Abstract
Direct recording of tight junction permeability is of pivotal importance to many biologic fields. Previous approaches bear an intrinsic disadvantage due to the difficulty of separating tight junction conductance from nearby membrane conductance. Here, we propose the design of Double whole-cell Voltage Clamp - Ion Conductance Microscopy (DVC-ICM) based on previously demonstrated potentiometric scanning of local conductive pathways. As proposed, DVC-ICM utilizes two coordinated whole-cell patch-clamps to neutralize the apical membrane current during potentiometric scanning, which in models described here will profoundly enhance the specificity of tight junction recording. Several potential pitfalls are considered, evaluated and addressed with alternative countermeasures.
Keywords: conductance, ion channel, patch clamp, SICM, tight junction
Abbreviations
- a
nanopore inner radius (eqn. 2-3)
- BE
balance electrode
- CE
counter electrode
- Cma
apical membrane capacitance
- Cmb
basolateral membrane capacitance
- CS
capacitance of patch clamp pipet
- d
nanopore diameter (Fig. 4B caption)
- Dps
probe-surface distance
- DVC-ICM
double whole-cell voltage clamp ion conductance microscopy
- E
electric field (eqn.1)
- Erest
resting membrane potential
- G
conductance (eqn.1)
- GND
electrical ground
- h
cylindrical nanopore length (Fig. 4B caption)
- I
current across Cmb1 (eqn. 5-6)
- i.d.
inner radius
- iBE
balance electrode current
- iGJ
gap junctional current
- ima
apical membrane current
- imb
basolateral membrane current
- ipara
paracellular current
- itrans
transcellular current
- JAMs
Junctional Adhesion Molecules
- MDCK
Madin-Darby canine kidney
- PE
pipet electrode
- P-SICM
potentiometric scanning ion conductance microscopy
- r
distance from the axis of symmetry (eqn.3)
- RC
resistor capacitor circuit or product of resistance and capacitance
- RE
reference electrode
- RGJ
gap junction resistance
- RL
leak resistance (or seal resistance)
- Rma
apical membrane resistance
- Rmb
basolateral membrane resistance
- Rpore
nanopore resistance (Fig. 4B caption)
- RS
series resistance
- RTE
transepithelial resistance
- RTJ
tight junction resistance
- S/N
signal-to-noise ratio
- SICM
scanning ion conductance microscopy
- TEER
transepithelial electrical resistance
- TJs
tight junctions
- UE
potential electrode
- V
command voltage at balance electrode
- V’
actual intracellular potential (holding potential)
- VTE
transepithelial potential
- WE
working electrode
- ΔV
potential deflection (eqn.1)
- z
normal distance from the pore (eqn.2-4)
- Δz
pipet vertical displacement (eqn. 1)
- ξ, η
rotational elliptic coordinates (eqn. 2-4)
- ρ
- Φ
potential at a certain position above nanopore (eqn. 4)
- Φ0
potential at the nanopore surface (eqn.4)
Figure 4.
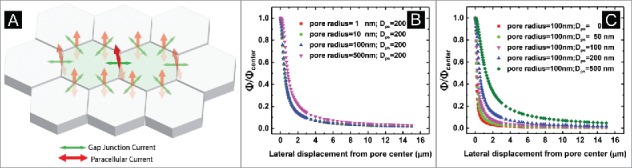
(A) Illustration of possible current flow between the two cells involved in DVC-ICM measurements (green cells in the middle) and their neighboring cells (gray cells in the periphery). Gap junctional currents flow within the epithelial cell layer (green arrow) and paracellular currents flow through tight junctions perpendicular to the cell layer (red arrow). (B) Lateral distribution of relative potential field (normalized to the potential amplitude over nanopore center) at 200 nm above a nanopore for nanopores with inner radius of 1, 10, 100 and 500 nm. The pore resistance (Rpore) of a cylindrical nanopore can be calculated by the following equation: , in which p represents the solution resistivity, d represents the pore diameter, and h represents the pore length. (C) Lateral distribution of relative potential field (same as in (B)) at different heights above a radius 100 nm nanopore (Dps = 0, 20, 100, 200, and 500 nm).
Tight Junctions
Tight junctions (TJs) form a continuous paracellular seal between adjacent epithelial cells and control solute transport between apical and basolateral fluids. In thin section electron microscopy, TJs appear as series of direct membrane contacts, where membranes from adjacent cells fuse together,1 and freeze-fracture electron microscopy has further revealed TJs to exist as extended protein strands that form transmembrane networks.2 Known integral membrane proteins of the tight junction include occludin,3 Junctional Adhesion Molecules (JAMs),4 and claudins.5 Claudins have been shown to confer ion selectivity to tight junctions. For instance, claudin-4, -5, -8, -11 and -14 selectively decrease cation permeability,6-10 whereas claudin-15 and -17 are anion selective.11,12 Permeability studies suggest paracellular channels 4-7 Å in diameter are formed from claudins at tight junction contacts and are responsible for the selectivity in ion transport.13,14 The first crystal structure of claudin has recently been published,15 which primes the field to study the structure and function relationship of tight junction. The present study offers a new approach for assessing the conductive properties of tight junction with unprecedented resolution.
