathed in Ringer solution containing 5 mM K+ and 0.5 mM Na+ at 0 min and 5 mM K+ and 93 mM Na+ at 5, 10 and 30 min; E, cation selectivity of oocytes clamped continuously at −92 mV and bathed in Ringer solution containing 0.5 mM K+ and 0.5 mM Na+ and superfused with 5 mM test cation; F, cation selectivity of oocytes clamped continuously at −93 mV and bathed in Ringer solution containing 0.5 mM K+ and 0.5 mM Na+ and superfused with 10 mM test cation. Note the high currents in AeKir1 compared to AeKir2B. Adapted from Piermarini et al.22
Expressed in Xenopus oocytes, AeKir1 is sensitive to Na+ while AeKir2B is not (Figs. 3C, D). At a holding membrane voltage of −120 mV and extracellular [K+] and [Na+] of respectively 5 mM and 0.5 mM, the membrane conductance of AeKir1-expressing oocytes is only 15 µS (Fig. 3C). The isosmotic increase of the extracellular [Na+] to 93 mM leads to the progressive increase of the inward conductance to 134 µS after 15 min (Fig. 3C). The conductance returns to control values (also in the time of 15 min) after returning the extracellular [Na+] to 0.5 mM. The delayed effects of extracellular [Na+] on AeKir1 conductance is consistent with a membrane or intracellular binding site for Na+. Na+ binding motifs are present in the cytosolic C-terminus of mammalian Kir3.x channels, where the binding of Na+ mimics the stimulating effects of G proteins.27 Even though the Na+ binding motif of mammalian Kir3.x channels is present in both AeKir1 and AeKir2B,22 Na+ activates only AeKir1 (Figs. 3C, D). Thus, the Na+ binding motif may be a necessary but not sufficient condition for activating channel activity.
Expressed in Xenopus oocytes, the 2 AeKir channels exhibit the highest electrical conductance to thallium (Tl+) and K+ and the lowest conductance to Na+ and Li+ (Figs. 3E, F). In addition, AeKir2B conducts Cs+ while AeKir1 does not.22 In mammalian Kir channels where Cs+ usually blocks channels,23 the Cs+ conductance can be induced by mutations of residues in the selectivity filter.28,29 Since the residues of the selectivity filter are identical in AeKir1 and AeKir2B,22 the Cs+ permeability of AeKir2B may reflect the geometry and charge distribution in the selectivity filter of AeKir2B that are suitable for the passage of Cs+. The same geometry and charge distribution may not be present in the selectivity filter of AeKir1 and restrict the passage of Cs+. The cation selectivity of the basolateral membrane of principal cells in isolated Malpighian tubules is Tl+ > K+ > Rb+.22 It resembles the selectivity sequence of both AeKir1 and AeKir2B expressed in Xenopus oocytes (Fig. 3 E, F).
Localization of AeKir channels in Malpighian tubules of Aedes aegypti
AeKir1 and AeKir2B are markedly segregated in the epithelial cells of the tubule (Fig. 4). Antibodies raised against the subunit of AeKir1 exclusively label stellate cells, and antibodies raised against the subunit of AeKir2B exclusively label principal cells.15 Moreover, both AeKir1 and AeKir2B are located exclusively at basolateral membranes of tubule epithelial cells consistent with their hypothesized roles in transepithelial K+ secretion (Figs. 4, 5).
Figure 4.
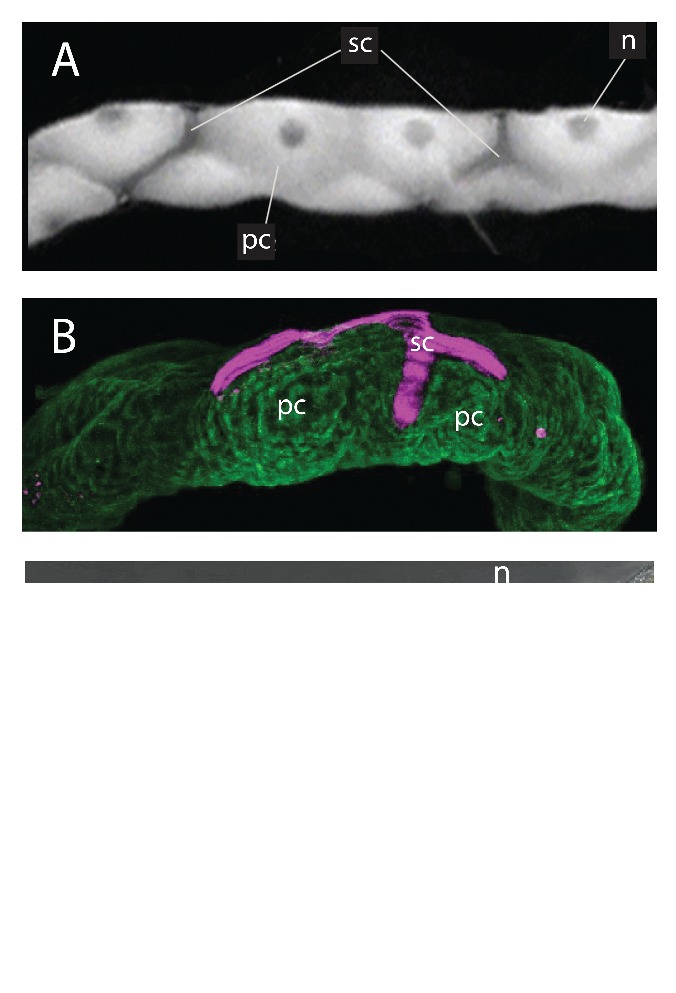
Malpighian tubules of the yellow fever mosquito Aedes aegypti. A, light microscopy of the tubule showing principal cells (pc), stellate cells (sc) and the nuclei (n) of principal cells. B. Immunolocalization of AeKir1 (magenta) in stellate cells and AeKir2B (green) in principal cells. C. Intracellular and intranuclear immunolocalization of AeKir 3 (yellow spots); npc and nsc, nuclei of principal and stellate cells respectively. Adapted from Piermarini et al.15
Principal cells have long been considered to secrete K+ and Na+ into the tubule lumen of Malpighian tubules of insects.17,20,30-36 Chloride, the usual counter-ion of Na+ and K+ (Fig. 2), is thought to pass through stellate cells in Drosophila Malpighian tubules,32,36-38 especially under diuretic conditions.35 Chloride passes through the paracellular pathway in Aedes Malpighian tubules, especially again under diuretic conditions stimulated by leucokinin or aedeskinin.39-43 The localization of AeKir1 in stellate cells of Aedes Malpighian tubules suggests a role of these cells in transepithelial K+ secretion which challenges the notion of the cellular separation of cation and anion transport in respectively principal cells and stellate cells in mosquito Malpighian tubules.
AeKir3 is found in the cytoplasm of principal cells and in the nucleus of stellate cells in Aedes Malpighian tubules (Fig. 4C). The distinct, punctate localization of AeKir3 in the cytoplasm suggests its presence in the vesicular, mineralized concretions that render principal cells opaque when viewed through the light microscope – in contrast to the clear, translucent stellate cells which do not contain concretions (Fig. 4). The intracellular concretions are storage sites for divalent and monovalent cations in Malpighian tubules of Drosophila.44 The localization of AeKir3 near the nucleus of stellate cells (Fig. 4C) suggests it may also be associated with the endoplasmic reticulum (ER) and/or Golgi complex.
The intracellular location of AeKir3 in Aedes Malpighian tubules is consistent with the lack of channel activity when AeKir3 is heterologously expressed in Xenopus oocytes.22 The oocytes do express AeKir3 protein, and the localization of this protein around the nucleus can be prevented with preadsorption of the antigenic peptide. 45 Apparently, AeKir3 is not targeted to plasma membranes but to intracellular sites. Our observations of AeKir3 in Xenopus oocytes are similar to expression studies of Drosophila Kir3 in an endogenous insect cell line where Drosophila Kir3 does not yield K+ channel activity.46
Updated model of transepithelial electrolyte transport in Aedes Malpighian tubules
In view of the identification of AeKir1 and AeKir2B in respectively stellate cells and principal cells, the model of transepithelial KCl and NaCl secretion in the distal upper secretory portion of Aedes Malpighian tubules can be updated as shown in Figure 5. In brief, the transepithelial secretion of K+ is active, requiring cellular energy to move K+ from the hemolymph to the tubule lumen against the electrochemical potential.47 AeKir2B and NKCC allow the entry of K+ from the hemolymph into the cytoplasm of principal cells, while AeKir1 and the Na/K ATPase serve this role in stellate cells. NHA and KCC serve the exit of K+ from the cytoplasm to the tubule lumen in principal cells, and NHA may have this role in stellate cells. Gap junctions are assumed to couple principal and stellate cells such that K+ entering one cell can exit through the other cell.48 In support of this model, the increase in the peritubular [K+] profoundly increases the rate of transepithelial K+ secretion (Fig. 2), while barium, ouabain, and bumetanide added to the peritubular side all reduce the rate of transepithelial K+ secretion.9,49,50 Together, barium and bumetanide bring the transepithelial secretion of K+ and fluid to a complete halt which indicates that the combined inhibition of Kir channels and NKCC also inhibits the role the Na/K ATPase might have in transepithelial K+ secretion.50
Next to serving extracellular K+ homeostasis, the transepithelial secretion of K+, Cl− and water generates a urinary space (volume) into which metabolic wastes and xenobiotics can be dumped. Thus, interference in K+ secretion, i.e. interference in the first step of urine formation, disrupts not only K+ homeostasis with negative consequences to the function of nerves and muscles; it also reduces the excretory capacity of xenobiotics. Accordingly, K+ excretion presents a specific target for inducing renal failure in the mosquito as a possible mechanism of pest control. This idea has motivated our search of “small molecules” that interfere in the transepithelial secretion of K+ in Aedes Malpighian tubules.
