Abstract
Despite the importance of individual problem solvers for group- and individual-level fitness, the correlates of individual problem-solving success are still an open topic of investigation. In addition to demographic factors, such as age or sex, certain personality dimensions have also been revealed as reliable correlates of problem-solving by animals. Such correlates, however, have been little-studied in chimpanzees. To empirically test the influence of age, sex, estrous state, and different personality factors on chimpanzee problem-solving, we individually tested 36 captive chimpanzees with two novel foraging puzzles. We included both female (N = 24) and male (N = 12) adult chimpanzees (aged 14–47 years) in our sample. We also controlled for the females’ estrous state—a potential influence on cognitive reasoning—by testing cycling females both when their sexual swelling was maximally tumescent (associated with the luteinizing hormone surge of a female’s estrous cycle) and again when it was detumescent. Although we found no correlation between the chimpanzees’ success with either puzzle and their age or sex, the chimpanzees’ personality ratings did correlate with responses to the novel foraging puzzles. Specifically, male chimpanzees that were rated highly on the factors Methodical, Openness (to experience), and Dominance spent longer interacting with the puzzles. There was also a positive relationship between the latency of females to begin interacting with the two tasks and their rating on the factor Reactivity/Undependability. No other significant correlations were found, but we report tentative evidence for increased problem-solving success by the females when they had detumescent estrous swellings.
Keywords: Pan troglodytes, Chimpanzees, Personality, Problem-solving, Enrichment, Sexual swelling, Estrous state
Introduction
At the group level, innovation has been defined as ‘a process that results in new or modified learned behavior and that introduces novel behavioral variants into a population’s repertoire’ (Reader and Laland 2003, p. 14) and such innovation cannot arise without individual problem solvers (Ramsey et al. 2007). The importance of problem solvers is undisputed as they enable the emergence of new behavior patterns, and potentially cultures, fuelling investigations of animal innovation both in captivity (e.g., Kendal et al. 2005) and the wild (e.g., Lefebvre et al. 2004; Sol et al. 2005). Empirical research suggests that those animals that cannot outcompete others are more likely to develop novel solutions to problems (e.g., Laland and Reader 1999b, but see Boogert et al. 2006), although innovation may also arise from environmental pressures, like food scarcity (e.g., Laland and Reader 1999a, but see Kummer and Goodall 1985) or ‘spare time’ (e.g., in captivity or during juvenile dependency).
In addition to environmental correlates of novel problem-solving, individual characteristics such as body size (e.g., in Poecilia reticulata, Laland and Reader 1999a, see also Webster and Lefebvre 2001; Cole and Quinn 2011) and specific ‘personality’ traits (Dall et al. 2004; or ‘behavioral syndromes,’ Sih et al. 2004) have also been revealed as reliable correlates of problem-solving in some species (e.g., Carter et al. 2013, but see Cole et al. 2011). Indeed, from their field experiment with meerkats (Suricata suricata), Thornton and Samson (2012) concluded that ‘certain intrinsic individual characteristics may make some individuals particularly likely to innovate’ (p. 1464).
Personality traits, ‘the existence of behavioral and physiological differences among individuals of the same species, which are stable over time and across different contexts or situations’ (Carere and Maestripieri 2013b, p. 1), are robust for a number of nonhuman animal species (for reviews see Gosling 2001; Weiss et al. 2011; Carere and Maestripieri 2013a). The study of personality is important because, among nonhuman primates, for example, specific personality traits have been shown to be correlates of well-being, such as poor digestive health in Rhinopithecus roxellana (the trait ‘excitable,’ in an interaction with age, Jin et al. 2013), longevity in Gorilla gorilla gorilla (the trait ‘extraversion,’ Weiss et al. 2013), and stereotypic behavior in Macaca mulatta (the trait ‘gentle,’ in an interaction with environmental correlates, Gottlieb et al. 2013). Whether primate personality traits correlate with problem-solving and innovation, however, is less well understood.
There has been a long history of studying primate problem-solving since Köhler’s (1925) early studies, which reported that chimpanzees (Pan trogolodytes) spontaneously combined tools and stacked boxes to reach otherwise unattainable food. Following Köhler, a number of cases of chimpanzee problem-solving have been documented in both wild and captive environments, including studies of causal understanding (e.g., Premack and Premack 1994; Hanus and Call 2011), tool use (e.g., Visalberghi et al. 1995; Sanz et al. 2009), and foraging puzzles (e.g., Clark and Smith 2013; Manrique et al. 2013, for reviews see Boakes 1984; Tomasello and Call 1997). However, despite one recent study that presented chimpanzees with tool-use tasks in a group setting (Massen et al. 2013), and which reported two ‘behavioral syndromes’ to be descriptive of their problem-solving abilities, there are limited data available to answer whether specific personality factors are predictive of an individual chimpanzee’s problem-solving ability.
The relationship between personality traits and problem-solving or foraging skill has been studied in a broad array of species, including humans (Goldsmith 1984), birds (e.g., Parus major, Titulaer et al. 2012; Taeniopygia guttata, Brust et al. 2013), ungulates (e.g., Dama dama, Bergvall et al. 2011), hyenas (Crocuta crocuta, Benson-Amram and Holekamp 2012), cavies (Cavia aperea, Guenther et al. 2013), and other nonhuman primates (e.g., Microcebus murinus, Dammhahn and Almeling 2012; Otolemur garnettii, Watson and Ward 1996). Given these data, it is also likely that chimpanzee personality factors should correlate with their problem-solving ability. It is known that chimpanzee personality ratings are robust over time (Uher et al. 2008), predict species-typical behaviors (Pederson et al. 2005) and affiliative relationships (Massen and Koski 2013), and can reflect an individual’s well-being (Weiss et al. 2012b). The aim of this study, therefore, was to assess whether certain personality traits are also predictive of chimpanzee problem-solving success. To test this, we first rated the personalities of 36 captive chimpanzees (as part of an earlier study, Freeman et al. 2013) and then presented the chimpanzees with two novel problem-solving tasks in order to evaluate their consistency in success across more than one task (c.f. Thornton and Samson 2012; Massen et al. 2013).
In their recent study, Massen et al. (2013) presented two groups of zoo-housed chimpanzees with 10 novel stimuli, of which five represented foraging puzzles that required different methods and tools to be used in order to gain the food rewards (the remaining stimuli were classed as ‘predator models’ and ‘novel food/objects’). A principle components analysis revealed that the metrics Massen et al. (2013) used to measure the chimpanzees’ interactions with the stimuli (e.g., ‘time manipulating’ and ‘success in obtaining rewards’) loaded onto two factors: ‘exploration-persistence’ and ‘boldness.’ Of these two factors, only exploration-persistence described the chimpanzees’ interactions with the foraging puzzles. Given these results, we wished to determine whether chimpanzee personality factors would also correlate with chimpanzee interactions with foraging puzzles if (a) the chimpanzees were tested individually, rather than in a group setting and (b) the personality measure was generated independently of the problem-solving interactions, and a priori, from human ratings and behavioral data.
To control for demographic predictors of success, we also tested the importance of demographic state-dependent factors (e.g., age, sex, and estrous state). While both age (Bard et al. 1995) and sex (Lonsdorf et al. 2004) may predict a chimpanzee’s tool-use skills, a meta-analysis of studies of chimpanzees in the wild revealed that these factors also correlate with chimpanzees’ proclivity to innovate novel foraging strategies (i.e., foraging innovations are more likely to arise from male and/or subordinate individuals, Reader and Laland 2001). There is also tentative evidence that a female chimpanzee’s estrous state may mediate her ability with cognitive tasks, potentially relating to changes in problem-solving success (Inoue and Matsuzawa 2011; Lacreuse et al. 2013, but see Wagner and Ross 2013 who reported that female chimpanzee cognition was not influenced by the estrous state of an individual, although female and male performance on a sequencing task, conducted in a group setting, decreased when two or more group members were maximally tumescent). Although the evidence for the impact of a female’s estrous state is mixed for chimpanzees, given that estrous state and sex hormone levels have been shown to influence cognitive abilities and behavioral strategies for a number of species (e.g., Rattus norvegicus, Korol et al. 2004; M. mulatta, Rapp et al. 2003; Homo sapiens, Kimura and Hampson 1994), we controlled for the female chimpanzees’ estrous state in the present study by testing cycling female chimpanzees with the two novel foraging puzzles in a counterbalanced manner across their estrous cycles.