Measurement of Tight Junction Conductance
Tight junction permeability is often experimentally assessed by electrical measurements of transepithelial ion transport. For instance, in the classic Ussing chamber,16-20 the transepithelial electrical resistance (TEER) is measured to determine the transepithelial resistance (RTE, inverse of epithelial conductance) of tissue slices and the dilution potential is recorded to determine the ion selectivity of the conductive pathways. This approach can also be translated to cells cultured on polymer membranes, where hundreds to thousands of cells can be measured in a single TEER measurement, with RTE values recorded that are typically in the range of 10-1000s of Ω∙cm2. Although useful, TEER measurements represent the aggregate response of all ion-conductive pathways, which include transcellular ion channels as well as paracellular channels. Approaches to separate the two conductivity pathways (transcellular, paracellular) are difficult to achieve experimentally. Two-path impedance spectroscopy21 has been perhaps most successful in distinguishing between paracellular and transcellular conductances, but still lacks local information for studying heterogeneously conductive epithelium. In particular, delineation of paracellular conductance from an epithelium proves especially challenging. If we consider patch-clamp techniques22 as the “gold standard” to measure ion transport of protein channels in the transcellular pathway, no comparable approach exists to study components of paracellular conductance. Patch–clamp approaches appear unsuitable for direct measurement of tight junctions because of the inability to form gigaohm seals between pipet and the cell perimeter where the tight junction resides.
Frömter and co-workers pioneered a little used, but particularly intriguing method to separate paracellular and transcellular conductances through voltage scanning (or conductance scanning) technique, in which local voltage deflections over individual cell junctions are measured.23,24 Voltage scanning makes use of a pair of microelectrodes that are positioned in space above a cell or tissue monolayer. With application of a pulsed current across the epithelial cell layer, the microelectrodes can be used to record spatially resolved conductance. However, limitations of this technique exist due to lack of accurate electrode position control and low signal-to-noise ratios (S/N), making voltage scanning only applicable to very flat epithelia and cases where tight junction conductance differs significantly from the transcellular conductance. We have adapted the general conductance scanning concepts of Frömter into scanning ion conductance microscopy (SICM)25 with a goal of improving resolution through better probe control and experimental flexibility. SICM utilizes an ion conductance feedback mechanism to scan an electrolyte filled nanopipet over a surface. SICM provides relatively high-resolution surface topography (<100 nm), which is then used to position the nanopipet probe accurately over cell-cell junctions. Additionally, the robust feedback control of SICM allows precise control of probe-cell surface distance (at the nanometer scale) during measurement and not only flat but also rough epithelial surfaces to be studied. To record tight junction conductance with SICM, the nanopipet is positioned over a tight junction and local ion current is recorded as a transepithelial potential is applied across the epithelium. To compensate for limits in S/N in the current-based measurement due to the high resistance of the probe, we introduce an additional electrode that measures the potential at the pipet tip, in an SICM mode we termed potentiometric scanning ion conductance microscopy (P-SICM).26
Potentiometric Scanning Ion Conductance Microscopy (P-SICM)
P-SICM utilizes a dual barrel nanopipet to scan and measure epithelial cell layers grown on a filter mounted between 2 chambers of a perfusion well (Fig. 1). The tip diameter of each barrel of the pipet typically ranges from 10 - 100 nanometers, as determined with scanning electron micrographs. The pipet electrode (PE, biased at 100 mV vs. reference electrode (RE)) present in one barrel monitors the probe-surface distance (Dps) dependent ion current to control probe position. A potential electrode (UE) in the second barrel measures the local potential deflection (also with respect to RE). A platinum counter electrode (CE) drives the majority of transepithelial current to avoid potential fluctuations of RE when a transepithelial potential (VTE) is applied at the working electrode (WE) placed in the bottom chamber. VTE drives ion current through cell-cell junctions as well as membrane channels and results in a potential gradient in the vicinity of these ion conductive pathways. Local potential variations captured by UE can then be used to determine apparent local conductance (G) values from Equation 1.27,28
| (1) |
Figure 1.
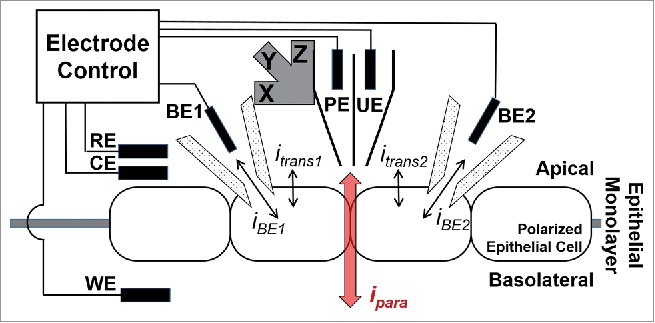
Illustration of the DVC-ICM setup. Double barrel pipet for topographical mapping (PE) and local potential measurement (UE) is attached to a piezo positioner. Reference electrode (RE), counter electrode (CE) and working electrode (WE) are controlled with home-built electrode controller to apply transmembrane potential across the epithelial monolayer. Two balance electrodes (BE1 and BE2) are used to control the intracellular potentials of 2 patched cells and to record the current flowing from or to BE1 or BE2 (iBE1 or iBE2) respectively. Upon the application of transmembrane potential, ion current flows across the epithelial cell layer through both paracellular pathways (ipara) and transcellular pathways (itrans1 and itrans2), and both itrans are undesired background signal for the measurement of paracellular conductance.