Small molecules are synthetic organic compounds with molecular weights less than 600 daltons. The low molecular weight aims to facilitate their entry into cells without the need of transporters. As will be shown below, it is now possible to find and/or develop a small molecule that affects Kir channels in the mosquito, causing renal failure and death. The same small molecule has greatly diminished effect on mammalian Kir channels.
Discovery of small molecules at Vanderbilt University
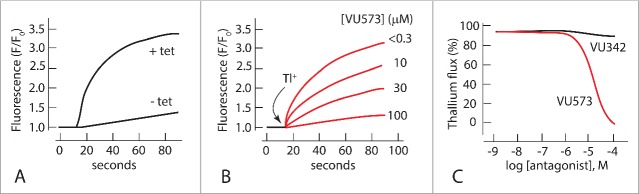
Vanderbilt University is well known for its Institute of Chemical Biology (VICB) that aims to discover new organic molecules with increased selectivity and potency over established pharmaceuticals. One of us (Denton) has utilized the high-throughput facility of this Institute to screen about 30,000 small molecules from the chemical library of VICB for the inhibition of Kir channels identified in mosquito Malpighian tubules.51 In brief, AeKir1 was expressed in T-REx-HEK-293 cells and submitted to high throughput screens using 384-well plates that tested the effects of small molecules on the influx of thallium (Tl+), a tracer of K+ passing through K+ channels (Fig. 6).
Figure 6.
Methods of the high throughput screen of small molecules. (A). Inward Tl+ flux in T-REx-HEK293 cells expressing AeKir1 induced by tetracycline (+ tet); data adapted from.51 (B). Inhibition of the AeKir1-mediated Tl+ influx by VU573 ranging in concentration from <0.3 µM to 100 µM. T-REx-HEK293 cells were pre-incubated with different concentrations of VU573 before Tl+ was added (arrow); data adapted from Raphemot et al.53 (C). Concentration response curves of active (VU573) and inactive (VU342) small molecules obtained in Tl+ influx assays; the IC50 is 15 µM for VU573. Data adapted from Raphemot et al.53
To detect the influx of Tl+ into AeKir1-expressing T-Rex-HEK293 cells, the cells are pre-loaded with the Tl+-sensitive dye Thallos-AM (TEFlabs, Austin, TX). The Tl+ flux assay was used to screen approximately 30,000 small molecules and identified 283 that diminished the influx of Tl+ into T-REx-HEK293 cells.51 To date, we have extensively characterized the pharmacology of 3 of these small molecules, VU573, VU590, and VU625 in 1) whole cell patch-clamp experiments in T-REx-HEK293 cells expressing AeKir1, 2) 2-electrode voltage clamp (TEVC) experiments in Xenopus oocytes expressing AeKir1 or AeKir2B, 3) fluid secretion and TEVC experiments in isolated Malpighian tubules of Aedes aegypti, and 4) toxicology experiments in intact mosquitoes.
VU573
VU573 is known to inhibit mammalian G protein-coupled inward rectifier K (GIRK) channels expressed in T-REx-HEK293 cells.52 Two-electrode voltage clamp studies of Xenopus oocytes expressing mammalian Kir channels confirmed channel block. VU573 inhibited neuronal and cardiac GIRK (Kir3.1/3.4) with equal potency (IC50, 1.9 µM) and preferentially inhibited GIRK, Kir2.3 and Kir7.1 over Kir1.1 and Kir2.1.52
We used VU573 on mosquito epithelial Kir channels to prove the concept that small molecule inhibitors of mosquito Kir channels can induce renal failure and death as a potential new approach to controlling mosquitoes. In T-REx-HEK293 cells expressing mosquito AeKir1, VU573 inhibited 1) the Tl+ influx with an IC50 of 15 µM (Fig. 6C), and 2) whole cell currents with an IC50 of 5.14 µM. The block of an open channel is expected to yield similar values of IC50 for permeating ions. Different IC50 values probably reflect the measurement of Tl+ flux in one study and whole cell current in the other study. Moreover, Tl+ flux was measured at unknown resting membrane voltage whereas whole cell current was measured at known clamp voltages of −120 mV, leaving uncertain the magnitude of the electro-diffusive driving force on Tl+ flux in the Tl+ flux studies. The inactive analog, VU342, inhibited AeKir1 currents with an IC50 of 112 µM compared to 5.14 µM in the case of VU573.53
While AeKir1 can be functionally expressed in T-REx-HEK293 cells, AeKir2B cannot. For this reason we switched to the Xenopus oocyte expression system.22 Here, VU573 (50 µM) inhibited the inward K+ current passing through AeKir1 (Fig. 8A), consistent with the studies on T-REx-HEK293 cells (Fig. 6). In contrast, VU573 increased the inward K+ current passing through AeKir2B (Fig. 8B). Thus, VU573 is an agonist of AeKir2B and an antagonist of AeKir1 when these 2 Aedes channels are expressed in Xenopus oocytes.53,54
Figure 8.
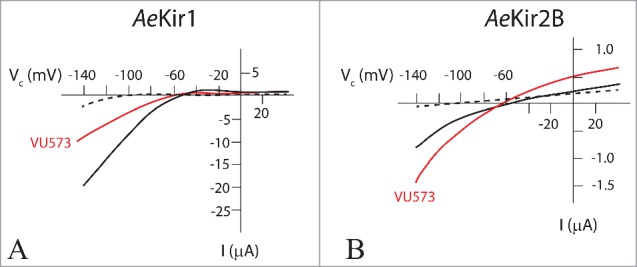
Effect of VU573 on AeKir and AeKir2B expressed in Xenopus oocytes. Oocytes were bathed in Ringer solution containing 0.5 mM K+ (broken line), and 10 mM K+ (solid lines) in the absence (black) and presence (red) of VU573 (50 µM). Note the different current scales in A and B. VU573 inhibits AeKir1 (A) and stimulates AeKir2b (B). Data adapted from Rouhier et al.54
Malpighian tubules of Aedes aegypti express both AeKir1 and AeKir2B (Fig. 4). To evaluate the effects of VU573 on the transepithelial secretion of electrolytes and fluid, we studied tubules isolated from the same mosquito. The study of paired tubules from the same mosquito eliminates the inter-mosquito variation of the data. One tubule serves as the sham control tubule, and the “sister” tubule as the experimental tubule treated with VU573 (Fig. 9A).
Figure 9.