Through this study, our aim was to assess chimpanzees at the level of the individual (e.g., Herrmann and Call 2012), rather than looking for species-level correlates of problem-solving (e.g., Reader and Laland 2001). As we tested chimpanzees individually, we do not address whether these newly learned behaviors would be adopted by naïve group members and transmitted throughout their home group—a requirement of some innovation definitions (Reader and Laland 2003; see also Kendal et al. 2007). Given the results of Massen et al. (2013), we expected to find inter-individual variation but intra-individual consistency, indicative of differences in skill according to specific personality factors.
Methods
Subjects
Thirty-six chimpanzees, socially housed in highly enriched and spacious indoor/outdoor enclosures at the Michale E. Keeling Center for Comparative Medicine and Research, UT MD Anderson Cancer Center, USA (‘UT MD Anderson’ hereafter), were the subjects in this study. The subjects for this study were members of 14 social groups at UT MD Anderson, which were comprised of 5–11 individuals (average group size of groups tested = 7.2 chimpanzees). The chimpanzee subjects included 24 females (average age 26.8 years, age range 14–47 years), on a variety of contraceptive plans, and 12 reproductively intact males (average age 26.7 years, age range 17–39 years).
Not every female member of every group participated in this study. Females were preselected according to the birth control that they were prescribed in order to control for any impact that a specific birth control may have had on the females’ performance. The majority of males in these 14 groups were tested, although as we only tested those that voluntarily separated from their group, we were unable to test all potential males (for example, certain dominant males were unwilling to separate from their group, especially when females in their group were in estrous). Full demographic information for the 36 chimpanzees that were tested is provided in the Electronic Supplementary Materials (Table S1).
In addition to their daily allowance of commercial chimpanzee chow, the chimpanzees were fed three fresh produce meals a day and had continual access to water. Chimpanzees were not food or water deprived at any time.
Apparatus
The two novel foraging puzzles were modified ‘dog activity toys’ (the DogCasino—renamed ‘Pin-Release Puzzle’—and the DogDomino—renamed ‘Slide-Release Puzzle’—from Nina Ottoson, http://www.nina-ottosson.com/). Neither foraging toy had been presented to any of the chimpanzees prior to the commencement of this study. However, we note that these chimpanzees live in a highly enriched environment and are regularly provided with enrichment devices that encourage their natural foraging behaviors (e.g., ‘termite’ fishing tasks, Hopper et al. 2007), and so they are familiar with manipulating devices to retrieve food rewards.
The first task, the Pin-Release Puzzle, was a 5-cm-deep board (20 × 36 cm) that had four trays protruding from each of the longer sides (eight trays total). These trays could be pulled out by the chimpanzees to reveal a food reward hidden in a well of each tray. Food rewards could be retrieved from the Pin-Release Puzzle according to two levels of complexity. The first, Phase A, is as described; the chimpanzee had to pull out each of the eight trays in order to get all eight rewards. In the second level, Phase B, eight pins were inserted into holes on the front of the Pin-Release Puzzle, each holding its corresponding tray in place. In this Phase B, to pull out a tray, the chimpanzee first had to remove the corresponding pin to release the tray (Fig. 1). The second task, the Slide-Release Puzzle, was also a 5-cm-deep board (20 × 38 cm), on which were five inverted cups. Each cup slotted into its own socket and contained a food reward. For the chimpanzee to remove a cup to get the food reward, it had to first slide one or more of the four sliding panels that ran along the center line on the front of the Slide-Release Puzzle. Sliding these panels along their tracks allowed each cup to be released from its socket in turn (Fig. 1).
Fig. 1.
The two novel foraging puzzles. a The Pin-Release Puzzle (Phase B) is shown in the start position with all eight trays and their corresponding pins locked in place. In Phase A, the Pin-Release Puzzle was presented to the chimpanzee in the same manner except that each of the eight pins was removed entirely. b Each pin could be removed and then the associated tray pulled out revealing a food treat placed in the tray. c The Slide-Release Puzzle in the start position with all five cups held in place by the sliding panels. d Once the sliding panels are moved to either the left or right each cup in turn could be removed revealing a food treat placed in the white cup
For use with the chimpanzees, both tasks were (1) structurally reinforced with a plastic fascia, (2) modified to include larger pins (Pin-Release Puzzle) or cups (Slide-Release Puzzle), which could be more easily manipulated by the chimpanzees, and (3) mounted on a plastic frame which allowed each puzzle to be hung on the chimpanzees’ cage mesh in such a way that the chimpanzees had sufficient room to reach through the mesh and manipulate the task.
Personality ratings
Personality ratings were collected for each of the 36 chimpanzees as part of a wider assessment of the personalities of the chimpanzees housed at UT MD Anderson. Full details of the procedures used to generate the personality rating scale are described in Freeman et al. (2013).
The development of the chimpanzee personality scale consisted of two phases. Firstly, an item pool of potential descriptors was generated from three sources: (1) previously used scales developed for both human and nonhuman primates, (2) personality traits nominated by experts familiar with chimpanzee behavior, and (3) behavioral categories listed in chimpanzee behavioral ethograms. Secondly, and in order to reduce redundancy and increase clarity, three experts selected a subset of the descriptors from the generated item pool that this generated, for inclusion in the final scale. This process resulted in a list of 41 items (‘traits’). Furthermore, to ensure comparability with the human personality literature, Freeman et al. (2013) ensured that all five dimensions of the human five factor model (FFM; John et al. 2008) were included in the final list of traits. In addition to the FFM traits, a sixth personality dimension, dominance, has been identified for chimpanzees (King and Figueredo 1997), so traits highlighting dominance were retained in the item set.
The questionnaire created by Freeman et al. (2013), requiring staff members to rate chimpanzees against a list of 41 traits, was given to 17 staff members at UT MD Anderson. These staff had worked directly with the chimpanzees for a minimum of 6 months (range 6 months–21 years). Ratings were completed at weekly meetings from 2006 to 2008, and raters were instructed not to speak to each other about the ratings during or outside of the meetings. From the responses to the personality questionnaire, factor analysis of the 41 traits revealed six dimensions of chimpanzee personality: Methodical, Extroversion, Agreeableness, Openness (to experience), Reactivity/Undependability, and Dominance (Table 1).
Table 1.
Each of the six chimpanzee personality (varimax-rotated) factors identified by Freeman et al. (2013) and the traits that loaded on to each
| Reactivity/Undependability | Dominance | Extraversion | Openness | Agreeableness | Methodical |
|---|---|---|---|---|---|
| Irritable | Fearful (−) | Solitary (−) | Human oriented | Protective | Self-caring |
| Autistic | Timid (−) | Depressed (−) | Persistent | Considerate | Methodical |
| Deceptive | Cautious (−) | Active | Inventive | ||
| Impulsive | Dominant | Playful | Intelligent | ||
| Defiant | Dependent (−) | Sexual | Affectionate/friendly | ||
| Mischievous | Anxious (−) | Affiliative | Inquisitive/curious | ||
| Jealous | Bold | ||||
| Manipulative | Relaxed | ||||
| Stingy | |||||
| Bullying | |||||
| Aggressive | |||||
| Eccentric | |||||
| Socially inept | |||||
| Calm (−) | |||||
| Excitable | |||||
| Temperamental/moody |
If a trait is associated with ‘(−),’ it signifies that that trait negatively correlated with that factor, e.g., the trait ‘fearful’ negatively correlated with the factor Dominance
To provide behaviors against which the personality dimensions would be validated, Freeman et al. (2013) collected behavioral data on 60 of the personality-rated chimpanzees, while the animals interacted freely in their large enclosures (see Weiss et al. 2012a, for a discussion on the importance of validating rating scales against behavioral data). It was found that the traits significantly correlated with observed chimpanzee behavior and we summarize these correlations in Table 2, referring the reader to full details in Freeman et al. (2013). Methodical, however, was not positively correlated with any behaviors measured through the behavioral observations, but Freeman et al. (2013) proposed that a better measure of this trait would be through the implementation of cognitive (tool-use) tasks. Thus, the present study provides an opportunity to further validate the traits identified by Freeman et al. (2013).