In this equation, E represents the electric field (potential gradient) induced by transepithelial potential VTE and ρ is the specific resistance of the bath electrolyte. In P-SICM measurements, UE records local potentials at two Dps (typically 0.2 µm and 12.5 µm above the cell junction or cell body), which are then used to calculate the electric field, knowing the pipet vertical displacement (Δz = 0.2 µm to 12.5 µm). To avoid polarization of the cell plasma membrane, an alternating transepithelial potential (VTE) is utilized, at a frequency (typically 1 Hz) selected to minimize capacitive contributions. The magnitude of VTE is usually held to less than 50 mV to avoid cell damage and disruption of the cell membrane.29,30
We have previously shown that P-SICM results in significantly enhanced S/N of approximately an order of magnitude for potential measurement (compared to current measurement).26,31 We have also demonstrated P-SICM as a sensitive tool to distinguish small differences in paracellular and transcellular conductance in cell monolayers and proposed that P-SICM can be utilized in leaky epithelia that cannot be measured by other techniques.
Double Whole-cell Voltage Clamp Ion Conductance Microscopy (DVC-ICM)
Despite advantages in tight junction recordings with P-SICM, the multitude of competing conductance pathways and the use of a nonzero probe-surface distance (Dps) may result in systematic errors that arise from transcellular conductance artifacts. To be specific, in the P-SICM configuration, the application of transepithelial potential (VTE) drives ions to move across the cell layer through both paracellular (cell junctions) and transcellular (membrane ion channels) pathways. The resultant currents are indicated as ipara and itrans in Figure 1. An unknown fraction of itrans (mainly from the two cells forming the cell junction under study) will inevitably be picked up by the pipet (or from the potential gradient point of view, itrans can interfere with local gradients at cell-cell junctions), especially at a Dps of ∼12.5 µm, where background conductance is measured (Equation 1). To more accurately record tight junction conductance, the background signal - itrans from neighboring cells, has to be subtracted either through pharmacologic inhibition or mathematical deduction. However, pharmacologic inhibition would likely affect natural physiological processes, while the exact contribution from transcellular transport within neighboring cells of the measured tight junction needs to be known for the mathematical deduction approach, which turns out to be very difficult.
Here, we propose a new method to electrically offset contributions of itrans from neighboring cells by coupling double whole-cell patch-clamps (commonly referred to as a double whole-cell patch-clamp)32,33 with P-SICM measurements. The general idea of this method is to “zero out” the driving force for itrans (due to the application of VTE) by controlling the intracellular potential (membrane potential) of cells on either side of the tight junction with whole-cell patch-clamps. One reasonable assumption of this method is that itrans from the two adjoining cells making the tight junction contribute to the major background signal in the previous P-SICM measurements at cell-cell junction compared to other cells that are further away from the junction (see detailed discussion below in “practical concerns” section).
Instrumentation
Figure 1 illustrates adaptation of the P-SICM instrumentation we have previously developed26,31 to incorporate the double whole-cell patch-clamp. As in the previous P-SICM configuration, a double barrel pipet controls the probe position through the pipet electrode (PE) and measures local potential deflection at the potential electrode (UE). VTE is applied to working electrode (WE) located on the basolateral side of the cell layer and a counter electrode (CE) close to the reference electrode (RE) drives the majority of the bulk ion current. The major instrumental advance is the addition of two micropipets that are patched to the two adjacent cells forming the cell junction being measured with P-SICM. We term these patched electrodes as “balance electrodes” (BEs). Each BE is controlled with a patch-clamp amplifier operating in voltage-clamp mode. During measurement, BE holds the intracellular potential at the resting membrane potential (Erest) by providing a counter current (iBE) through BE to eliminate the changes in intracellular potential due to the application of VTE.
Equivalent circuit analysis
The origin of itrans is the VTE driven ion movement, which disrupts the natural resting membrane potential, which maintains a net itrans of zero (although cellular processes generate a nonzero flux of specific ions). Therefore, the effect of VTE on cell membranes must be “zeroed out” to eliminate the itrans signal. To achieve this, DVC-ICM will clamp the membrane potential at Erest value during the application of VTE.
Figure 2A shows a proposed equivalent electric circuit for the DVC-ICM. Cell 1 and cell 2 are two adjoining cells that form a tight junction. At the cell junction, paracellular ion transport that occurs perpendicular to the epithelial cell layer is mediated through the tight junction, and the resistance of the TJ is represented as RTJ. Gap junctions are also located in the cell junction and provide the primary path for ion transport between 2 cells. The resistance of the gap junction is RGJ. For the cells, resistance of the apical membrane is indicated with Rmaj (j=1 or 2, refers to cell 1 and cell 2 respectively) and resistance of the basolateral membrane is indicated by Rmbj (j=1 or 2). For each patch pipet, a series resistance RSj (j=1 or 2), which consists of the resistances of BE and the broken membrane patch, exists. Depending on the pipet geometry and electrolyte conductivity, RS is typically on the order of 1-10 MΩ and draws a non-negligible voltage drop when current is passed through the patch pipet. The voltage drop across RS causes Vj’ (j=1 or 2), the actual intracellular potential or so-called holding potential, to differ from the command voltage at BE (V1 and V2 in Figure 2A). Current flowing through each resistor is labeled with the same subscript as shown in Figure 2B.