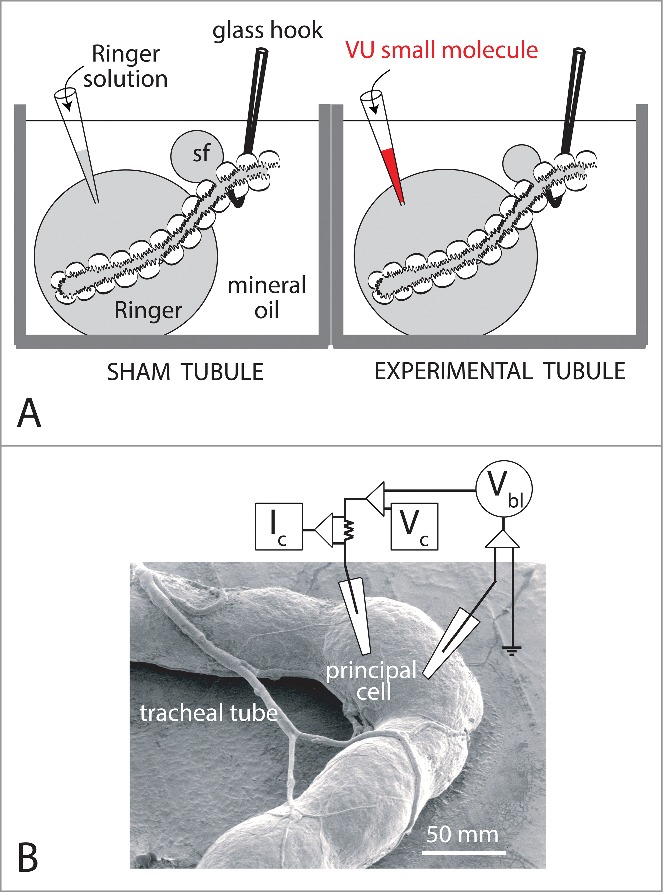
Methods for studying Malpighian tubules in vitro. A. The modified Ramsay fluid secretion assay.78 “Sister tubules” isolated from the same female yellow fever mosquito are studied in parallel. The blind, secretory end of each tubule is bathed in a Ringer droplet (50 µl) under light mineral oil. The open end of the tubule is pulled into mineral oil. A nick in the epithelium allows fluid secreted by the tubule to escape the lumen as a droplet separate from the Ringer bath. To begin, both tubules are first studied for a 30 min control period. Thereafter, 5 µl of Ringer solution is replaced with an equal volume of Ringer solution plus 1) 0.5 % DMSO (vehicle) in the sham tubule, and 2) 0.5% DMSO and 100 µl VU small molecule. Secreted fluid was collected for the analysis of its composition by electron probe.79,80 B. TECV studies of a principal cell of an Aedes Malpighian tubule.61 Both voltage and current microelectrodes impale a single principal cell of the tubule. Vbl, basolateral membrane voltage; Vc, voltage command; Ic, clamp current. Stellate cells are too thin (<10 µM) for stable measurements with intracellular microelectrodes. Data from Wu et al.81 and Hine et al.9
The VU573 concentration of 10 µM in the peritubular bath of experimental tubules first stimulated and then inhibited the transepithelial secretion of fluid (Fig. 10A). After the initial stimulation during the first hour, rates of fluid secretion declined to net inhibition.55 The electron probe analysis of secreted fluid revealed that stimulation of fluid secretion stems from the stimulation of transepithelial KCl secretion (Fig. 10B), and the inhibition stems from the inhibition of transepithelial KCl secretion (Fig. 10C). In stimulating and inhibiting KCl secretion, VU573 had no effect on the secretion of NaCl. The temporal sequence, first stimulation then inhibition, suggests not only different mechanism of action of VU573 in the 2 AeKir channels of the tubule, but also differences in the ease of access to the active sites of channels. While VU573 closes gates in AeKir1 and opens gates in AeKir2B (Fig. 8), it may reach active sites in AeKir2B earlier than in AeKir1. Furthermore, VU573 binding to AeKir2B may enhance the interaction of this channel with NKCC (Na/K/2Cl co-transport) to increase the transepithelial secretion of KCl. Consistent with this hypothesis is the observation that bumetanide, the well-known blocker of Na/K/2Cl co-transport56 blocks the stimulation of transepithelial KCl secretion by VU573.55 The late inhibition of transepithelial KCl and fluid secretion in the presence of VU573 (Fig. 10C) can be attributed to the inhibition of AeKir1 we have observed in T-REx-HEK293 cells and Xenopus oocytes expressing AeKir1 (Fig. 8A).52,54
Figure 10.
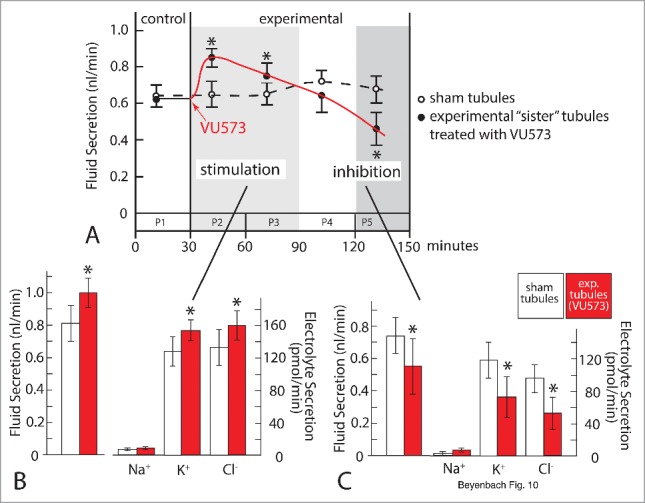
The effects of VU573 (10 µM) on transepithelial electrolyte and fluid secretion in isolated Malpighian tubules of the yellow fever mosquito. Isolated Malpighian tubules were bathed in K+-rich Ringer solution as in Figs. 2B, 5 and 9A. Rates of fluid secretion were assessed in 5 periods (P) lasting 30 min each. A. Initial stimulation in P2 and P3 and late inhibition of fluid secretion in P5. B. Initial stimulation of KCl and water secretion. C) Late inhibition of KCl and water secretion. Data are from paired sister tubules. Asterisk indicates statistical significance. For more detail see Rouhier et al.55
Electrophysiological studies of Malpighian tubules revealed only inhibitory effects of VU573. Two-electrode voltage clamp studies of single principal cells of the tubule (Fig. 9B) showed that VU573 (10 µM) significantly increased 1) the basolateral membrane voltage from −45.4 mV to −52.4 mV, and 2) the cell input resistance from 249.6 kΩ to 255.3 kΩ.53 Both effects are consistent with the block of K+ channels, similar to the effects of Ba2+, the universal blocker of K+ channels. However, the effects of VU573 are small compared to those of Ba2+. For example, peritubular Ba2+ increased the cell input resistance by 62% compared to 2% in the presence of VU573.53 Moreover, the inhibitory effects of VU573 develop gradually in contrast to the sudden on-effect of Ba2+.
In mosquitoes, the hemolymph injection of VU573 incapacitated them with an ED50 of 53.5 pmol within 24 h (Fig. 11 A, B). Incapacitated mosquitoes are either flightless or dead.53 Importantly, the hemolymph injection of VU342, the inactive analog of VU573, did not significantly incapacitate mosquitoes (Fig. 11A). In vivo measurements of diuretic capacity point to renal failure as the cause of incapacitation. Under the usual laboratory conditions, mosquitoes voided urine at a rate of 0.41 nl/min (Fig. 11C, control). When the hemolymph was injected with a K+ load in a volume of 900 nl, the urine excretion rate shot up to 3.54 nl/min, which represents the normal, diuretic response (Fig. 11C, vehicle DMSO). When VU573 was co-injected with the K+ load, the diuresis was inhibited (Fig. 11C, VU573). In contrast, the co-injection of VU342 with the K+ load did not inhibit diuresis (Fig. 11C, VU342).
Figure 11.
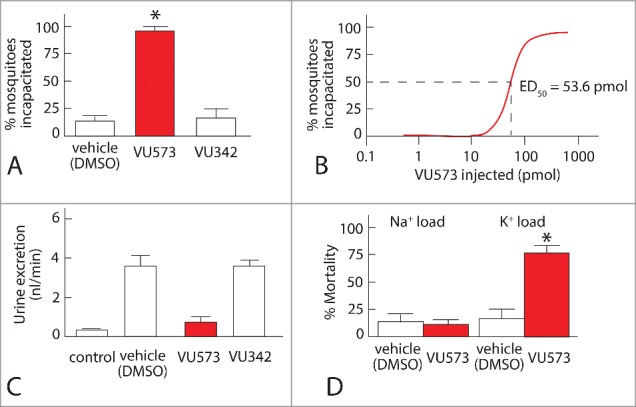
Effects of injecting VU573 and VU342 into the hemolymph of adult female yellow fever mosquitoes. A. Effects on “incapacitating” mosquitoes, i.e., rendering them flightless or dead within 24 after the hemolymph injection of VU573. Mosquitoes were injected with phosphate-buffered saline containing DMSO, the vehicle of VU small molecules, 10 mM VU573, or 10 mM VU342 (10 mM). B. Dose-response curve of the incapacitating effect of VU573 revealing an ED50 of 53.6 pmol. C. Renal failure in mosquitoes induced by VU573. Urine flow per mosquito is shown. Control mosquitoes did not receive hemolymph injections. All others were injected with a K+ load plus DMSO (vehicle DMSO), or K+ load plus VU573 or VU342 in DMSO. D. K+ specificity of the fatal effect of VU573. Most mosquitoes survive the effects of VU573 when injected with a Na+ load but not with a K+ load. For more detail see Raphemot et al.53
By blocking the renal excretion of the K+ load, VU573 brought about the demise of most mosquitoes (Fig. 11D). Mortality was low (> 15%) in mosquitoes injected with only a K+ load or a Na+ load (Fig. 11D). In contrast, most mosquitoes (˜78%) died when the injected K+ load included VU573. However, VU573 did not increase mortality when it was injected with a Na+ load (Fig. 11D). These results demonstrate that VU573 kills mosquitoes by interfering in specifically K+ excretion, i.e., by inducing the renal failure of K+ homeostasis (Fig. 11D).
Some mosquitoes displayed the obvious signs of renal failure within 24 h after the hemolymph co-injection VU573 and the K+ load: grossly distended, bloated abdomens (Fig. 12B). Bloating stems from the retention of fluid. The mosquitoes drag their abdomens loaded down by the increased weight, and they are unable to fly. Pricking the bloated abdomen with forceps deflates the abdomen with the sudden exodus of fluid. In some cases, the swelling of the abdomen exceeded its elastic limit, rupturing the abdomen and releasing its aqueous contents (Piermarini, personal observation). However, not more than 10% of the injected mosquitoes displayed distended abdomens, indicating again that VU573 induces more of a failure of K+ homeostasis than volume homeostasis.”
Figure 12.