Table 2.
The behaviors that significantly correlated (positively and negatively) with each of the six personality factors identified by Freeman et al. (2013)
| Personality factor | Behavior
|
|
|---|---|---|
| Positive correlation | Negative correlation | |
| Reactivity/Undependability | Aggressive (intervene and sexual behavior) | Affiliatory (post-conflict affiliation) |
| Dominance | Aggressive (contact aggression, display, intervene, noncontact aggression, sexual behavior) Affiliatory (proximity) |
Submissive (submissive) |
| Openness (to experience) | Submissive (submissive) Affiliatory (play) |
Affiliatory (proximity) |
| Extraversion | Aggressive (contact aggression) Submissive (begging) Affiliatory (play) |
– |
| Agreeableness | Affiliatory (affiliation) | Aggressive (displace) |
| Methodical | – | – |
Each correlation provides the category of behavior followed by the specific behavioral codes in brackets. Methodical did not correlate with any of the chimpanzees’ observed behaviors
Procedure
In order to ensure that we were testing each chimpanzee’s individual problem-solving skills—as opposed to their ability to learn via social influences—we presented individual chimpanzees with novel foraging puzzles, out-of-sight of the rest of their social group. Additionally, to reduce the chance of experimenter ‘cueing’ (regarding end states or reward locations), we baited the puzzles with food rewards prior to entering the testing area.
For testing, each chimpanzee was called into their inside enclosure by the experimenter, enabling entirely voluntary participation in the study. Given this choice and the stimulation provided by the foraging test itself (e.g., Clark and Smith 2013), these sessions created environmental variety and cognitive stimulation for these chimpanzees. Because participation was voluntary and the chimpanzees had a positive/familiar relationship with the researcher, all subjects were calm when individually housed for these brief (≤15 min) tests. Once inside, the researcher rewarded the chimpanzee with a small food reward (e.g., a slice of apple) for coming inside and the puzzle was presented to the chimpanzee by hanging it on the outside of their enclosure.
The Pin-Release Puzzle was first presented in the easier state (Phase A). Each chimpanzee was given 5 min during which to interact with the puzzle, beginning from the time it was hung on their cage. At the end of the 5-min period, if a subject had not retrieved any of the food rewards (by sliding out one or more of the eight trays), the puzzle was removed, and the session terminated, at which time the chimpanzee returned to its social group. If the chimpanzee was able to obtain one or more of the eight food rewards from the Pin-Release Puzzle, the session continued for an additional 5 min. Thus, each session that contained the successful attainment of at least one reward ended when 10 min had elapsed or when the chimpanzee had successfully removed all eight food rewards from the Pin-Release Puzzle, whichever occurred first. Unsuccessful chimpanzees were only given 5 min to interact with the task because, a pilot study, and our own previous research (e.g., Hopper et al. 2007) indicated that chimpanzees do not persist in exploring the task if unrewarded for 5 min.
For those chimpanzees that were able to retrieve one or more of the eight rewards from the Pin-Release Puzzle in Phase A, the puzzle was removed, the eight pins were added, out-of-sight of the chimpanzee, and the rebaited puzzle was represented to the chimpanzee for Phase B. Phase B for the Pin-Release Puzzle followed the same parameters as Phase A, such that if the chimpanzees were successful within the first 5 min, the session continued for 10 min or until all rewards had been retrieved. At the end of its test session, the chimpanzee immediately returned to its social group.
The Slide-Release Puzzle was presented following the same protocol as that for Phase A of the Pin-Release Puzzle.
Although the majority of the female chimpanzees were on some form of contraceptive (Electronic Supplementary Materials: Table S1), nine of the 24 females exhibited a regular estrous cycle, as measured by the size of their sexual swelling. During their cycle, a female chimpanzee’s sexual swelling size and shape will change in ways that can be visually assessed using a 0–4 scale (Graham 1981), which takes into consideration several factors, including the overall size and color of the swelling (Reichert et al. 2002). While acknowledging that those females that exhibited sexual swelling changes following a regular cycle may not have been experiencing typical fluctuations in hormone levels (Proctor et al. 2011, but see Bettinger et al. 1997), we note that the benefit of this visual scale is that it can be used as an indicator of hormone levels in the absence of hormonal assays (Deschner et al. 2004). In the Electronic Supplementary Materials (ESM1), we provide details of how we recorded the females’ sexual swelling cycles and information about the impact of birth control on the females’ cycling. The presentation of the two tasks was counterbalanced across the cycling females such that four were tested with the Pin-release task when their sexual swelling was maximally tumescent (rated as ‘4’) and with the Slide-release task when their sexual swelling was detumescent (rated as ‘0’, Electronic Supplementary Materials: Table S1). The remaining five cycling females received the tasks in the opposite order.
The time period between the presentation of the two tasks was dictated by each female’s estrous cycle: Across the nine females who exhibited a regular cycle, the average delay between presentations of each novel puzzle was 15.7 days. In order to allow comparisons between the cycling females and both the noncycling females and the males, the remaining 15 females and 12 males were presented with the two tasks in a counterbalanced order with a 2-week period between task presentations. For noncycling females, the order of presentation of each task was also counterbalanced within the birth control method that they were prescribed (Electronic Supplementary Materials: Table S1).
Coding and analysis
All test sessions were videotaped with a Canon ZR900 camcorder (Canon USA, Inc.) and the footage coded using EthoLog 2.2 (Ottoni 2000). We recorded the number of defenses (trays, pins, and cups) that each chimpanzee removed (Fig. 1). For the Pin-Release Puzzle in Phase A, the maximum number of defenses and rewards that could be removed was eight: the eight trays, each of which held a food reward. For the Pin-Release Puzzle in Phase B, the maximum number of defenses was 16: the eight trays, as described for Phase A, plus the eight pins which corresponded with each of the trays. In Phase B, to remove any tray, the chimpanzee had to first remove the associated pin and so the minimum number of defense removals required to gain a food reward was two. For the Slide-Release Puzzle, the maximum number of defenses that could be removed was five: the five food cups, each of which contained a food reward.
Following Massen et al. (2013), we also coded the latency for each chimpanzee to begin interacting with the task (defined as first contact with either a hand or mouth), the latency for the chimpanzee to successfully remove the first defense and get the food reward, and the length of time they spent actively interacting with the task (using their hands or mouth to manipulate any part of the task, not exclusive to the defenses, and thus in contact with the task, rather than just in proximity to it).
The reliability of our ratings was assessed by computing intra-class correlation coefficients (ICC, Shrout and Fleiss 1979) between the ratings completed by S.A.P. and ratings completed by a research assistant who was blind to the conditions of this study but familiar with chimpanzee behavior. S.A.P. coded all the test sessions, while the blind rater coded 20 % of the test sessions. The subset of test sessions that were blind coded was pseudo-randomly selected in order to include test sessions with both male and female chimpanzees and also presentations of both the Pin-Release and Slide-Release Puzzles. There was high inter-rater reliability for all measures; ICC (2,1) for the number of defenses removed = 1.000 (P <0.001), ICC (2,1) for the latency of the chimpanzees’ first success = 0.998 (P <0.001), and the ICC (2,1) for the number of seconds the chimpanzees actively manipulated the puzzles = 0.994 (P <0.001).