Figure 2.
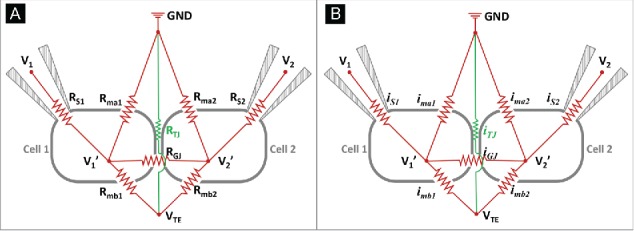
(A) Equivalent circuit of the DVC-ICM system. Here, tight junction conductance (reciprocal of tight junction resistance, RTJ) of the junction formed by cell 1 and cell 2 is being measured with P-SICM (electrodes not shown). Cell 1 and cell 2 are voltage-clamped with two patch clamp pipets controlled with an individual patch clamp amplifier respectively. The intracellular potential of cell 1 (cell 2) is held at V1’ through BE1 (BE2), where the holding voltage by patch clamp amplifier is V1 (V2). Series resistance RS1 (RS2) consists the resistances of BE and broken membrane patch from cell 1 (cell 2). Apical membrane resistance and basolateral membrane resistance are indicated separately with Rma1 and Rmb1 for cell 1 and Rma2 and Rmb2 for cell 2. RGJ refers to the gap junction resistance through which ion current can pass between cell 1 and 2. (B) Ion current passing each resistor in (A) is labeled with the same subscript.
To simplify the circuit, RGJ is omitted from the discussion, because the intracellular potential of cell 1 is considered to be equal to that of cell 2 in a uniform epithelial cell layer. Furthermore, the effects on the intracellular potentials of both cells from VTE should be equivalent if 2 cells are considered “electrically” the same (gap junctional reversal potential is not considered here). Therefore iGJ = 0 always holds, which means RGJ can be considered an open circuit, and can be neglected in further circuit analysis.
With no external driving force - VTE, the balance electrode potentials at patch pipets (V1 and V2) and the intracellular potentials remain at resting membrane potential value (Erest, ∼ −70 mV) and the net ion currents across cell membranes (ima1, ima2, imb1, imb2) are zero. In this circuit, with the application of VTE, ion movement will take place either through the paracellular pathway (RTJ) or the transcellular pathway (Rmb1+ Rma1, and Rmb2+ Rma2). The value of V1′ (or V2′) will deviate from Erest due to the non-zero imb1 (or imb2) driven by VTE, and result in transmembrane current at the apical membrane side (ima1 and ima2). This is the case for previous conductance scanning and P-SICM measurements. In the DVC-ICM configuration, the two patch pipets inject compensating currents through the BEs to clamp V1′ and V2′ at Erest in the voltage clamp feedback loop of the patch clamp amplifiers. Since V1′ = V2′ = Erest, the driving force for ima1 and ima2 due to the application of VTE is eliminated and hence ima1 = ima2 = 0. Under this condition, the P-SICM probe positioned over this “electrically isolated” cell-cell junction will only measure the signal generated from the paracellular conductive pathway. Another way of analyzing current flow in the circuit is shown in Figure 3. The two BEs that provide compensation current can be viewed as much less resistive pathways in the circuit, which serves to shunt the majority of current flow (compared to the apical membranes) coming from the basolateral membrane.
Figure 3.
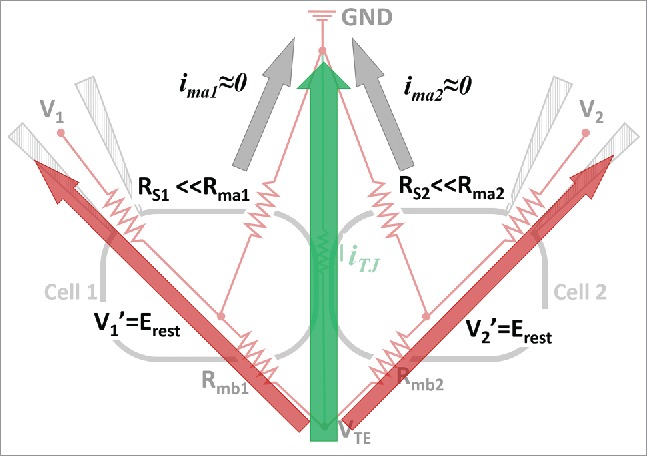
Intracellular potentials (V1’ and V2’) for both cell 1 and cell 2 are held at their Erest to yield zero transmembrane current at the apical membranes (ima1 and ima2) during the application of transmembrane potential VTE. Majority of VTE induced basolateral membrane current (imb1 and imb2) is drawn through BE1 and BE2 (red arrows). Note: RGJ is omitted from this circuit because V1’ and V2’ are always kept the same and there is no current through RGJ.
