Abstract
Purpose
Proteomics technologies are well suited for harnessing the immune response to tumor antigens for diagnostic applications as in the case of breast cancer. We previously reported a substantial impact of hormone therapy (HT) on the proteome. Here we investigated the effect of HT on the immune response toward breast tumor antigens.
Experimental design
Plasmas collected 0-10 months prior to diagnosis of ER+ breast cancer from 190 post-menopausal women and 190 controls that participated in the Women's Health Initiative (WHI) Observational Study were analyzed for the effect of HT on IgG reactivity against arrayed proteins from MCF-7 or SKBR3 breast cancer cell line lysates following extensive fractionation.
Results
HT user cases exhibited significantly reduced autoantibody reactivity against arrayed proteins compared to cases who were not current users. An associated reduced level of IL-6 and other immune-related cytokines was observed among HT users relative to non-users.
Conclusion and clinical relevance
Our findings suggest occurrence of a global altered immune response to breast cancer derived proteins associated with HT. Thus a full understanding of factors that modulate the immune response is necessary to translate autoantibody panels into clinical applications.
1 Introduction
The development of diagnostic tests for cancer is complicated by substantial disease and subject heterogeneity. Even more challenging is the identification of cancer biomarkers that have the potential for early detection. A promising approach for which proteomics technologies are well suited is the harnessing of the immune response to tumor antigens in the form of Ig based immunoreactivity [1]. However subjects whose tumors express a particular tumor antigen(s) exhibit varied seropositivity to such antigens. Understanding the factors that modulate the immune response to tumor antigens has substantial relevance to the development of diagnostic tests based on seropositivity. We have previously demonstrated a substantial impact of hormone therapy (HT) on the serum and plasma proteome and a confounding effect of HT in protein biomarker studies [2-4]. Several cohort studies have sought to determine the beneficial and adverse effects of postmenopausal hormone therapy. The large Women's Health Initiative (WHI) randomized, placebo controlled hormone therapy trials of 0.625 mg/day conjugated equine estrogen (E-alone) [5] or of this same estrogenic preparation plus 2.5 mg/day medroxyprogesterone acetate (E+P) [6], over respective average intervention periods of 6.8 and 5.6 years have shown multiple effects of HT of public health importance. The observed effects were similar for the two preparations for some outcomes, including stroke [7, 8] and hip fracture [9, 10] while E+P effects were unfavorable (p<0.05) compared to those for E-alone for other outcomes, notably breast cancer [11, 12]. The basis for adverse effects on breast cancer associated with E+P remains poorly understood.
In studies by others [13] the relationship between HT-regulated gene profiles and tumor characteristics was examined in postmenopausal women with breast cancer. HT use in patients with estrogen receptor (ER) protein positive tumors was associated with an altered regulation of 276 genes. Expression profiles based on these genes clustered ER-positive tumors into two molecular subclasses, one of which was associated with HT use. Additional evidence suggested that gene regulation in tumors associated with HT was negatively correlated with gene regulation induced by short-term estrogen exposure.
HT is known to exert a multitude of effects on the inflammatory response [14, 15], and has been shown to affect the levels of autoantibodies to some proteins in healthy individuals [16]. While many previous studies have identified a number of circulating autoantibodies in breast cancer [17, 18], the effect of HT on the immune response to tumor antigens including specifically the humoral response manifested through circulating autoantibodies to tumor antigens has not been previously investigated. Here we report on the effects of HT on immunoglobulin reactivity to native breast tumor proteins from breast cancer cell lysates and associated cytokine levels. The use of tumor lysate arrays allows for analysis of immune response to native proteins as previously investigated for lung [19-21], colon [22, 23], prostate [24] and pancreatic [25] cancer. In this study, we investigated using a proteomic approach, the effect of HT on the immune response to tumor antigens in breast cancer. Data was based on the analysis of pre-diagnostic plasma and matched controls from the WHI.
2 Materials and Methods
Plasma Samples
Prediagnostic plasma samples from 190 patients with Er+/Pr+ breast cancer and 190 healthy controls were collected as part of the Women's Health Initiative (WHI) trial. The case and control groups were further divided into three groups: 68 cases and 91 controls who were not currently taking hormone replacements (Not-Current), 55 cases and 52 controls who were taking only estrogen (E-alone), and 67 cases and 47 controls who took both estrogen and progesterone (E+P). HT use was self-reported on the WHI Observational Study baseline survey. The distribution of histology, time from blood draw to diagnosis, and other characteristics are provided in Table 1. Samples were stored at -80°C for comparable amounts of time.