A Shapiro–Wilk test revealed that the data were not normally distributed (by sex or task). Given this and due to small sample sizes, nonparametric statistics were used throughout. Related samples Wilcoxon tests were used to compare the chimpanzees’ success across the two puzzles, while Pearson’s correlations were used to correlate the chimpanzees’ success with their individual ratings on each of the six personality factors identified by Freeman et al. (2013). Comparisons between male and female chimpanzees were conducted with Mann–Whitney U tests. All analyses reported were two-tailed unless otherwise stated. To account for familywise errors arising from multiple comparisons, we used a false discovery rate control (Benjamini and Hochberg 1995; Storey 2002), which calculates the expected proportion of ‘false positives’ among all the discoveries (i.e., rejected null hypotheses). Using this method, we identified the largest value for k (for α = 0.05) thus: (when m is the number of comparisons made and P is the reported P value). We then rejected all H(i) for i = 1, …, k. All analyses were run in IBM SPSS version 20, including the false discovery rate control via a script provided by IBM (http://www-01.ibm.com/support/docview.wss?uid=swg21476447).
Results
Personality factors as correlates of success
The proportion of possible defenses removed by a chimpanzee from the Pin-Release Puzzle (Phase A) was significantly positively correlated with the proportion removed from the Slide-Release Puzzle (related samples Wilcoxon, T+ = 292.0, N = 36, P = 0.003 and individual success on Phases A and B combined of the Pin-Release Puzzle significantly positively correlated with success with the Slide-Release Puzzle, T+ = 49.5, N = 36, P = 0.001). Such intra-individual consistency suggested that the chimpanzees’ success may be indicative of individual traits or personality factors. To assess this, we correlated the chimpanzees’ personality factor ratings with their interactions with the two tasks. Given that it has been shown that male and female chimpanzees are typified by different personality factors (King et al. 2008, see also Titulaer et al. 2012; Sussman et al. 2013), we analyzed each sex individually.
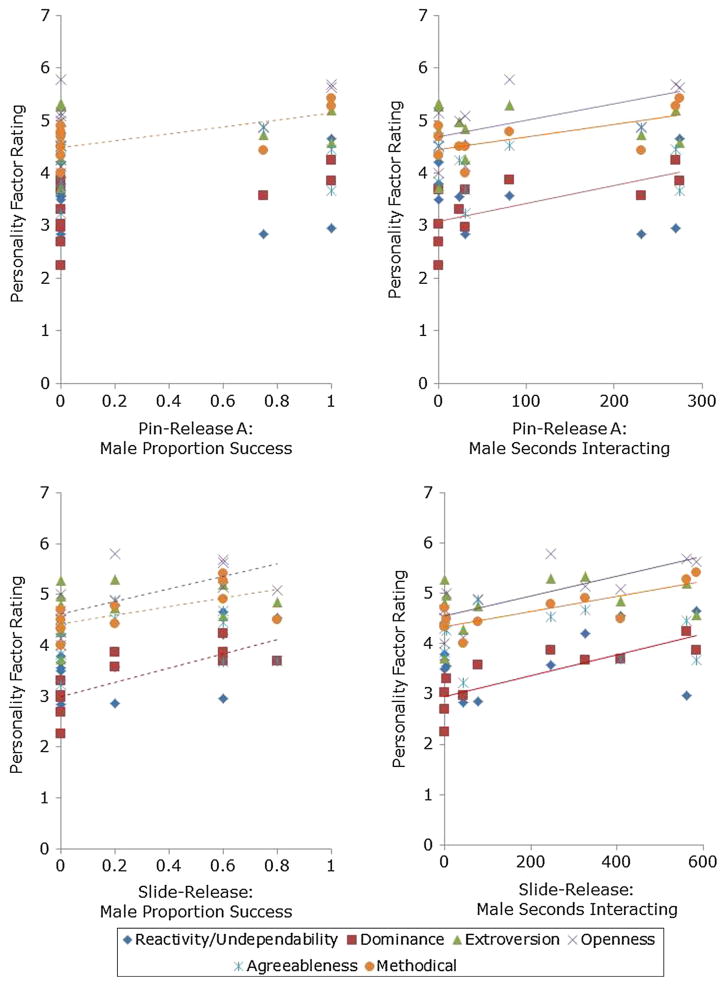
For males, the proportion of defenses removed from both tasks significantly correlated with the traits Methodical (r = 0.756, N = 12, P = 0.007) and Dominance (r = 0.725, N = 12, P = 0.01) and showed a trend toward a positive correlation with Openness (to experience) (r = 0.635, N = 12, P = 0.036), but given the application of the false discovery rate control, this was not considered significant (Fig. 2). For all other correlations, P > 0.05 (see Table 3). The time males spent interacting with the tasks, also showed significant correlations with the factors Dominance, Openness (to experience), and Methodical (see Table 3).
Fig. 2.
Male chimpanzee personality as a correlate of problem-solving success for which the rating was measured on a 7-point Likert scale. a The proportion of defenses that male chimpanzees successfully removed from the Pin-Release Puzzle (Methodical: r = 0.76, P = 0.006) and c Slide-Release Puzzle (Methodical: r = 0.64, P = 0.03; Openness: r = 0.64, P = 0.03; Dominance: r = 0.74, P 0.009). The length of time (seconds) that male chimpanzees spent actively interacting with the b Pin-Release Puzzle (Methodical: r = 0.78, P = 0.005) and d Slide-Release Puzzle (Methodical: r = 0.80, P = 0.003; Openness: r = 0.76, P = 0.007; Dominance: r = 0.81, P 0.003). Solid line a significant correlation. Dotted lines significant correlation that was nonsignificant after the false discovery rate control was applied
Table 3.
The correlations between the male chimpanzees’ personality factor scores and success (proportion of defenses removed), latency (latency to interact), and manipulating (proportion of session actively manipulating the puzzle) when presented with the Pin-Release and the Slide-Release Puzzle (Fig. 2)
| Personality factor | Latency | Manipulating | Success |
|---|---|---|---|
| Pin-release | |||
| Dominance | r = −0.38, N = 12, P = 0.35 | r = 0.68, N = 12, P = 0.02** | r = 0.58, N = 12, P = 0.06 |
| Reactivity/Undependability | r = −0.30, N = 12, P = 0.01* | r = 0.14, N = 12, P = 0.68 | r = −0.10, N = 12, P = 0.76 |
| Extraversion | r = −0.43, N = 12, P = 0.87 | r = 0.07, N = 12, P = 0.83 | r = 0.04, N = 12, P = 0.92 |
| Openness | r = −0.37, N = 12, P = 0.53 | r = 0.67, N = 12, P = 0.02** | r = 0.52, N = 12, P = 0.11 |
| Agreeableness | r = 0.23, N = 12, P = 0.17 | r = −0.04, N = 12, P = 0.92 | r = 0.12, N = 12, P = 0.72 |
| Methodical | r = −0.42, N = 12, P = 0.93 | r = 0.78, N = 12, P = 0.01** | r = 0.67, N = 12, P = 0.02* |
| Slide-release | |||
| Dominance | r = −0.40, N = 12, P = 0.33 | r = 0.81, N = 12, P = 0.00** | r = 0.74, N = 12, P = 0.01* |
| Reactivity/Undependability | r = 0.02, N = 12, P = 0.10 | r = 0.41, N = 12, P = 0.21 | r = 0.54, N = 12, P = 0.09 |
| Extraversion | r = 0.00, N = 12, P = 0.99 | r = 0.27, N = 12, P = 0.42 | r = 0.32, N = 12, P = 0.34 |
| Openness | r = −0.15, N = 12, P = 0.73 | r = 0.76, N = 12, P = 0.01** | r = 0.64, N = 12, P = 0.03* |
| Agreeableness | r = −0.07, N = 12, P = 0.88 | r = −0.12, N = 12, P = 0.73 | r = 0.95, N = 12, P = 0.10 |
| Methodical | r = −0.29, N = 12, P = 0.49 | r = 0.80, N = 12, P = 0.00** | r = 0.64, N = 12, P = 0.03* |
A correlation where P < 0.05 and
those correlations considered significant after the application of the false discovery rate control. Personality factor scores were originally calculated by Freeman et al. (2013)
For the females, no correlations between personality factor and success (as measured by the proportion of possible defenses removed) were found for either of the two puzzles. There was, however, a positive relationship between the latency of females to begin interacting with the Pin-Release task (in Phase A) and their rating in the factor Reactivity/Undependability (r = 0.685, N = 24, P = 0.002), such that those who were rated highly in Reactivity/Undependability were slower to interact with the task. This same pattern was revealed for the Slide-Release Puzzle (r = 0.593, N = 24, P = 0.012), but the correlation was not considered significant after the application of a false discovery rate control. No other significant correlations were found between females’ latency to begin interacting with the foraging puzzles or the duration of time spent interacting with them and the chimpanzees’ personality ratings.