Practical Concerns
Effects from neighboring cell-cell junctions
In the equivalent circuit shown in Figure 2A, only the resistance of the measured cell junction and the membrane resistance for 2 cells (cell 1 and cell 2) involved in forming this junction are considered. In a confluent epithelial cell layer, neighboring cells that are coupled to cell 1 and cell 2 by gap junctions will inevitably induce current flow to or from these 2 cells (Fig. 4A, green arrows). Additionally, the paracellular current at cell-cell junctions formed by cell 1 (or cell 2) with the neighboring cells (Fig. 4A, red arrows) may also be picked up by the P-SICM UE and hence contribute to background noise and unwanted signal for P-SICM measurements. Influence from these 2 effects is discussed here.
During P-SICM measurement, when a transepithelial potential (VTE) is applied to the working electrode (WE, placed in the bottom chamber of the perfusion well) the intracellular potential for all cells in the epithelial layer will be altered by VTE. In the proposed DVC-ICM model, only the two cells forming the junction under study are voltage-clamped at Erest, which results in a difference in intracellular potential between either of the clamped cells (central green cells in Figure 4A, V1′ or V2′ in Figure 2A) and their closest neighbors (gray cells in the periphery in Figure 4A). Such intracellular potential differences may induce current flow through gap junctions (indicated with light green arrows around the 2 central cells in Figure 4A), which may interrupt V1′ or V2′ in a manner similar to imb1 or imb2 discussed above. Nevertheless, these effects will be detected and compensated by the patch clamp amplifiers connected to the BEs. In other words, the BEs will eventually offset additional gap junctional current flow between cell 1 and cell 2, as V1′ and V2′ are always held at Erest. Therefore, the gap junction current through neighboring cells will not contribute to itrans in the proposed DVC-ICM model. The junctional reversal potential due to any type of asymmetry (e.g., voltage gating property of connexins) is not considered here but may also be eliminated by clamping V1′=V2′ at Erest.
Another possible effect arises from the paracellular ion current at tight junctions formed by either cell 1 or cell 2 with other cells adjacent to them. These paracellular currents are indicated with light red arrows (vs. the dark red arrow at the cell 1-cell 2 junction) oriented perpendicular to the epithelial layer in Figure 4A. Although the paracellular channel density may vary at different cell-cell junctions to result in intrinsic variation of the measured conductance, the signals from neighboring junctions would have to be estimated and minimized to make the recording as accurate and localized as possible. First, UE is held over the center of a bicellular cell junction ("vs". the edge or corner of junctions) where the lateral distance between UE and the closest neighboring cell junctions is the largest (e.g., ∼5-10 µm for epithelial cells such as MDCK). To estimate the relative potential field strength 5-10 µm away from versus at the field center (in this case, the tight junction), a previous mathematical model for calculation of potential distribution above a disk electrode is used.34 Here, the tight junction channel at one individual cell-cell junction is treated as ideal nanopore geometry for simplicity. In this model, rotational elliptic coordinates , and (nanopore inner radius) are used to express (the normal distance from the pore) and (distance from the axis of symmetry) as shown in Equations (2-3).
| (2) |
| (3) |
The potential at a certain position can be determined as a function of (Equation 4, is the potential at the nanopore surface).
| (4) |
Figure 4B shows the relative potential distribution (normalized to the potential value above nanopore center) at Dps of 200 nm above the surface for different sized nanopores (pore radii are 1, 10, 100, 500 nm). Generally, the shape of the curve is not influenced by nanopore radius and the potential drops rapidly (by ∼90%) through distances within 2 µm from the nanopore center. At 5-10 µm from the pore center, the potential gradient changes very little and is approximately equal to the baseline value for the potential measured at a farther away distance (Dps = 12.5 µm, simulation results not shown). Thus, at scales and potentials described here, we expect that contributions from nearby cell-cell junctions can be negligible. Controlling Dps provides an additional route to minimize the signal from neighboring cell-cell junctions. As shown in Figure 4C, as Dps decreases, the potential gradient above a 100 nm radius pore becomes sharper and results in a much more localized potential field.
Voltage-Gated Membrane Channels
Voltage-gated membrane channels are transmembrane ion channels that allow ion transport in response to changes in the membrane potential.35-37 In P-SICM, VTE is typically swept from -50 mV to +50 mV at 1 Hz. From previous studies, this potential range (at least at +50 mV) is sufficient to excite many types of voltage-gated channels, which will also contribute to itrans. Because 1 Hz (the frequency VTE is swept at) is much slower than the frequency needed to activate voltage-gated channels,38 clamping the cell intracellular potentials at resting membrane potential (Erest) can effectively avoid activation of voltage-gated membrane channels in the apical membrane and hence minimize the itrans from these channels.
How Effectively can V1′ and V2′ be Clamped?
The equivalent electric circuit in Figure 2A includes key resistance components in the DVC-ICM system, other resistors and capacitors are added in Figure 5 and need to be considered to evaluate the accuracy and response speed in clamping the intracellular potentials of the two cells (V1′ and V2′).
Figure 5.