Table 1.
Characteristics of WHI samples.
| Number | Age | Days-to Diagnosis | Stage 1 | Stage 2 | Stage 3 | Stage 4 | ||
|---|---|---|---|---|---|---|---|---|
| Not Current | Cases | 68 | 66.0 ± 7.3 | 123 ± 71 | 0 | 54 | 13 | 1 |
| Controls | 91 | 66.1 ± 6.8 | - | - | - | - | - | |
|
| ||||||||
| E+P | Cases | 55 | 61.9 ± 6.8 | 151 ± 79 | 0 | 52 | 15 | 0 |
| Controls | 52 | 61.4 ± 6.5 | - | - | - | - | - | |
|
| ||||||||
| E-Alone | Cases | 67 | 65.7 ± 6.3 | 125 ± 78 | 0 | 41 | 14 | 0 |
| Controls | 47 | 64.6 ± 7.1 | - | - | - | - | - | |
Protein microarrays
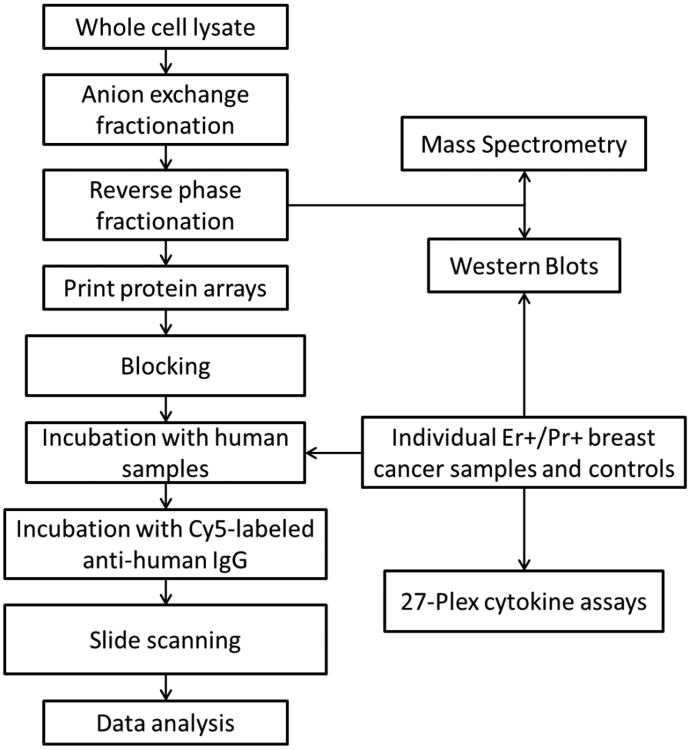
Experimental workflow can be seen in Figure 1. A total of 150 mg of proteins from each of the human breast adenocarcinoma cell lines MCF-7 and SKBR3 lysate was first separated by anion exchange high-performance liquid chromatography (HPLC), followed by reverse-phase chromatography as described previously [26]. Following reverse phase chromatography 1,950 protein fractions were collected from the MCF7 cell line and 2,600 protein fractions were collected from the SKBR3 cell line. Fractions were lyophilized and resuspended in 25 μL of printing buffer (250 mM of Tris-HCl, pH 6.8, 0.5% sodium dodecyl sulfate, 25% glycerol, 0.05% Triton X-100, 62.5 mM of dithiothreitol (DTT)). 10 μL of each fraction was aliquoted into a 384-well plate. All fractions, together with printing buffer as negative controls and purified human IgG as positive controls, were printed onto nitrocellulose-coated slides using a contact printer as previously described [27, 28].
Figure 1. Experimental workflow.
Sample slides were blocked for 1 hour at 4 °C with 3% BSA in 150 mM PBS, 0.1% Tween-20, 5% glycerol. Plasma samples were hybridized with an individual protein microarray at a dilution of 1/150 in probe buffer (150 mM PBS, 5 mM MgCl2, 0.5 mM DTT, 0.05% Triton X-100, 5% glycerol, 1% BSA) (PB) for 3 hours at 4 °C. Slides were washed 3 × 10 min with PB. Slides were then hybridized with Cy5-labeled anti-human IgG for 1 hour at 4 °C. Slides were washed 3 × 10 min with PB and dried by spinning at 800 × g for 5 minutes. IgG reactivity was assessed quantitatively using an indirect immune-fluorescence protocol, and local background-subtracted median spot intensities for downstream statistical analysis were generated using GenePix Pro 6.1. Background-subtracted median spot intensities were log2 transformed prior to analysis with no cut-off applied to the data. Most fractions had signal-to-noise ratios above 10, with few below 3. All statistical analyses were performed using R 2.9.0. Reported p-values are from a Mann-Whitney Wilcoxon test. Plasma samples were randomly allocated to the arrays both within and between batches to eliminate potential bias due to position within a particular batch.