There were also no significant correlations found, for either males or females, between their personality rating and the number of ‘paired defenses’ (i.e., a pin and its associated tray) removed from the Pin-Release Puzzle in Phase B in order to gain a food reward (Table 4).
Table 4.
The correlations between the male and female chimpanzees’ personality factor scores and the number of paired defenses (i.e., a pin and tray) removed from the Pin-Release Puzzle in Phase B
| Personality factor | Paired defenses removed from Pin-Release in Phase B
|
|
|---|---|---|
| Males | Females | |
| Dominance | r = 0.83, N = 12, P = 0.38 | r = −0.32, N = 24, P = 0.44 |
| Reactivity/Undependability | r = 0.55, N = 12, P = 0.63 | r = −0.31, N = 24, P = 0.46 |
| Extraversion | r = 0.27, N = 12, P = 0.83 | r = 0.14, N = 24, P = 0.74 |
| Openness | r = 0.99, N = 12, P = 0.04* | r = 0.44, N = 24, P = 0.28 |
| Agreeableness | r = −0.76, N = 12, P = 0.45 | r = 0.08, N = 24, P = 0.90 |
| Methodical | r = 0.99, N = 12, P = 0.08 | r = 0.38, N = 24, P = 0.36 |
A correlation where P < 0.05 (although note that this was not considered significant after the application of the false discovery rate control). Personality factor scores were originally calculated by Freeman et al. (2013)
Estrous state, age, and sex as correlates of success
We also analyzed the impact of a chimpanzee’s age, sex, and estrous state (for females) on their ability to retrieve the food rewards from either of the two tasks. We found tentative evidence for differing success across some of the females related to their estrous state. Regardless of what form of birth control a female was prescribed, for the Slide-Release Puzzle, observably cycling females removed a greater proportion of defenses when their swelling was detumescent and, rated 0, than females whose swellings were fully tumescent and rated 4 (U = 1.50, N0 = 4, N4 = 5, P = 0.034). This was not true, however, for the Pin-Release Puzzle in Phase A (U = 10.0, N0 = 4, N4 = 5, P = 1.00) or in Phase B (U = 10.0, N0 = 4, N4 = 5, P = 1.00). No impact of a chimpanzee’s age or sex was found (i.e., males were not able to retrieve any more food rewards than females; see Table 5 for and the Electronic Supplementary Materials (ESM3) for complete details).
Table 5.
Comparison of the chimpanzees’ responses by their sex and age when presented with the Pin-Release in Phase A (P-R A), Pin-Release in Phase B (P-R B), and the Slide-Release (S-R)
| Task | Latency | Manipulating | Success |
|---|---|---|---|
| Sex | |||
| P-R A | U = 98.0, Nf = 24, Nm = 12, P = 0.82 | U = 97.5, Nf = 24, Nm = 12, P = 0.12 | 14/24 versus 3/12, P = 0.08 |
| P-R B | U = 22.0, Nf = 14, Nm = 3, P = 0.90 | U = 27.0, Nf = 14, Nm = 3, P = 0.45 | 14/24 versus 3/12, P = 0.08 |
| S-R | U = 97.0, Nf = 24, Nm = 12, P = 0.79 | U = 143.5, Nf = 24, Nm = 12, P = 0.99 | 14/24 versus 7/12, P = 1.00 |
| Age | |||
| P-R A | r = −0.24, N = 36, P = 0.19 | r = 0.18, N = 36, P = 0.25 | r = 0.19, N = 36, P = 0.27 |
| P-R B | r = −0.39, N = 17, P = 0.13 | r = 0.30, N = 17, P = 0.29 | r = 0.19, N = 17, P = 0.26 |
| S-R | r = −0.22, N = 36, P = 0.23 | r = 0.02, N = 36, P = 0.89 | r = −0.02, N = 36, P = 0.91 |
Between-sex comparisons were run with Mann–Whitney U tests for the ‘latency to interact’ and ‘duration manipulating’ and with Fisher’s exact tests for the number of successful individuals (‘success’). Correlations between the chimpanzees’ age and their ‘latency to interact,’ ‘duration manipulating’ and ‘success’ were run with Pearson’s correlation
Discussion
This study showed that certain chimpanzee personality factors correlated with measures of the chimpanzees’ interactions with novel foraging puzzles, however, different personality factors explained the responses of the males and females. The problem-solving abilities of the male chimpanzees correlated with the personality factors Methodical and Dominance; those male chimpanzees who had higher scores on these factors removed more defenses from both novel foraging puzzles. Additionally, males rated highly on the factors Methodical, Dominance, and Openness (to experience) spent more time actively interacting with the two tasks (i.e., foraging).
The personality factors Methodical and Openness (to experience) are comprised of traits that describe problem-solving, inquisitive or methodical chimpanzee personalities. Thus, we provide further behavioral support for these questionnaire-generated personality factors within the context of a cognitive test (in addition to the behavioral observation correlates reported by Freeman et al. 2013). However, given the traits associated with the factor Openness, it is surprising that this factor was only related to time spent interacting with one of the puzzles rather than with more measures of the chimpanzees’ success (unlike Methodical and Dominance). Perhaps the reason that Methodical was a more reliable correlate of the males’ success relates to the nature of the specific novel foraging puzzles, both of which required methodical stepwise responses to enable success (e.g., Tecwyn et al. 2013). It is possible that those chimpanzees rated highly on Openness (to experience) would show greater success in more dynamic, or social, problem-solving settings.
Considering the traits that loaded onto Dominance, it may not be immediately apparent why male chimpanzees that rated highly on this factor also spent more time interacting with the puzzles, but we propose that it may be that these animals were calmer and more confident when tested in an individual setting and so could focus more on the task at hand. An alternative explanation is that Dominance is simply a predictor of an innovative personality. For humans, for example, innovation has been shown to correlate positively with personality traits such as ‘sensation seeking’ and ‘risk taking’ and negatively with ‘dogmatism’ (Goldsmith 1984; Jacoby 1971), and it is notable how these traits reflect the chimpanzee traits associated with Dominance.
For female chimpanzees, we found evidence that, for one of the tasks, the Pin-Release Puzzle, there was a positive correlation between the animals’ rating on the factor of Reactivity/Undependability and their latency to commence interacting with the task such that those who were rated highly in Reactivity/Undependability were slower to interact with the task. (a nonsignificant trend in the same direction was also found for their latency to interact with the Slide-Release Puzzle.) Contrary to our finding, it may be predicted that female chimpanzees whom were rated highly in Reactivity/Undependability may be more impulsive and so would begin interacting with novel objects rapidly. When considering the traits that loaded onto the Reactivity/Undependability factor, it appears that the personality construct is composed of traits that might relate more to social situations, rather than interactions with inanimate objects (e.g., irritable, jealous, bullying, manipulative). Therefore, it might be that these females are indeed more reactive in social contexts, but that this measure does not relate to their interactions with individual problem-solving tasks, such as those presented in this study. Comparably, a previous study investigating the personalities of great tits (P. major) found differences in the individual’s responses to their environment when they were in social compared with nonsocial situations that also varied by sex (van Oers et al. 2005). Future studies should take into consideration how personality factors might vary with regard to social compared with nonsocial situations, as it is possible that chimpanzees (and thus their learning strategies, Kendal et al. 2005, 2009) exhibit consistent trade-offs in their attention to social and nonsocial contexts or depending on the reliability of information available to them (as has been shown for Papio ursinus (Carter et al. 2013)).