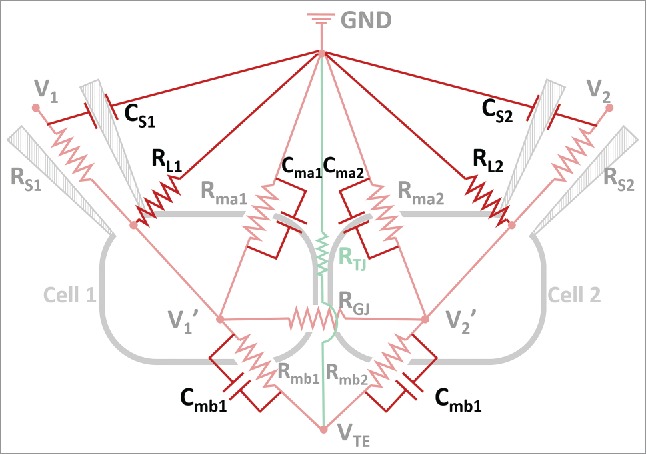
A more complete equivalent circuit of DVC-ICM system. CS1 and CS2 represent the capacitance of the patch clamp pipets glass wall. RL1 and RL2 are the “gigaohm seal” resistances at the sealed spaces between patch pipets and cell membranes. The cell membrane capacitances are indicated with Cma1, Cma2 (for apical membrane) and Cmb1, Cmb2 (for basolateral membrane).
First, the voltage drop across RS1 (or RS2) due to current flow through BE causes a difference between V1 (or V2), the amplifier command voltage, and V1′ (or V2′), the actual intracellular potential. This effect becomes more significant when RS1 (or RS2) is larger and cell membrane resistance becomes smaller. For instance, typical values for RS1 (or RS2) are on the order of 1-10 MΩ, and the cell membrane resistance can range from GΩ39 to tens of MΩ,40-42 dependent on the cell type. One way to accurately hold V1′ (or V2′) at Erest is to set the value of V1 (or V2) as the sum of Erest and the voltage drop across BE. To do this, the value of RS1 (or RS2) and the BE current need to be known. Ion current at each BE is constantly monitored under V-clamp mode by the patch clamp amplifiers, while RS1 (or RS2) can be obtained by application of a test pulse of known amplitude between BE and RE (GND in Figure 5) and measurement of the corresponding current before and after formation of the gigaohm seal.
Second, in principle, the resistance of the sealed space (between pipet tip and cell membrane) should be infinite to guarantee no leakage current from the seal. In practice, however, the seal resistance (or leak resistance as RL1 and RL2 in Figure 5) is on the order of GΩ or hundreds of MΩ for a poor seal. RL1 and RL2 are in parallel with cell membrane resistances as shown in circuit in Figure 5. For highly resistive cell membranes, the seal resistances become comparable to membrane resistances and may need to be considered when setting the holding potential values. RL1 and RL2 can be measured in a manner similar to that described for RS1 and RS2 through the application of a test pulse.
Third, the RC response of the system, or the speed at which potentials are built up in the equivalent circuit should be evaluated. For example in cell 1, the changes in VTE induce changes in V1′, which is clamped by V1 through the patch pipet RS1. As shown in Figure 5, circuit components involved in this current path include V1, RS1, V1′, Rmb1, Cmb1 and VTE (capacitances for Ag/AgCl BEs are omitted because capacitive charging current is negligible for Ag/AgCl electrode). The response speed of the abovementioned current path is dominated by the RC circuit formed by RS1 and Cmb1. Rmb1 is not included in the estimation of response speed because Rmb1 is a purely resistive path for ion current flow and hence the time-dependent current across basolateral membrane due to the sweeping of VTE passes through Cmb1 instead of Rmb1. Consider RS1 = 10 MΩ and Cmb1 = 30 pF, the RC time constant can be calculated by the product of RS1 and Cmb1, which yields 0.3 ms. VTE is swept at 1 Hz in P-SICM measurement and as such is much slower than the response speed of this RC circuit.
An alternative way to estimate the rate-dependent effect exerted on V1′ by sweeping VTE is to calculate the potential change on V1′. Initially before any potential is applied to WE, V1 and V1′ are held at Erest. As VTE starts to change, a time-dependent current passes through Cmb1 and this current (I) can be calculated from the fundamental description of a capacitor and Equation 5 below.
| (5) |
In Equation 5, the product of the capacitance and voltage difference across a capacitor equals the charge on the capacitor. In this case, changes in VTE () during time induce the voltage difference across Cmb1 from the initial state, with representing current through Cmb1. Rearrangement of Equation 5 yields:
| (6) |
where represents the sweeping rate of VTE. As described previously, VTE is swept from −50 mV to +50 mV at 1 Hz and therefore
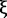 . The current
. The current 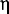 is calculated to be 6 pA according to Equation 6 if 30 pF is used for Cmb1. The current
is calculated to be 6 pA according to Equation 6 if 30 pF is used for Cmb1. The current  then flows through RS1 to the amplifier and V1 = V1′ no longer holds. If RS1 = 10 MΩ, a 60 µV () voltage difference is induced by VTE through the RC circuit, which is small enough to be compensated by patch clamp amplifier. One thing worth mentioning is that, the patch clamp amplifier response speed in controlling V1 is not discussed because the frequency of VTE is much smaller than the upper limit of signal frequency (tens to hundreds of kHz) accessible by most patch clamp amplifiers.
then flows through RS1 to the amplifier and V1 = V1′ no longer holds. If RS1 = 10 MΩ, a 60 µV () voltage difference is induced by VTE through the RC circuit, which is small enough to be compensated by patch clamp amplifier. One thing worth mentioning is that, the patch clamp amplifier response speed in controlling V1 is not discussed because the frequency of VTE is much smaller than the upper limit of signal frequency (tens to hundreds of kHz) accessible by most patch clamp amplifiers.