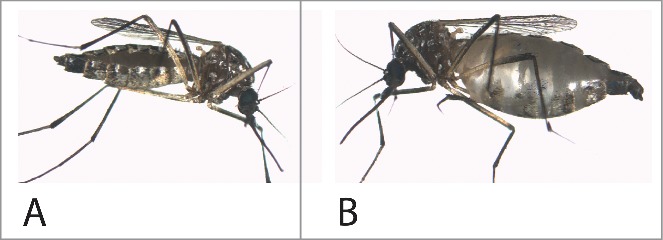
The effect of VU573 on fluid retention in some mosquitoes. A, normal mosquito maintained in the laboratory. B, incapacitated mosquito after the injection of a small volume (69 nl) of phosphate-buffered saline containing VU573 in DMSO. For further detail see Raphemot et al.53
VU590
VU590 was discovered by the Vanderbilt team searching for new modulators of Kir channels. Using the Tl+ flux-based high throughput screen of more than 126,000 small molecules, Lewis et al. found VU590 to inhibit the renal outer medullary K+ channel (ROMK, Kir1.1) with nanomolar affinity.57 ROMK is one of 6 Kir channels expressed in the mammalian kidney. Located in apical membranes of epithelial cells of the distal nephron, ROMK plays a major role in fine-tuning extracellular K+ homeostasis by controlling the secretion of K+ into the lumen of the connecting tubule and the cortical collecting duct. Further upstream in the thick ascending limb of the Loop of Henle, ROMK mediates the secretory flux of K+ into the tubule lumen, thereby recycling K+ that has entered the cell via NKCC2 (Na/K/2Cl transport). The expression of ROMK in T-REx-HEK293 cells enabled Tl+ flux studies that revealed the inhibition of ROMK with an IC50 of 294 nM by VU590.57 Electrophysiological and computational modeling studies indicate that VU590 inhibits ROMK by blocking the selectivity filter from the intracellular side.57,58
In mosquitoes, VU590 was found to be a potent and selective inhibitor of AeKir1; it had no effect on AeKir2B.54 In T-REx-HEK293 cells expressing AeKir1, VU590 blocked the Tl+-flux in a dose-dependent manner with an IC50 of 5.6 µM,54 and in Xenopus oocytes, VU590 inhibited the inward K+ current of AeKir1 but not Kir2B (Fig. 13).
Figure 13.
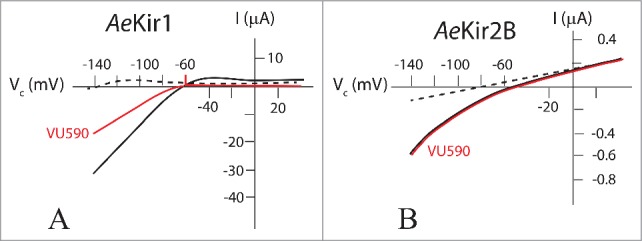
Effect of VU590 on AeKir and AeKir2B expressed in Xenopus oocytes. Oocytes were bathed in Ringer solution containing 0.5 mM K+ (broken line), and 10 mM K+ (solid lines) in the absence (black) and presence (red) of VU590 (50 µM). Note the different current scales in A and B. VU590 inhibits AeKir1 (A) but has no effect on AeKir2B (B). Data adapted from Rouhier et al.54
In isolated tubules bathed in a Ringer solution containing 34 mM K+ (to simulate K+ loads in vivo), VU590 (10 μM) in the peritubular bath inhibited transepithelial fluid secretion 120 min later.15 The delayed inhibition was partial, lowering rates of transepithelial KCl and fluid secretion to 54% of the control, similar to the late inhibition of KCl and fluid secretion in the presence of VU573 (Fig. 10C). Furthermore, like VU573, VU590 inhibited the secretion of KCl and not NaCl. The similarity of the inhibitory effects of VU590 and VU573 suggests that VU573 inhibits the transepithelial secretion of KCl by inhibiting AeKir1 in stellate cells.
VU590 had no effect on the basolateral membrane voltage (Vbl) of principal cells (Fig. 9B), but it significantly reduced the cell input conductance (gin) to values 63% of the control within ˜90 min. Accordingly, the inhibition of AeKir1 in stellate cells has little effect on the basolateral membrane voltage in principal cells where AeKir2B is located (Fig. 4B). The effect of VU590 on gin supports the presence of gap junctions that electrically couple principal cells and stellate cells. However, effects that VU590 may have on Vbl in stellate cells may not be detected in principal cells in view of 1) the 5:1 ratio of principal vs stellate cells in mosquito Malpighian tubules,59,60 and 2) the small surface of the basolateral membrane of stellate cells compared to the large surface in principal cells (Fig. 4). The electrophysiological effects of VU590 developed slowly and gradually15 in contrast to the rapid on/off channel block of barium.61
In mosquitoes, the hemolymph injection of VU590 with a K+ load killed mosquitoes within 24 hours with an LD50 of 1.56 nmol.54 About 17% of the mosquitoes killed by VU590 had swollen abdomens as observed after the injection of VU573 (Fig. 12). Swollen abdomens reflect again the inability to excrete KCl and water. Mosquitoes injected with a K+ load in a volume of 900 nl responded appropriately by excreting 95% of this volume within 1 hour.54 When the hemolymph injection included VU590, the output of urine dropped significantly but only ˜32%, consistent with inhibition of only one of the 2 Kir channels present in Malpighian tubules.54 VU608, the inactive analog of VU590, had no effect on volume retention or mortality.54 Altogether, the in vitro and in vivo data confirm that AeKir1 in stellate cells plays a key role in the regulation of hemolymph K+ homeostasis, and strengthen the case that the pharmacological block of renal K+ channels offers a promising new mode of action for killing mosquitoes.
VU625
VU625 is the first submicromolar inhibitor of a mosquito K+ channel. The Denton laboratory discovered it in high throughput screen of approximately 30,000 small molecules for modulators of AeKir1 and chose it for optimization because of its high affinity for AeKir1.51 In particular, VU625 is 80 times more selective for AeKir1 than for 7 mammalian Kir channels, including Kir1.1, Kir2.1, Kir2.2, Kir2.3, Kir7.1 and Kir 6.2/Sur1, and is over 20 times more selective for AeKir1 than for mammalian Kir3.1/3.2.51 Furthermore, at a concentration of 10 µM, VU625 had significant effects on only 3 of 68 mammalian molecular targets that included a selection of voltage-gated ion channels, ion transporters, neurotransmitters, peptide receptors and G protein-coupled receptors.51 Thus, VU625 is the most selective small molecule that we have found so far.
In T-REx-HEK293 cells expressing AeKir1, VU625 inhibited the Tl+ influx with an IC50 of 350 nM, and it inhibited AeKir1-mediated currents with an IC50 of 96.8 nM (Fig. 14A), making it the most potent small molecule inhibitor of AeKir1. Since AeKir2B does not express in T-REx-HEK293 cells, both AeKir1 and AeKir2B were studied upon expression in Xenopus oocytes. As shown in Fig. 14B, VU625 inhibited the AeKir1-mediated current with an IC50 of 3.8 µM and the AeKir2B-mediated current with an IC50 of 45.1 µM.51 Accordingly, VU625 inhibits both AeKir1 and AeKir2B but with a 12-fold greater affinity for AeKir1.
Figure 14.
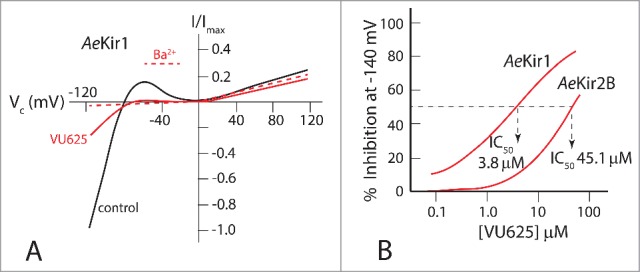
The effect of VU625 on mosquito Kir channels expressed in T-REx-HEK293 cells (A) and Xenopus oocytes (B). A. Strong inward rectification of K+ current (control) in T-REx-HEK293 cells expressing AeKir1. The inward current is markedly reduced by VU625 (0.9 µM), similar to the channel block by barium (Ba2+, 2 mM). B. Dose-response curves of currents mediated by AeKir1 and AeKir2B expressed in Xenopus oocytes and inhibited by VU625. Adapted from Raphemot et al.51
In isolated Malpighian tubules, VU625 (10 µM) together with probenecid (100 µM) reduced the transepithelial secretion of fluid by more than 60% (Fig. 15A). The inhibition stems from the inhibition of specifically transepithelial K+ and Cl− secretion, both of which dropped below 60% of control rates. VU625 did not affect transepithelial Na+ secretion (Fig. 15A). Compared to the effect of VU590 which reduced transepithelial K+ secretion to 54% of control, VU625 reduced the secretion to values less than 40% of control (Fig. 15A). The increased potency of VU625 reflects its inhibition of both AeKir1 and AeKir2B (Fig. 14B) compared to the inhibition of only AeKir1 by VU590 (Fig. 13).
Figure 15.