Considering the personality results globally, it is unclear why we found specific differences in the personality factors that correlated with the males’ compared to the females’ responses, but we note that previous studies have also found sex differences with regard to primate personality (King et al. 2008, see also Titulaer et al. 2012; Sussman et al. 2013; Manson and Perry 2013). In addition to considering differences between social and nonsocial contexts, future studies could also investigate why certain personality factors are predictive of different behavioral responses in males and females.
We wish to emphasize that this finding—a correlation between the chimpanzees’ personality factors and their interactions with the novel foraging puzzles—is not circular. Firstly, the personality rating scale was developed from staff ratings collected between 2006 and 2008 (at least 3 years before the present study, run in 2011), suggesting that the personality factors represent stable ratings, which are consistent and meaningful over time (Uher et al. 2008). Secondly, the factors that emerged from the rating scale (Freeman et al. 2013) were correlated against data from behavioral observations that were collected between 2004 and 2006. This is important because it provides evidence that the personality ratings were not only consistent across human raters but also correlated strongly with appropriate independent behavioral measures (Weiss et al. 2012a). Thirdly and perhaps most importantly, those staff members who completed the original personality rating questionnaires were different from those who collected the observational data reported in Freeman et al. (2013), all of whom were different again from the experimenter (S.A.P.) who collected the data for this present study. S.A.P. collected all the behavioral data for the present study and although other authors of this study were also co-authors for Freeman et al. (2013), none acted as raters for the generation of the chimpanzee personality traits.
In addition to investigating the importance of the chimpanzees’ personality ratings on their interactions with the two novel foraging puzzles, we also considered the chimpanzees’ age, sex, and, for females, their estrous state. We found some evidence that a female chimpanzee’s estrous state influenced her success for the more complex Slide-Release Puzzle. However, no influence of what form of birth control a female was prescribed, or whether the female cycled regularly, was found (see the Electronic Supplementary Materials: ESM2 for full details of these analyses). Although some data exist for a link between a female chimpanzee’s ability on tests of memory (Inoue and Matsuzawa 2011) or spatial awareness (Lacreuse et al. 2013), the lack of a strong relationship between estrous state and the chimpanzees’ responses in our study may have been because our puzzles did not test these skills, but rather tested physical cognition and causal understanding.
Neither the chimpanzees’ age nor sex correlated with any of our measures of success, latency to begin interacting with the puzzle, or time spent interacting with either puzzle (the Electronic Supplementary Materials: ESM2). Although some studies have suggested that primate problem-solving behaviors are correlated with age, at least in group contexts (e.g., Kendal et al. 2005; Reader and Laland 2001), a recent longitudinal study with chimpanzees revealed no overall effect of age on physical cognition—tests comparable to our foraging puzzles—although age did influence their responses in other domains (e.g., for tests of social cognition, Lacreuse et al. 2013). It is also notable that the only age-related changes in physical cognition that Lacreuse and colleagues did report related to those chimpanzees that were over 50 years old, while the oldest chimpanzee included in our study was 47 years of age. Data are comparably mixed for the influence of sex on primate problem-solving with some revealing evidence for sex effects (e.g., Reader and Laland 2001), while others do not (e.g., Cameron and Rogers 1999). However, it might be that age and sex may be more relevant for chimpanzees when in a group setting rather than when tested individually (Reader and Laland 2001; Massen et al. 2013) or we might have found age effects if we had been able to include a wider age range (e.g., infants and juveniles and/or individuals over 50 years old) in our study.
This study also provided insights into chimpanzees’ ability for building upon previously gained knowledge. Of the 17 chimpanzees that successfully removed one or more trays from the Pin-Release Puzzle in Phase A, 14 were also able to remove one or more of the eight pairs of defenses (i.e., a pin and its associated tray) in Phase B (Electronic Supplementary Material ESM3). In this way, when ‘forced’ into adding a step to their repertoire, the chimpanzees showed step-by-step problem-solving (Manrique et al. 2013; Tecwyn et al. 2013). This is notable because instead of indicating that the chimpanzees’ responses were ‘conservative’ (e.g., Hrubesch et al. 2009; Marshall-Pescini and Whiten 2008), the chimpanzees showed flexible learning in which they built upon their previous efforts in a cumulative manner (Lehner et al. 2011). Given these data, in conjunction with other recent studies showing flexible problem-solving by chimpanzees (e.g., Dean et al. 2012; Hopper et al. 2013; Manrique et al. 2013; Yamamoto et al. 2013), we question a blanket classification of chimpanzees as ‘conservative.’ Therefore, we welcome future studies identifying the individual, social, and environmental predictors of chimpanzee problem-solving which foster cumulative learning.
Through this study, we have identified specific personality factors (i.e., Methodical, Dominance, and Openness) that are predictive of the persistence shown by male chimpanzees when foraging. Conversely, for the females, we found evidence that the personality factor Reactivity/Undependability correlated with their latency to begin foraging. This study also enabled us to validate the chimpanzee factor Methodical, tentatively proposed by Freeman et al. (2013). Further investigations into the importance of individual factors as predictors of innovation are vital for a detailed investigation of cumulative culture; as Ramsey et al. (2007) stated: ‘a complete understanding of culture requires an understanding of innovation’ (p. 394). By using an individualistic approach (Herrmann and Call 2012), we have begun to identify those factors that could explain a chimpanzee’s problem-solving ability and such methods may also enable us to identify those chimpanzees that are the likely innovators of novel cultural technologies. Furthermore, by considering animals at the individual level, rather than just the group level, researchers can investigate the potential impact of certain individuals upon their group’s behavior, such as in the context of innovation (Hoffman 1959) or learning (Hopper et al. 2011).
Supplementary Material
Acknowledgments
S.A.P. and R.L.K. were funded by a Royal Society Dorothy Hodgkin Fellowship awarded to R.L.K., and at the time of writing, L.M.H. was supported by the Leo S. Guthman Foundation. The chimpanzee colony at the UT MD Anderson facility is supported by a NIH U42 (RR-15090) grant. We also wish to show our appreciation for all the staff at the UT MD Anderson facility for their support and help and for providing the highest level of care for the chimpanzees housed there. In particular, we thank Tyrel McAdams and Ricky Merino for making the required modifications to the two foraging puzzles to make them suitable for use with chimpanzees and Veterinary Technician Jeffrey Haller for providing expert knowledge on the chimpanzee swelling ratings. We are also grateful to Laura Kurtycz for acting as our blind coder, our anonymous reviewers and the journal editor.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10071-013-0715-y) contains supplementary material, which is available to authorized users.