Conclusion
We have described an approach to isolate and measure paracellular ionic permeability, with minimal contributions from transcellular pathways. The approach is based on previous developments in potentiometric scanning ion conductance microscopy and voltage clamp methods. Ultimately, we expect the approach described here will lead to definitive measurements of ion permeation through tight junctions, especially at nanometer scales, and with the potential of identifying discrete conductance events of the paracellular ion channels. This new approach will allow revealing previously unknown aspects of the tight junction in physiological and pathophysiological processes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We acknowledge Andy Alexander of Indiana University EIS for enlightening discussions.
Funding
This work is supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases - RO1DK084059 and Department of Defense - HDTRA1-11-16-BRCWMD-BAA.
References
- 1.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17, 375-412; http://dx.doi.org/ 10.1083/jcb.17.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodenough DA, Revel JP. A. Fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol 1970; 45:272-90; PMID:4105112; http://dx.doi.org/ 10.1083/jcb.45.2.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123:1777-88; PMID:8276896; http://dx.doi.org/ 10.1083/jcb.123.6.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci 2004; 117:19-29; PMID:14657270; http://dx.doi.org/ 10.1242/jcs.00930 [DOI] [PubMed] [Google Scholar]
- 5.Furuse M, Fujita K, Hiiragi T, Fujimoto K, TsuKita S. Claudin-1 and-2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141:1539-50; PMID:9647647; http://dx.doi.org/ 10.1083/jcb.141.7.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alan S, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 2003; 278:17350-9; PMID:12615928; http://dx.doi.org/ 10.1074/jbc.M213286200 [DOI] [PubMed] [Google Scholar]
- 7.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet 2003; 12:2049-61; PMID:12913076; http://dx.doi.org/ 10.1093/hmg/ddg210 [DOI] [PubMed] [Google Scholar]
- 8.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol.: Cell Physiol 2002; 283:C142-7; PMID:12055082; http://dx.doi.org/ 10.1152/ajpcell.00038.2002 [DOI] [PubMed] [Google Scholar]
- 9.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 2001; 107:1319; PMID:11375422; http://dx.doi.org/ 10.1172/JCI12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen H, Watry DD, Marcondes MCG, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol 2004; 24:8408-17; PMID:15367662; http://dx.doi.org/ 10.1128/MCB.24.19.8408-8417.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 2003; 285:F1078-1084; PMID:13129853; http://dx.doi.org/ 10.1152/ajprenal.00116.2003 [DOI] [PubMed] [Google Scholar]
- 12.Conrad MP, Piontek J, Gunzel D, Fromm M, Krug SM. Molecular basis of claudin-17 anion selectivity. Cell Mol Life Sci 2015; PMID:26194246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J 2003; 84:1660-73; PMID:12609869; http://dx.doi.org/ 10.1016/S0006-3495(03)74975-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 2008; 121:298-305; PMID:18198187; http://dx.doi.org/ 10.1242/jcs.021485 [DOI] [PubMed] [Google Scholar]
- 15.Saitoh Y, Suzuki H, Tani K, Nishikawa K, Irie K, Ogura Y, Tamura A, Tsukita S, Fujiyoshi Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015; 347:775-8; PMID:25678664; http://dx.doi.org/ 10.1126/science.1261833 [DOI] [PubMed] [Google Scholar]
- 16.Madara JL. Increases in guinea pig small intestinal transepithelial resistance induced by osmotic loads are accompanied by rapid alterations in absorptive-cell tight-junction structure. J Cell Biol 1983; 97:125-36; PMID:6863387; http://dx.doi.org/ 10.1083/jcb.97.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappenheimer J. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J Membr Biol 1987; 100:137-48; PMID:3430570; http://dx.doi.org/ 10.1007/BF02209146 [DOI] [PubMed] [Google Scholar]
- 18.Ussing HH, Zerahn K. Active Transport of Sodium as the Source of Electric Current in the Short‐circuited Isolated Frog Skin. Acta Physiol Scand 1951; 23:110-27; PMID:14868510; http://dx.doi.org/ 10.1111/j.1748-1716.1951.tb00800.x [DOI] [PubMed] [Google Scholar]
- 19.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 2001; 107:1319-27; PMID:11375422; http://dx.doi.org/ 10.1172/JCI12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 2005; 118:5109-18; PMID:16234325; http://dx.doi.org/ 10.1242/jcs.02631 [DOI] [PubMed] [Google Scholar]