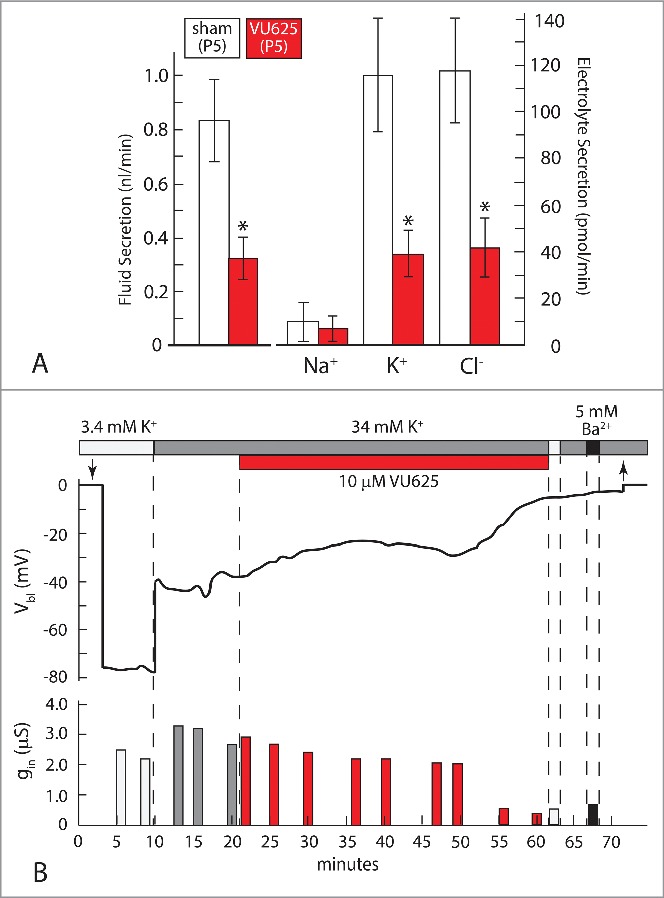
Effect of VU625 (10 µM) on Malpighian tubules isolated from the yellow fever mosquito. (A). Effects on the transepithelial secretion of fluid, Na+, K+, and Cl− in tubules bathed in 34 mM K+ Ringer solution 120 min after the addition of VU625 (period 5, P5, Fig. 10) Experiments were performed in sister tubules isolated from the same mosquito (see Fig. 9). (B). Effects on the basolateral membrane voltage (Vbl) and cell input conductance (gin). The representative tubule was bathed in Ringer solution containing 3.4 mM or 34 mM K+, plus or minus VU625 or barium (Ba2+). Probenecid was added to the peritubular bath to block the tubular secretion of VU625. Downward and upward arrows indicate respectively the entry and exit of the voltage-sensing microelectrode into a principal cell of the tubule. * indicates statistical significance. Adapted from Piermarini et al.15
Electrophysiological studies confirm the inhibition of K+ channels. After treating the representative tubule shown in Figure 15B with VU625 for 40 min, the large K+ diffusion potential (Δ 40 mV) observed upon the 10-fold increase in peritubular [K+] at the start of the experiment, was no longer observed. Furthermore, the basolateral membrane voltage (Vbl) of principal cells no longer responded to Ba2+ after treatment with VU625. The absence of K+ diffusion potentials and the lack of Ba2+ effect indicate the absence of open K+ channels, i.e. both AeKir1 and AeKir2B are closed.18,61 As observed in studies of VU573 and VU590, the shutdown of K+ channels by VU625 developed slowly with time (Fig. 15B). As Vbl depolarized, the cell input conductance (gin) decreased in parallel, reflecting in part the closing of basolateral membrane K+ channels (Fig. 15B).
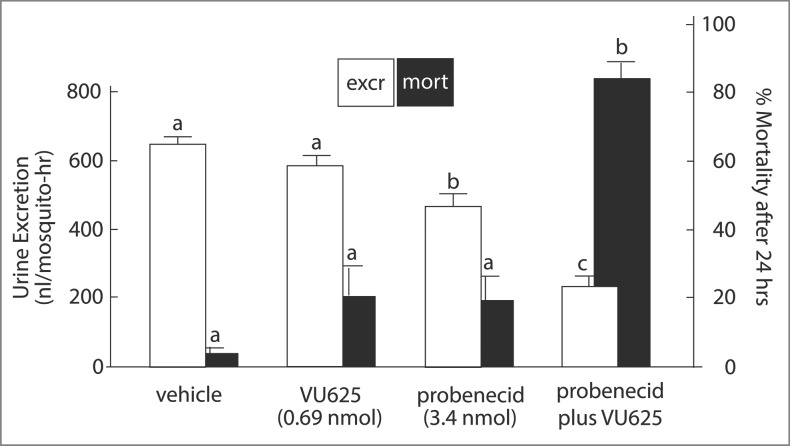
Mosquitoes survive the hemolymph injection of K+ loads as long as they are able to excrete K+ (Fig. 16, vehicle). The hemolymph injection of a K+ load in a volume of 900 nl was largely excreted (644 nl) within the first hour of injection, and only 3% of the injected mosquitoes died within one day (Fig. 16, vehicle). In contrast, after injecting the K+ load with VU625 and probenecid, the mosquito excreted only 237 nl within the hour, and more than 83 % of the injected mosquitoes died within the day. These observations indicate the inability to excrete K+ as the cause of death. It is important to note that for VU625 to induce renal failure in vivo, it had to be co-injected with probenecid (Fig. 16). Probenecid is known to inhibit the multidrug resistance protein1 (MRP1) and other organic anion transporters that are known to remove foreign organic substances including sulfonamides and xenobiotics from the circulation.62-64 VU625 and probenecid are both sulfonamides (Fig. 7). Accordingly, the hemolymph injection of probenecid at a dose 5 times greater than that of VU62551 appears to competitively inhibit MRP-like transporters that must be present in Malpighian tubules of Aedes aegypti. The competitive inhibition blocks the tubular secretion of VU625, thereby enhancing its inhibition of tubular secretion of K+. The inhibition prevents the renal excretion of the K+ load which leads to renal failure and death (Fig. 16). The presence of MRP-like transporters in Malpighian tubules of Aedes aegypti is supported by the observation that, in the absence of probenecid, VU625 does not inhibit transepithelial K+ and fluid secretion in isolated Malpighian tubules (Beyenbach, personal observations).
Figure 16.
The effect of the hemolymph injection of VU625 on urine excretion and mortality in the yellow fever mosquito. White bars show urine flow rate per female mosquito in the first hour after the hemolymph injection of a K+ load in 900 nl of phosphate based saline (PBS, vehicle), vehicle plus VU625, vehicle plus probenecid, or vehicle plus probenecid and VU625. Data are mean ± SE of 6 to 18 trials of 5 mosquitoes each. Black bars show percent mortality of an independent set of mosquitoes 24 h after the hemolymph injection of a K+ load in 69 nl of PBS. Lower-case letters (a,b,c) indicate statistical categorization of the means as determined by one-way ANOVA with a Newman-Keuls post test (p <0.05). For further detail see Raphemot et al.51
Figure 7.
VU inhibitors of mosquito Kir channels. The analogs active in mosquito Kir channels are shown in the top row with their respective inactive analogs in the bottom row. Replacement of the aryl ether of VU573 with a morpholine moiety produces the inactive analog VU342. Positively charged VU590 and VU608 share the same macrocycle core; they differ in the replacement of the 2 nitrobenzyl groups in VU590 with (methylsulfonyl)benzyl groups in VU608. VU625 and VU688 are sulfonamides that differ in the replacement of the methyl group in VU625 with an aryl group in VU688.54
Lessons learned from the use of VU small molecules
Our study of VU small molecules has 1) advanced our understanding of K+ secretion by Malpighian tubules of the yellow fever mosquito, 2) documented the renal system as a promising target for the development of new insecticides, and 3) encouraged the search of small molecules that exploit species-specific subunit differences at the molecular level to target one insect species while sparing other species.