Contributor Information
Lydia M. Hopper, Lester E. Fisher Center for the Study and Conservation of Apes, Lincoln Park Zoo, Chicago, IL, USA. Michale E Keeling Center for Comparative Medicine and Research, UT MD Anderson Cancer Center, Bastrop, TX, USA
Sara A. Price, Michale E Keeling Center for Comparative Medicine and Research, UT MD Anderson Cancer Center, Bastrop, TX, USA. Language Research Center, Georgia State University, Atlanta, GA, USA. Centre for Coevolution of Biology and Culture, Department of Anthropology, Durham University, Durham, UK
Hani D. Freeman, Lester E. Fisher Center for the Study and Conservation of Apes, Lincoln Park Zoo, Chicago, IL, USA. Michale E Keeling Center for Comparative Medicine and Research, UT MD Anderson Cancer Center, Bastrop, TX, USA
Susan P. Lambeth, Michale E Keeling Center for Comparative Medicine and Research, UT MD Anderson Cancer Center, Bastrop, TX, USA
Steven J. Schapiro, Michale E Keeling Center for Comparative Medicine and Research, UT MD Anderson Cancer Center, Bastrop, TX, USA. Department of Experimental Medicine, University of Copenhagen, Copenhagen, Denmark
Rachel L. Kendal, Email: rachel.kendal@durham.ac.uk, Centre for Coevolution of Biology and Culture, Department of Anthropology, Durham University, Durham, UK
References
- Bard KA, Fragaszy D, Visalberghi E. Acquisition and comprehension of a tool-using behavior by young chimpanzees (Pan troglodytes): effects of age and modeling. Int J Comp Psychol. 1995;8:47–68. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- Benson-Amram S, Holekamp KE. Innovative problem solving by wild spotted hyenas. Proc R Soc Lond B. 2012;279:4087–4095. doi: 10.1098/rspb.2012.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergvall UA, Schäpers A, Kjellander P, Weiss A. Personality and foraging decisions in fallow deer, Dama dama. Anim Behav. 2011;81:101–112. [Google Scholar]
- Bettinger TD, Cougar D, Lee DR, Lasley BL, Wallis J. Ovarian hormone concentrations and genital swelling patterns in female chimpanzees with norplant implants. Zoo Biol. 1997;16:209–223. [Google Scholar]
- Boakes R. Psychology and the minds of animals. Cambridge University Press; Cambridge: 1984. From Darwin to behaviourism. [Google Scholar]
- Boogert NJ, Reader SM, Laland JN. The relation between social rank, neophobia and individual learning in starlings. Anim Behav. 2006;72:1229–1239. [Google Scholar]
- Brust V, Wuerz Y, Krüger O. Behavioural flexibility and personality in zebra finches. Ethology. 2013;119:559–569. [Google Scholar]
- Cameron R, Rogers LJ. Hand preference of the common marmoset (Callithrix jacchus): problem solving and responses in a novel setting. J Comp Psychol. 1999;113(2):149–157. [Google Scholar]
- Carere C, Maestripieri D. Animal personalities: behavior, physiology, and evolution. The University of Chicago Press; Chicago, IL: 2013a. [Google Scholar]
- Carere C, Maestripieri D. Animal personalities: who cares and why? In: Carere C, Maestripieri D, editors. Animal personalities: behavior, physiology, and evolution. University of Chicago Press; Chicago: 2013b. pp. 1–9. [Google Scholar]
- Carter AJ, Marhsall HH, Heinsohn R, Cowlishaw G. Personality predicts decision making only when information in unreliable. Anim Behav. 2013;86:633–639. [Google Scholar]
- Clark FE, Smith LJ. Effect of cognitive challenge device containing food and non-food rewards on chimpanzee well-being. Am J Primatol. 2013;75:807–816. doi: 10.1002/ajp.22141. [DOI] [PubMed] [Google Scholar]
- Cole EF, Quinn JL. Personality and problem-solving performance explain competitive ability in the wild. Proc R Soc Lond B. 2011;279:1168–1175. doi: 10.1098/rspb.2011.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EF, Cram DL, Quinn JL. Individual variation in spontaneous problem-solving performance among wild great tits. Anim Behav. 2011;81:491–498. [Google Scholar]
- Dall SRX, Houston AI, McNamara JM. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett. 2004;7:734–739. [Google Scholar]
- Dammhahn M, Almeling L. Is risk taking during foraging a personality trait? A field test for cross-context consistency in boldness. Anim Behav. 2012;82:613–618. [Google Scholar]
- Dean LG, Kendal RL, Schapiro SJ, Thierry B, Lalnd KN. Identification of the social and cognitive processes underlying human cumulative culture. Science. 2012;335:1114–1118. doi: 10.1126/science.1213969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm Behav. 2004;46(2):204–215. doi: 10.1016/j.yhbeh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Freeman HD, Brosnan SF, Hopper LM, Lambeth SP, Schapiro SJ, Gosling SD. Developing a comprehensive and comparative questionnaire for measuring personality in chimpanzees using a simultaneous top-down/bottom-up design. Am J Primatol. 2013 doi: 10.1002/ajp.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith RE. Personality characteristics associated with adaption-innovation. J Psychol. 1984;117(2):159–165. [Google Scholar]
- Gosling SD. From mice to men: what can we learn about personality from animal research? Psychol Bull. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, McCowan B. Risk factors for stereotypic behavior and self-Biting in rhesus macaques (Macaca mulatta): animal’s history, current environment, and personality. Am J Primatol. 2013;75(10):995–1008. doi: 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CE. Reproductive biology of the great apes: comparative and biomedical perspectives. Academic Press; London: 1981. [Google Scholar]
- Guenther A, Brust V, Dersen M, Trillmich F. Learning and personality types are related in cavies (Cavia aperea) J Comp Psychol. 2013 doi: 10.1037/a0033678. [DOI] [PubMed] [Google Scholar]
- Hanus D, Call J. Chimpanzee problem-solving: contrasting the use of causal and arbitrary cues. Anim Cognit. 2011;14:871–878. doi: 10.1007/s10071-011-0421-6. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Call J. Are there geniuses among the apes? Philos Trans R Soc Lond B. 2012;367:2753–2761. doi: 10.1098/rstb.2012.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LR. Homogeneity of member personality and its effect on group problem-solving. J Abnorm Soc Psychol. 1959;58(1):27–32. doi: 10.1037/h0043499. [DOI] [PubMed] [Google Scholar]
- Hopper LM, Spiteri A, Lambeth SP, Schapiro SJ, Horner V, Whiten A. Experimental studies of traditions and underlying transmission processes in chimpanzees. Anim Behav. 2007;73:1021–1032. [Google Scholar]
- Hopper LM, Schapiro SJ, Lambeth SP, Brosnan SF. Chimpanzees’ socially maintained food preferences indicate both conservatism and conformity. Anim Behav. 2011;81:1195–1202. doi: 10.1016/j.anbehav.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ, Brosnan SF. When given the opportunity, chimpanzees maximize personal gain rather than “level the playing field”. Peer J. 2013;1:e165. doi: 10.7717/peerj.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubesch C, Preuschoft S, van Schaik CP. Skill mastery inhibits adoption of observed alternative solutions among chimpanzees (Pan troglodytes) Anim Cognit. 2009;12(2):209–216. doi: 10.1007/s10071-008-0183-y. [DOI] [PubMed] [Google Scholar]
- Inoue S, Matsuzawa T. Correlation between menstrual cycle and cognitive performance in a chimpanzee (Pan troglodytes) J Comp Psychol. 2011;125(1):104–111. doi: 10.1037/a0020870. [DOI] [PubMed] [Google Scholar]
- Jacoby J. Personality and innovation proneness. J Mark Res. 1971;8(2):244–247. [Google Scholar]
- Jin J, Su Y, Tao Y, Guo S, Yu Z. Personality as a predictor of general health in captive golden snub-nosed monkeys (Rhinopithecus roxellana) Am J Primatol. 2013;75(6):524–533. doi: 10.1002/ajp.22127. [DOI] [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big-Five Trait Taxonomy: history, measurement, and conceptual issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: theory and research. Guilford Press; New York: 2008. pp. 114–158. [Google Scholar]
- Kendal RL, Coe RL, Laland KN. Age differences in neophilia, exploration, and innovation in family groups of callitrichid monkeys. Am J Primatol. 2005;66:167–188. doi: 10.1002/ajp.20136. [DOI] [PubMed] [Google Scholar]