- 21.Krug SM, Fromm M, Günzel D. Two-Path Impedance Spectroscopy for Measuring Paracellular and Transcellular Epithelial Resistance. Biophys. J 2009; 97:2202-11; PMID:19843452; http://dx.doi.org/ 10.1016/j.bpj.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neher E, Sakmann B, Steinbach J. The extracellular patch clamp: A method for resolving currents through individual open channels in biological membranes. Pflügers Arch 1978; 375:219-28; PMID:567789; http://dx.doi.org/ 10.1007/BF00584247 [DOI] [PubMed] [Google Scholar]
- 23.Fromter E, Diamond J. Route of passive ion permeation in epithelia. Nat New Biol 1972; 235:13; http://dx.doi.org/ 10.1038/newbio235009a0 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JT Jr, Cesaro L, Gebler B, Frömter E. Electrical properties of Amphibian urinary bladder epithelia. Pflügers Arch 1975; 358:41-56; PMID:808794; http://dx.doi.org/ 10.1007/BF00584568 [DOI] [PubMed] [Google Scholar]
- 25.Hansma P, Drake B, Marti O, Gould S, Prater C. The scanning ion-conductance microscope. Science 1989; 243:641-3; PMID:2464851; http://dx.doi.org/ 10.1126/science.2464851 [DOI] [PubMed] [Google Scholar]
- 26.Chen CC, Zhou Y, Morris CA, Hou J, Baker LA. Scanning ion conductance microscopy measurement of paracellular channel conductance in tight junctions. Anal Chem 2013; 85:3621-8; PMID:23421780; http://dx.doi.org/ 10.1021/ac303441n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cereijido M, Stefani E, Palomo AM. Occluding junctions in a cultured transporting epithelium: Structural and functional heterogeneity. J Membr Biol 1980; 53, 19-32; PMID:7373646; http://dx.doi.org/ 10.1007/BF01871169 [DOI] [PubMed] [Google Scholar]
- 28.Gitter AH, Bertog M, Schulzke JD, Fromm M.. Measurement of paracellular epithelial conductivity by conductance scanning. Pflügers Arch - Eur J Physiol 1997; 434:830-40; http://dx.doi.org/ 10.1007/s004240050472 [DOI] [PubMed] [Google Scholar]
- 29.Bindslev N, Tormey JM, Wright EM. The effects of electrical and osmotic gradients on lateral intercellular spaces and membrane conductance in a low resistance epithelium. J Membr Biol 1974; 19:357-80; PMID:4549221; http://dx.doi.org/ 10.1007/BF01869986 [DOI] [PubMed] [Google Scholar]
- 30.Reuss L. Electrical properties of the cellular transepithelial pathway inNecturus gallbladder: III. Ionic permeability of the basolateral cell membrane. J Membr Biol 1979; 47:239-59; PMID:480334; http://dx.doi.org/ 10.1007/BF01869080 [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Chen CC, Weber AE, Zhou L, Baker LA. Potentiometric-scanning ion conductance microscopy. Langmuir 2014; 30:5669-75; PMID:24773406; http://dx.doi.org/ 10.1021/la500911w [DOI] [PubMed] [Google Scholar]
- 32.Churchill D, Caveney S. Double whole-cell patch-clamp characterization of gap junctional channels in isolated insect epidermal cell pairs. J Membr Biol 1993; 135:165-80; PMID:8411137; http://dx.doi.org/ 10.1007/BF00231442 [DOI] [PubMed] [Google Scholar]
- 33.Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature 1985; 317:331-5; PMID:2413362; http://dx.doi.org/ 10.1038/317331a0 [DOI] [PubMed] [Google Scholar]
- 34.Newman J. Resistance for Flow of Current to a Disk. J Electrochem Soc 1966; 113:501-2; http://dx.doi.org/ 10.1149/1.2424003 [DOI] [Google Scholar]
- 35.Hodgkin AL, Huxley AF. Action potentials recorded from inside a nerve fibre. Nature 1939; 144:710-1; http://dx.doi.org/ 10.1038/144710a0 [DOI] [Google Scholar]
- 36.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 1952; 117:500-44; PMID:12991237; http://dx.doi.org/ 10.1113/jphysiol.1952.sp004764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol 1949; 108:37-77; PMID:18128147; http://dx.doi.org/ 10.1113/jphysiol.1949.sp004310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keynes R. The kinetics of voltage-gated ion channels. Q Rev Biophys 1994; 27:339-434; PMID:7540309; http://dx.doi.org/ 10.1017/S0033583500003097 [DOI] [PubMed] [Google Scholar]
- 39.Rook M, Jongsma H, Van Ginneken A. Properties of single gap junctional channels between isolated neonatal rat heart cells. Am J Physiol: Heart Circ Physiol 1988; 255:H770-82 [DOI] [PubMed] [Google Scholar]
- 40.Joyner RW, Sugiura H, Tan RC. Unidirectional block between isolated rabbit ventricular cells coupled by a variable resistance. Biophys J 1991; 60:1038; PMID:1760503; http://dx.doi.org/ 10.1016/S0006-3495(91)82141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kameyama M. Electrical coupling between ventricular paired cells isolated from guinea‐pig heart. J Physiol 1983; 336:345-57; PMID:6875911; http://dx.doi.org/ 10.1113/jphysiol.1983.sp014585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar R, Wilders R, Joyner RW, Jongsma HJ, Verheijck EE, Golod DA, van Ginneken AC, Goolsby WN. Experimental model for an ectopic focus coupled to ventricular cells. Circulation 1996; 94:833-41; PMID:8772708; http://dx.doi.org/ 10.1161/01.CIR.94.4.833 [DOI] [PubMed] [Google Scholar]