As for the basic science of Malpighian tubules, VU small molecules have illuminated the role of the 2 epithelial Kir channels expressed in Malpighian tubules of Aedes aegypti. The selective inhibition of AeKir1 by VU590 reveals stellate cells as an important route of transepithelial K+ secretion. Nearly half (46%) of transepithelial K+ secretion depends on AeKir1 in stellate cells and only 20% depends on AeKir2B in principal cells when the tubules face a high (34 mM) peritubular K+ concentration.15 The strong inward rectification of K+ currents displayed by AeKir1 expressed in Xenopus oocytes (Figs.3 A,C, 5) appears to serve transepithelial K+ secretion particularly well in the presence of high peritubular [K+], because K+ entering stellate cells is not recycled to the peritubular medium. Instead, it leaves the cell across the apical membrane into the tubule lumen (Figs. 2B,5). The increasing role of basolateral membrane K+ channels with increasing peritubular [K+] has also been noted in Malpighian tubules of the ant Formica.20 On the other hand, the weak inward rectification displayed by AeKir2B expressed in Xenopus oocytes (Figs. 3B, D) may serve transepithelial K+ secretion under the normal, low [K+] in the peritubular bath or hemolymph (Fig. 2A,5). Allowing inward as well outward K+ flux suits AeKir2B for autoregulating transepithelial K+ secretion in principal cells of the tubule because intracellular and extracellular [K+] can rise and fall together. Indeed, intracellular K+ is near or at electrochemical equilibrium with extracellular K+ in principal cells of every Malpighian tubule where these measurements have been made.18,19,65-68
As to the mechanism of action of VU small molecules, all 3 small molecules have remarkable affinities for AeKir channels in spite of marked structural differences. VU573 inhibits AeKir1 but stimulates AeKir2B, VU590 inhibits AeKir1 with no effect on AeKir2B, and VU625 inhibits both AeKir1 and AeKir2B albeit with a preference for AeKir1. Studies in the Denton lab suggest that VU590 inhibits ROMK by blocking the selectivity filter from an intracellular side of the channel.57,58 It is therefore reasonable to speculate that VU590 also inhibits AeKir1 at a similar binding site. Time required for VU590 to access its site of action may explain its slow kinetics of channel block after it has been added to the peritubular bath of isolated Malpighian tubules.15 VU573 does not block AeKir1 as VU590 does.54 Instead VU573 may interact with regulatory or gating domains that differ between AeKir1 and AeKir2B. Binding of VU573 to AeKir1 could disrupt the interaction of the channel with an agonist, thereby inhibiting this channel. In contrast, binding of VU573 to AeKir2B could enhance the interaction between the channel and agonist, thereby stimulating this channel (Fig. 8). Known agonists of Kir channels include ATP, H+, Na+, G proteins, PIP2 and PO4.23 VU625 is the most potent inhibitor of AeKir1 found to date. Moreover, its high affinity for mosquito AeKir1 over mammalian Kir channels and membrane proteins make VU625 the most promising small molecule for further development into a target-specific mosquitocide.51
At the level of Malpighian tubules, VU590 and VU625 selectively inhibit transepithelial K+ secretion, leaving Na+ secretion unchanged. VU573 first stimulates and then inhibits transepithelial K+ secretion, again without changing Na+ secretion. The stimulation and inhibition mimic respectively the effects of VU573 on AeKir2B and AeKir1. The slow kinetics of the effect of all 3 small molecules in shutting down K+ channels and transepithelial K+ secretion in isolated Malpighian tubules is consistent with a membrane or intracellular site of action. Moreover, barium hyperpolarizes Vbl while VU small molecules depolarize Vbl, but both reduce the cell input conductance consistent with increased numbers of closed AeKir channels. Thus, the closing of AeKir channels by VU small molecules likely differs from channel block by Ba2+. Barium added to the peritubular medium reversibly hyperpolarizes Vbl and decreases the cell input conductance with fast kinetics, consistent with reversible, physical block of open AeKir channels.18,61 VU small molecules added to the peritubular medium irreversibly depolarize Vbl and decrease the cell input conductance with slow kinetics (Fig. 15B), consistent with the modulation of gating mechanisms at membrane or intracellular sites of AeKir channels. Moreover, depolarization rather than hyperpolarization of Vbl indicates that VU small molecules have secondary effects in intact Malpighian tubules, including effects on the interaction between AeKir channels with other membrane transporters in the cell. Expected networked interactions between AeKir at basolateral membranes with NHA and the H+ V-ATPase at apical membranes (Fig. 5) may serve steady-states by matching K+ influx (channel) with K+ efflux (pump). Thus, closing AeKir channels at basolateral membranes may decrease transport activities at apical membranes, thereby lowering the activity of the H+ V-ATPase and decreasing Vbl via electrical coupling of the 2 cell membranes.15 This would explain the depolarization of Vbl as VU small molecules close Kir channels in intact Malpighian tubules (Fig. 15B).
As to applied science brought to insect Malpighian tubules, VU small molecules have shown their potential as new pesticides. The injection of all 3 VU small molecules into the hemolymph of mosquitoes incapacitates and kills them within one day. Studies in vivo and in vitro indicate renal failure to excrete K+ as the primary cause of death. However, effects on extra-renal Kir channels cannot be excluded. Kir channels in the nervous system could also be affected by VU compounds as suggested by the decreased responsiveness, inability to fly, and paralysis prior to death. In Rhodnius and Glossina, rising hemolymph K+ concentrations are known to trigger the release of diuretic hormone.11,69 Inhibiting the release of diuretic hormone could contribute to the renal failure and bloating of mosquito abdomens we have observed in mosquitoes treated with VU compounds.
Renal functions in insects have long been overlooked as target of pest control. It would appear that renal functions in pestiferous insects are particularly vulnerable to intervention in view of the wide range of solute and water challenges these small animals experience: from long periods of dehydration to short bouts of gorging on the sap of plants or blood of animals.
VU573, VU590 and VU625 kill mosquitoes by disrupting renal K+ homeostasis. In the short span of 3 years we have discovered the promising small molecule VU625. This small molecule is 20-80 times more selective for AeKir1 than mammalian Kir channels, and it has limited effects on 3 of more than 60 diverse mammalian membrane proteins, including voltage-gated ion channels, ion transporters, neurotransmitters, peptide receptors and G protein-coupled receptors.51 Finding a highly selective antagonist of Aedes Kir channels, such as VU625, from a screen of over 30,000 small molecules without modifying its chemistry provide strong encouragement that species-specific insecticides can be developed. Further refinements of VU625, or entirely different small VU molecules, may discriminate against no only mammals and lower vertebrates, but also beneficial insects such as honeybees.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by a grant to P.M. Piermarini from the Foundation for the National Institutes of Health through the Vector-Based Transmission of Control: Discovery Research (VCTR) program of the Grand Challenges in Global Health Initiative. Work in the lab of J.S. Denton was supported in part by grant R01DK082884 from the National Institute of Diabetes and Digestive and Kidney Diseases, and work in the lab of K.W. Beyenbach was funded in part by grant IBN0078058 from the National Science Foundation.
References
- 1.Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 1990; 87:245-54; PMID:2176588; http://dx.doi.org/ 10.1289/ehp.9087245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijverberg HP, van den Bercken J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit Rev Toxicol 1990; 21:105-26; PMID:1964560; http://dx.doi.org/ 10.3109/10408449009089875 [DOI] [PubMed] [Google Scholar]
- 3.Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, et al.. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Poll Res Int 2015; 22:5-34; PMID:25233913; http://dx.doi.org/ 10.1007/s11356-014-3470-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, et al.. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Poll Res Int 2015; 22:68-102; PMID:25223353; http://dx.doi.org/ 10.1007/s11356-014-3471-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 2002; 82:893-922; PMID:12270947; http://dx.doi.org/ 10.1152/physrev.00013.2002 [DOI] [PubMed] [Google Scholar]
- 6.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 2003; 278:8516-25; PMID:12496265; http://dx.doi.org/ 10.1074/jbc.M210432200 [DOI] [PubMed] [Google Scholar]