- Kendal RL, Dean L, Laland KN. Objectivism should not be a casualty of innovation’s operationalization. Behav Brain Sci. 2007;30:413–414. [Google Scholar]
- Kendal RL, Kendal JR, Hoppitt W, Laland KN. Identifying social learning in animal populations: a new ‘option-bias’ method. PLoS ONE. 2009;4(8):e6541. doi: 10.1371/journal.pone.0006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D, Hampson E. Cognitive pattern in men and women is influenced by fluctuations in sex hormones. Curr Dir Psychol Sci. 1994;3:57–61. [Google Scholar]
- King JE, Figueredo AJ. The five-factor model plus dominance in chimpanzee personality. J Res Pers. 1997;31:257–271. [Google Scholar]
- King JE, Weiss A, Sisco MM. Aping humans: age and sex effects in chimpanzee (Pan troglodytes) and human (Homo sapiens) personality. J Comp Psychol. 2008;4:418–427. doi: 10.1037/a0013125. [DOI] [PubMed] [Google Scholar]
- Köhler W. The mentality of apes. Liveright; New York: 1925. [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kummer H, Goodall J. Conditions of innovative behaviour in primates. Philos Trans R Soc Lond B. 1985;308:2013–2021. [Google Scholar]
- Lacreuse A, Russell JL, Hopkins WD, Herndon JG. Cognitive and motor aging in female chimpanzees. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland KN, Reader SM. Foraging innovation in the guppy. Anim Behav. 1999a;57:331–340. doi: 10.1006/anbe.1998.0967. [DOI] [PubMed] [Google Scholar]
- Laland KN, Reader SM. Foraging innovation is inversely related to competitive ability in male but not female guppies. Behav Ecol. 1999b;10:270–274. [Google Scholar]
- Lefebvre L, Reader SM, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav Evol. 2004;63:233–246. doi: 10.1159/000076784. [DOI] [PubMed] [Google Scholar]
- Lehner SR, Burkart JM, van Schaik CP. Can captive orangutans (Pongo pygmeaeus abelii) be coaxed info cumulative build-up of techniques? J Comp Psychol. 2011;125:446–455. doi: 10.1037/a0024413. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Pusey AE, Eberly L. Sex differences in learning in chimpanzees. Nature. 2004;428:715–716. doi: 10.1038/428715a. [DOI] [PubMed] [Google Scholar]
- Manrique HM, Völter CJ, Call J. Repeated innovation in great apes. Anim Behav. 2013;85:195–202. [Google Scholar]
- Manson JH, Perry S. Personality structure, sex differences, and temporal change and stability in wild white-faced capuchins (Cebus capucinus) J Comp Psychol. 2013;127(3):299–311. doi: 10.1037/a0031316. [DOI] [PubMed] [Google Scholar]
- Marshall-Pescini S, Whiten A. Chimpanzees (Pan troglodytes) and the question of cumulative culture: an experimental approach. Anim Cognit. 2008;11:449–456. doi: 10.1007/s10071-007-0135-y. [DOI] [PubMed] [Google Scholar]
- Massen JJM, Koski SE. Chimps of a feather sit together: chimpanzee friendships are based on homophily in personality. Evol Hum Behav. 2013 doi: 10.1016/j.evolhumbehav.2013.08.008. [DOI] [Google Scholar]
- Massen JJM, Antonides A, Arnold A-MK, Bionda T, Koski SE. A behavioral view on chimpanzee personality: exploration tendency, persistence, boldness, and tool-orientation measured with group experiments. Am J Primatol. 2013;75:947–958. doi: 10.1002/ajp.22159. [DOI] [PubMed] [Google Scholar]
- Ottoni EB. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav Res Method Instr Comp. 2000;32(3):446–449. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- Pederson AK, King JE, Landau VI. Chimpanzee (Pan troglodytes) personality predicts behavior. J Res Pers. 2005;39:534–549. [Google Scholar]
- Premack D, Premack AJ. Levels of causal understanding in chimpanzees and children. Cognition. 1994;50(1–3):347–362. doi: 10.1016/0010-0277(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Proctor DP, Lambeth SP, Schapiro SJ, Brosnan SF. Male chimpanzees’ grooming rates vary by female age, parity, and fertility status. Am J Primatol. 2011;73:1–8. doi: 10.1002/ajp.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey G, Bastian ML, van Schaik C. Animal innovation defined and operationalized. Behav Brain Sci. 2007;30:393–437. doi: 10.1017/S0140525X07002373. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader SM, Laland KN. Primate innovation: sex, age, and social rank differences. Int J Primatol. 2001;22(5):787–805. [Google Scholar]
- Reader SM, Laland KN. Animal innovation: an introduction. In: Reader SM, Laland KN, editors. Animal innovation. Oxford University Press; Oxford: 2003. [Google Scholar]
- Reichert KE, Heistermann M, Hodges JK, Boesch C, Hohmann G. What females tell males about their reproductive status: are morphological and behavioural cues reliable signals of ovulation in bonobos (Pan paniscus)? Ethology. 2002;108:583–600. [Google Scholar]
- Sanz C, Call J, Morgan DB. Design complexity in termite-fishing tools of chimpanzees (Pan troglodytes) Biol Lett. 2009;5:293–296. doi: 10.1098/rsbl.2008.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc Natl Acad Sci USA. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. A direct approach to false discovery rates. J R Stat Soc B. 2002;63(3):479–498. [Google Scholar]
- Sussman AF, Ha JC, Bentson KL, Crockett CM. Temperament in rhesus, long-tailed, and pigtailed macaques varies by species and sex. Am J Primatol. 2013;75:303–313. doi: 10.1002/ajp.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecwyn EC, Thorpe SKS, Chappell J. A novel test of planning ability: great apes can plan step-by-step, but not in advance of action. Behav Proc. 2013;100:174–184. doi: 10.1016/j.beproc.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Thornton A, Samson J. Innovative problem solving in wild meerkats. Anim Behav. 2012;83:1459–1468. [Google Scholar]
- Titulaer M, van Oers K, Naguib M. Personality affects learning performance in difficult tasks in a sex-dependent way. Anim Behav. 2012;83:723–730. [Google Scholar]
- Tomasello M, Call J. Primate cognition. Oxford University Press; Oxford: 1997. [Google Scholar]
- Uher J, Asendorf JB, Call J. Personality in the behaviour of great apes: temporal stability, cross-situational consistency and coherence in response. Anim Behav. 2008;75:99–112. [Google Scholar]
- van Oers K, Klunder M, Drent PJ. Context dependence of personalities: risk-taking behavior in a social and a nonsocial situation. Behav Ecol. 2005;16:716–723. [Google Scholar]
- Visalberghi E, Fragaszy D, Savage-Rumbaugh S. Performance in a tool-using task by common chimpanzees (Pan troglodytes), bonobos (Pan paniscus), an orangutan (Pongo pygmaeus), and capuchin monkeys (Cebus apella) J Comp Psychol. 1995;109(1):52–60. doi: 10.1037/0735-7036.109.1.52. [DOI] [PubMed] [Google Scholar]
- Wagner KE, Ross SR. Female sexual state influences the cognitive test performance of zoo-living chimpanzees (Pan troglodytes) but not gorillas (Gorilla gorilla gorilla) Am J Primatol. 2013;75(S1):48. [Google Scholar]
- Watson SL, Ward JP. Temperament and problem solving in the small-eared bushbaby (Otolemur garnettii) J Comp Psychol. 1996;110(4):377–385. [Google Scholar]
- Webster SJ, Lefebvre L. Problem solving and neophobia in a columbiform-passeriform assemblage in Barbados. Anim Behav. 2001;62:23–32. [Google Scholar]
- Weiss A, King JE, Murray L. Personality and temperament in nonhuman primates. Springer; New York: 2011. [Google Scholar]
- Weiss A, Inoue-Murayama M, King JE, Adams MJ, Matsuzawa T. All too human? Chimpanzee and orang-utan personalities are not anthropomorphic projections. Anim Behav. 2012a;83:1355–1365. [Google Scholar]
- Weiss A, King JE, Inoue-Murayama M, Matsuzawa T, Oswal AJ. Evidence for a midlife crisis in great apes consistent with the U-shape in human well-being. Proc Natl Acad Sci USA. 2012b;109:19949–19952. doi: 10.1073/pnas.1212592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Gartner MC, Gold KC, Stoinski TS. Extraversion predicts longer survival in gorillas: an 18-year longitudinal study. Proc R Soc Lond B. 2013;280:1471–2954. doi: 10.1098/rspb.2012.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Humle T, Tanaka M. Basis for cumulative cultural evolution in chimpanzees: social learning of a more efficient tool-use technique. PLoS ONE. 2013;8(1):e55768. doi: 10.1371/journal.pone.0055768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.