- 7.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol 2006; 22:308-12; PMID:16713358; http://dx.doi.org/ 10.1016/j.pt.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 8.Dayan FE, Cantrell CL, Duke SO. Natural products in crop protection. Bioorg Med Chem 2009; 17:4022-34; PMID:19216080; http://dx.doi.org/ 10.1016/j.bmc.2009.01.046 [DOI] [PubMed] [Google Scholar]
- 9.Hine RM, Rouhier MF, Park ST, Qi Z, Piermarini PM, Beyenbach KW. The excretion of NaCl and KCl loads in mosquitoes. 1. Control data. Am J Physiol Regul Integr Comp Physiol 2014; 307:R837-49; PMID:25056103; http://dx.doi.org/ 10.1152/ajpregu.00105.2014 [DOI] [PubMed] [Google Scholar]
- 10.Williams JC, Hagedorn HH, Beyenbach KW. Dynamic changes in flow rate and composition of urine during the post-bloodmeal diuresis in Aedes aegypti (L.). J Comp Physiol ; A 1983; 153:257-65; http://dx.doi.org/ 10.1007/BF0068962925309022 [DOI] [Google Scholar]
- 11.Maddrell SH, O'Donnell MJ, Caffrey R. The regulation of haemolymph potassium activity during initiation and maintenance of diuresis in fed Rhodnius prolixus. J Exp Biol 1993; 177:273-85; PMID:8486999 [DOI] [PubMed] [Google Scholar]
- 12.Coast GM, Garside CS, Webster SG, Schegg KM, Schooley DA. Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae (Giles). J Exp Biol 2005; 208:3281-91; PMID:16109890; http://dx.doi.org/ 10.1242/jeb.01760 [DOI] [PubMed] [Google Scholar]
- 13.Wheelock GD, Petzel DH, Gillett JD, Beyenbach KW, Hagedorn HH. Evidence for hormonal control of diuresis after a blood meal in the mosquito Aedes aegypti. Arch Insect Biochem Physiol 1988; 7:75-90; http://dx.doi.org/ 10.1002/arch.940070202 [DOI] [Google Scholar]
- 14.Beyenbach KW, Petzel DH. Diuresis in mosquitoes: Role of a natriuretic factor. News Physiol Sci 1987; 2:171-5 [Google Scholar]
- 15.Piermarini PM, Dunemann SM, Rouhier MF, Calkins TL, Raphemot R, Denton JS, Hine RM, Beyenbach KW. Localization and role of inward rectifier K+ channels in Malpighian tubules of the yellow fever mosquito Aedes aegypti. Insect Biochem Mol Biol 2015; submitted; PMID:26079629 [DOI] [PubMed] [Google Scholar]
- 16.Beyenbach KW, Oviedo A, Aneshansley DJ. Malpighian tubules of Aedes-aegypti- Five tubules, one function. J Insect Physiol 1993; 39:639-48; http://dx.doi.org/ 10.1016/0022-1910(93)90069-4 [DOI] [Google Scholar]
- 17.Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol 2003; 206:3845-56; PMID:14506220; http://dx.doi.org/ 10.1242/jeb.00639 [DOI] [PubMed] [Google Scholar]
- 18.Beyenbach KW, Masia R. Membrane conductances of principal cells in Malpighian tubules of Aedes aegypti. J Insect Physiol 2002; 48:375-86; PMID:12770112; http://dx.doi.org/ 10.1016/S0022-1910(02)00057-4 [DOI] [PubMed] [Google Scholar]
- 19.Ianowski JP, Christensen RJ, O'Donnell MJ. Intracellular ion activities in Malpighian tubule cells of Rhodnius prolixus: evaluation of Na+-K+-2Cl- cotransport across the basolateral membrane. J Exp Biol 2002; 205:1645-55; PMID:12000809 [DOI] [PubMed] [Google Scholar]
- 20.Leyssens A, Dijkstra S, Van Kerkhove E, Steels P. Mechanisms of K+ uptake across the basal membrane of Malpighian tubules of Formica polyctena: The effect of ions and inhibitors. J Exp Biol 1994; 195:123-45; PMID:7964409 [DOI] [PubMed] [Google Scholar]
- 21.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, et al.. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 2007; 316:1718-23; PMID:17510324; http://dx.doi.org/ 10.1126/science.1138878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piermarini PM, Rouhier MF, Schepel M, Kosse C, Beyenbach KW. Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti. Insect Biochem Mol Biol 2013; 43:75-90; PMID:23085358; http://dx.doi.org/ 10.1016/j.ibmb.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function and physiological roles. . Physiol Rev 2010; 90:291-306; PMID:20086079; http://dx.doi.org/ 10.1152/physrev.00021.2009 [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol 2014; 206:151-62; PMID:25049269; http://dx.doi.org/ 10.1083/jcb.201404136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 2011; 477:495-8; PMID:21874019; http://dx.doi.org/ 10.1038/nature10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron 2002; 34:933-44; PMID:12086641; http://dx.doi.org/ 10.1016/S0896-6273(02)00725-0 [DOI] [PubMed] [Google Scholar]
- 27.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 2011; 147:199-208; PMID:21962516; http://dx.doi.org/ 10.1016/j.cell.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dibb KM, Rose T, Makary SY, Claydon TW, Enkvetchakul D, Leach R, Nichols CG, Boyett MR. Molecular basis of ion selectivity, block, and rectification of the inward rectifier Kir3.1/Kir3.4 K(+) channel. J Biol Chem 2003; 278:49537-48; PMID:14504281; http://dx.doi.org/ 10.1074/jbc.M307723200 [DOI] [PubMed] [Google Scholar]
- 29.Sackin H, Nanazashvili M, Li H, Palmer LG, Walters DE. A conserved arginine near the filter of Kir1.1 controls Rb/K selectivity. Channels 2010; 4:203-14; PMID:20458182; http://dx.doi.org/ 10.4161/chan.4.3.11982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiehart UI, Nicolson SW, Van Kerkhove E. K+ transport in Malpighian tubules of Tenebrio molitor L: a study of electrochemical gradients and basal K+ uptake mechanisms. J Exp Biol 2003; 206:949-57; PMID:12582137; http://dx.doi.org/ 10.1242/jeb.00200 [DOI] [PubMed] [Google Scholar]
- 31.Weltens R, Leyssens A, Zhang SL, Lohrmann E, Steels P, Van Kerkhove E. Unmasking of the apical electrogenic proton pump in isolated Malpighian tubules (Formica Polyctena) by the use of barium. Cell Physiol Biochem 1992; 2:101-16; http://dx.doi.org/ 10.1159/000154630 [DOI] [Google Scholar]
- 32.O'Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in Malpighian tubules of Drosophila melanogaster. J Exp Biol 1996; 199 (Pt 5):1163-75; PMID:8786336 [DOI] [PubMed] [Google Scholar]
- 33.Beyenbach KW. Mechanism and regulation of electrolyte transport in Malpighian tubules. J Insect Physiol 1995; 41:197-207; http://dx.doi.org/ 10.1016/0022-1910(94)00087-W [DOI] [Google Scholar]
- 34.Beyenbach KW, Piermarini PM. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol (Oxf) 2011; 202:387-407; PMID:20946239; http://dx.doi.org/ 10.1111/j.1748-1716.2010.02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JA. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci U S A 2014; 111:14301-6; PMID:25228763; http://dx.doi.org/ 10.1073/pnas.1412706111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, Kaiser K, Dow JA. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol 1998; 274:R1039-R49; PMID:9575967 [DOI] [PubMed] [Google Scholar]
- 37.Radford JC, Davies SA, Dow JA. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem 2002; 277:38810-7; PMID:12163486; http://dx.doi.org/ 10.1074/jbc.M203694200 [DOI] [PubMed] [Google Scholar]
- 38.Terhzaz S, O'Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JA. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J Exp Biol 1999; 202:3667-76; PMID:10574744 [DOI] [PubMed] [Google Scholar]
- 39.Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol 1993; 132:63-76; PMID:8459448; http://dx.doi.org/ 10.1007/BF00233052 [DOI] [PubMed] [Google Scholar]
- 40.Yu MJ, Beyenbach KW. Leucokinin activates Ca2+-dependent signal pathway in principal cells of Aedes aegypti Malpighian tubules. Am J Physiol Renal Physiol 2002; 283:F499-508; PMID:12167601; http://dx.doi.org/ 10.1152/ajprenal.00041.2002 [DOI] [PubMed] [Google Scholar]
- 41.Beyenbach KW. Regulation of tight junction permeability with switch-like speed. Curr Opin Nephrol Hypertens 2003; 12:543-50; PMID:12920403; http://dx.doi.org/ 10.1097/00041552-200309000-00010 [DOI] [PubMed] [Google Scholar]
- 42.Schepel SA, Fox AJ, Miyauchi JT, Sou T, Yang JD, Lau K, Blum AW, Nicholson LK, Tiburcy F, Nachman RJ, et al.. The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito. Am J Physiol Regul Integr Comp Physiol 2010; 299:R612-22; PMID:20538895; http://dx.doi.org/ 10.1152/ajpregu.00068.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyenbach KW. A dynamic paracellular pathway serves diuresis in mosquito Malpighian tubules. Ann N Y Acad Sci 2012; 1258:166-76; PMID:22731730; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wessing A, Zierold K, Hevert F. Two types of concretions in Drosophila Malpighian tubules as revealed by X-ray microanalysis: A study of urine formation. J Insect Physiol 1992; 38:543-54; http://dx.doi.org/ 10.1016/0022-1910(92)90080-W [DOI] [Google Scholar]
- 45.Doring F, Wischmeyer E, Kuhnlein RP, Jackle H, Karschin A. Inwardly rectifying K+ (Kir) channels in Drosophila. A crucial role of cellular milieu factors Kir channel function. J Biol Chem 2002; 277:25554-61; PMID:11964404; http://dx.doi.org/ 10.1074/jbc.M202385200 [DOI] [PubMed] [Google Scholar]
- 46.Williams JC, Beyenbach KW. Differential effects of secretagogues on the electrophysiology of the Malpighian tubules of the yellow fever mosquito. J Comp Physiol ; B 1984; 154:301-9; http://dx.doi.org/ 10.1007/BF0246441125309022 [DOI] [Google Scholar]
- 47.Weng XH, Piermarini PM, Yamahiro A, Yu MJ, Aneshansley DJ, Beyenbach KW. Gap junctions in Malpighian tubules of Aedes aegypti. J Exp Biol 2008; 211:409-22; PMID:18203997; http://dx.doi.org/ 10.1242/jeb.011213 [DOI] [PubMed] [Google Scholar]
- 48.Hegarty JL, Zhang B, Pannabecker TL, Petzel DH, Baustian MD, Beyenbach KW. Dibutyryl cAMP activates bumetanide-sensitive electrolyte transport in Malpighian tubules. Am J Physiol Cell Physiol 1991; 261:C521-C9 [DOI] [PubMed] [Google Scholar]
- 49.Scott BN, Yu MJ, Lee LW, Beyenbach KW. Mechanisms of K+ transport across basolateral membranes of principal cells in Malpighian tubules of the yellow fever mosquito, Aedes aegypti. J Exp Biol 2004; 207:1655-63; PMID:15073198; http://dx.doi.org/ 10.1242/jeb.00932 [DOI] [PubMed] [Google Scholar]
- 50.Raphemot R, Rouhier MF, Swale DR, Days E, Weaver CD, Lovell KM, Konkel LC, Engers DW, Bollinger SR, Hopkins C, et al.. Discovery and characterization of a potent and selective inhibitor of Aedes aegypti inward rectifier potassium channels. PLoS One 2014; 9:e110772; PMID:25375326; http://dx.doi.org/ 10.1371/journal.pone.0110772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raphemot R, Lonergan DF, Nguyen TT, Utley T, Lewis LM, Kadakia R, Weaver CD, Gogliotti R, Hopkins C, Lindsley CW, et al.. Discovery, characterization, and structure-activity relationships of an inhibitor of inward rectifier potassium (Kir) channels with preference for Kir2.3, Kir3.x, and Kir7.1. Front Pharmacol 2011; 2:75; PMID:22275899; http://dx.doi.org/ 10.3389/fphar.2011.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raphemot R, Rouhier MF, Hopkins CR, Gogliotti RD, Lovell KM, Hine RM, Ghosalkar D, Longo A, Beyenbach KW, Denton JS, et al.. Eliciting renal failure in mosquitoes with a small-molecule inhibitor of inward-rectifying potassium channels. PLoS ONE 2013; 8:e64905; PMID:23734226; http://dx.doi.org/ 10.1371/journal.pone.0064905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouhier MF, Raphemot R, Denton JS, Piermarini PM. Pharmacological validation of an inward-rectifier potassium (Kir) channel as an insecticide target in the yellow fever mosquito Aedes aegypti. PLoS One 2014; 9:e100700; PMID:24959745; http://dx.doi.org/ 10.1371/journal.pone.0100700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rouhier MF, Hine RM, Park ST, Raphemot R, Denton J, Piermarini PM, Beyenbach KW. Excretion of NaCl and KCl loads in mosquitoes. 2. Effects of the small molecule Kir channel modulator VU573 and its inactive analog VU342. Am J Physiol Regul Integr Comp Physiol 2014; 307:R850-61; PMID:25056106; http://dx.doi.org/ 10.1152/ajpregu.00106.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haas M, Forbusch BI. The Na-K-Cl cotransporters. J Bioenerg Biomembr 1998; 30:161-72; PMID:9672238; http://dx.doi.org/ 10.1023/A:1020521308985 [DOI] [PubMed] [Google Scholar]
- 56.Lewis LM, Bhave G, Chauder BA, Banerjee S, Lornsen KA, Redha R, Fallen K, Lindsley CW, Weaver CD, Denton JS. High-throughput screening reveals a small-molecule inhibitor of the renal outer medullary potassium channel and Kir7.1. Mol Pharmacol 2009; 76:1094-103; PMID:19706730; http://dx.doi.org/ 10.1124/mol.109.059840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swale DR, Sheehan JH, Banerjee S, Husni AS, Nguyen TT, Meiler J, Denton JS. Computational and functional analyses of a small-molecule binding site in ROMK. Biophys J 2015; 108:1094-103; PMID:25762321; http://dx.doi.org/ 10.1016/j.bpj.2015.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Satmary WM, Bradley TJ. The distribution of cell types in the Malpighian tubules of Aedes taeniorhynchus Diptera Culicidae. Int J Insect Morphol Embryol 1984; 13:209-14; http://dx.doi.org/ 10.1016/0020-7322(84)90037-0 [DOI] [Google Scholar]
- 59.Bradley TJ, Stuart AM, Satir P. The ultrastructure of the larval malpighian tubules of a saline-water mosquito. Tissue Cell 1982; 14:759-73; PMID:7170712; http://dx.doi.org/ 10.1016/0040-8166(82)90064-7 [DOI] [PubMed] [Google Scholar]
- 60.Masia R, Aneshansley D, Nagel W, Nachman RJ, Beyenbach KW. Voltage clamping single cells in intact malpighian tubules of mosquitoes. Am J Physiol Renal Physiol 2000; 279:F747-54; PMID:10997925 [DOI] [PubMed] [Google Scholar]
- 61.Bakos E, Evers R, Sinko E, Varadi A, Borst P, Sarkadi B. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol Pharmacol 2000; 57:760-8; PMID:10727523 [DOI] [PubMed] [Google Scholar]
- 62.Szeri F, Ilias A, Pomozi V, Robinow S, Bakos E, Varadi A. The high turnover Drosophila multidrug resistance-associated protein shares the biochemical features of its human orthologues. Biochim Biophys Acta 2009; 1788:402-9; PMID:19059376; http://dx.doi.org/ 10.1016/j.bbamem.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 63.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 2006; 86:849-99; PMID:16816140; http://dx.doi.org/ 10.1152/physrev.00035.2005 [DOI] [PubMed] [Google Scholar]
- 64.Petzel DH, Pirotte PT, Van Kerkhove E. Intracellular and luminal pH measurements of Malpighian tubules of the mosquito Aedes aegypti: the effects of cAMP. J Insect Physiol 1999; 45:973-82; PMID:12770272; http://dx.doi.org/ 10.1016/S0022-1910(99)00076-1 [DOI] [PubMed] [Google Scholar]
- 65.Neufeld DS, Leader JP. Electrochemical characteristics of ion secretion in Malpighian tubules of the New Zealand alpine weta (Hemideina maori). J Insect Physiol 1998; 44:39-48; http://dx.doi.org/ 10.1016/S0022-1910(97)00087-5 [DOI] [PubMed] [Google Scholar]
- 66.Nicolson SW, Isaacson LC. Transepithelial and intracellular potentials in isolated Malpighian tubules of tenebrionid beetle. Am J Physiol 1987; 252:F645-53; PMID:3031999 [DOI] [PubMed] [Google Scholar]
- 67.Leyssens A, Van Kerkhove E, Zhang SL, Weltens R, Steels P. Measurement of intracellular and luminal K+ concentrations in a Malpighian tubule (Formica): Estimates of basal and luminal electrochemical gradients. J Insect Physiol 1993; 39:945-58; http://dx.doi.org/ 10.1016/0022-1910(93)90004-B [DOI] [Google Scholar]
- 68.Maddrell SH, Gee JD. Potassium-induced release of the diuretic hormones of Rhodnius prolixus and Glossina austeni: Ca dependence, time course and localization of neurohaemal areas. J Exp Biol 1974; 61:155-71; PMID:4153437 [DOI] [PubMed] [Google Scholar]
- 69.Maddrell SHP, O'Donnell MJ, Caffrey R. The regulation of haemolymph potassium activity during initiation and maintenance of diuresis in fed Rhodnius prolixus. Journal of Experimental Biology 1993; 177:273-85; PMID:8486999 [DOI] [PubMed] [Google Scholar]
- 70.Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol 2010; 55:351-74; PMID:19961332; http://dx.doi.org/ 10.1146/annurev-ento-112408-085512 [DOI] [PubMed] [Google Scholar]
- 71.Piermarini PM, Grogan LF, Lau K, Wang L, Beyenbach KW. A SLC4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): evidence for a novel role of stellate cells in diuretic fluid secretion. Am J Physiol Regul Integr Comp Physiol 2010; 298:R642-60; PMID:20042685; http://dx.doi.org/ 10.1152/ajpregu.00729.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Connor KR, Beyenbach KW. Chloride channels in apical membrane patches of stellate cells of Malpighian tubules of Aedes aegypti. J Exp Biol 2001; 204:367-78; PMID:11136622 [DOI] [PubMed] [Google Scholar]
- 73.Xiang MA, Linser PJ, Price DA, Harvey WR. Localization of two Na+- or K+-H+ antiporters, AgNHA1 and AgNHA2, in Anopheles gambiae larval Malpighian tubules and the functional expression of AgNHA2 in yeast. J Insect Physiol 2012; 58:570-9; PMID:22206887; http://dx.doi.org/ 10.1016/j.jinsphys.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 74.Piermarini PM, Hine RM, Schepel M, Miyauchi J, Beyenbach KW. Role of an apical K,Cl cotransporter in urine formation by renal tubules of the yellow fever mosquito (Aedes aegypti). Am J Physiol Regul Integr Comp Physiol 2011; 301:R1318-37; PMID:21813871; http://dx.doi.org/ 10.1152/ajpregu.00223.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beyenbach KW. Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci 2001; 16:145-51; PMID:11479361 [DOI] [PubMed] [Google Scholar]
- 76.Beyenbach KW, Pannabecker TL, Nagel W. Central role of the apical membrane H+-ATPase in electrogenesis and epithelial transport in Malpighian tubules. J Exp Biol 2000; 203:1459-68; PMID:10751161 [DOI] [PubMed] [Google Scholar]
- 77.Ramsay JA. Active transport of water by the Malpighian tubules of the stick insect, Dixippus morosus (Orthoptera; Phasmidae). J Exp Biol 1954; 31:104-13 [Google Scholar]
- 78.Gupta BL, Hall TA, Maddrell SHP, Moreton RB. Distribution of Ions in a Fluid Transporting Epithelium Determined by Electron Probe X-Ray Micro Analysis. Nature (London) 1976; 264:284-7; http://dx.doi.org/ 10.1038/264284a0 [DOI] [PubMed] [Google Scholar]
- 79.Williams JC, Beyenbach KW. Differential effects of secretagogues on Na and K secretion in the Malpighian tubules of Aedes aegypti (L.). J Comp Physiol 1983; 149:511-7; http://dx.doi.org/ 10.1007/BF00690010 [DOI] [Google Scholar]
- 80.Wu DS, Beyenbach KW. The dependence of electrical transport pathways in Malpighian tubules on ATP. J Exp Biol 2003; 206:233-43; PMID:12477894; http://dx.doi.org/ 10.1242/jeb.00066 [DOI] [PubMed] [Google Scholar]