Identification of reactive antigens
Antigens of interest in significant fraction clusters were localized into reactive bands by SDS-PAGE following Western blotting with reactive plasma and mass spectrometry. Briefly, Western blot analysis was performed by blocking with 5% milk in PBS with 0.1% Tween-20 (PBST) for 1 hour at room temperature. Plasma samples were diluted 1:500 in 1% milk in PBST and incubated with membranes overnight at 4°C. Membranes were washed with PBST and incubated with HRP-labeled anti-human IgG diluted 1:500 in 1% milk in PBST for 1 hour at room temperature. Membranes were stripped and reactive bands were excised and subjected to mass spectrometry analysis.
The total protein content of each reactive fraction was also determined by mass spectrometry analysis. Unprocessed fractions were lyophilized, digested with trypsin and subjected to mass spectrometry analysis on an LTQ-FT, as previously described [21].
Cytokine Assays
A 27 multiplex cytokine assay from Bio-Rad was performed on 120 plasma samples distributed across study groups according to the manufacturer's protocol with provided reagents. 20 cases and 20 controls were randomly selected from each HT group. Half of the cases and controls had immune responses above slide median value and half were below the median. Briefly, 50μL of Bio-Plex beads were pipetted into each well of a 96-well filter plate. Wells were washed twice with provided wash buffer. 50μL of sample or provided standard was added to each well and incubated on a shaker at room temperature for 30 minutes. Samples were a 1:3 dilution of 12.5μL of individual plasma. Wells were washed three times with wash buffer. 25μL of detection antibody was added to each well and incubated on a shaker at room temperature for 30 minutes. Following incubation, wells were washed three times with wash buffer. 50μL of streptavidin-PE was added to each well and incubated on a shaker for 10 minutes at room temperature. Following incubation, wells were washed three times with wash buffer. 125μL of assay buffer was then added to each well. The plate was shaken for 30 seconds and data was acquired using Bio-Plex Manager software on a luminex system. All statistical analyses were performed using R version 2.9.0. Reported p-values are from a Mann-Whitney Wilcoxon test. Samples with values below the detectable range for a given protein were assigned the value of the lowest detectable standard.
3 Results
Reduced autoantibody response to arrayed MCF-7 proteins in subjects on HT
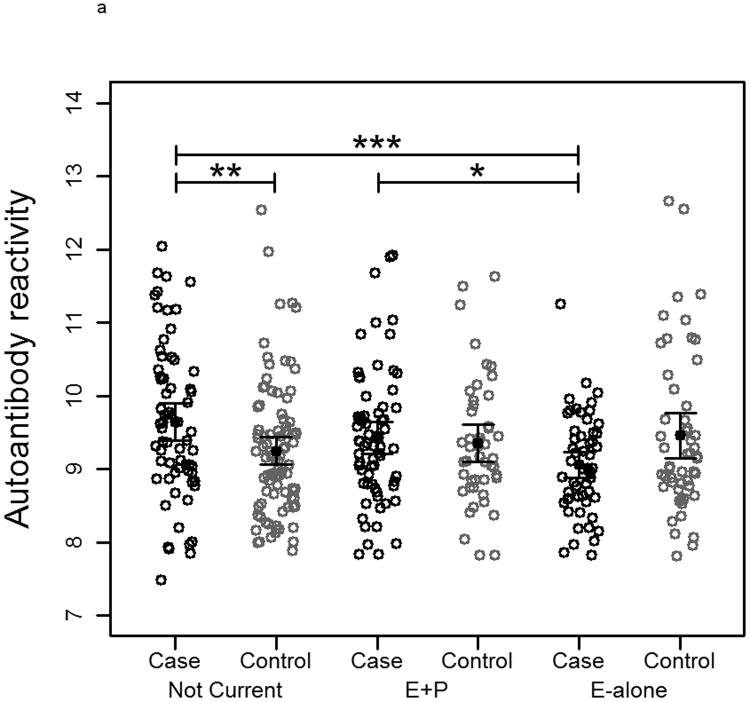
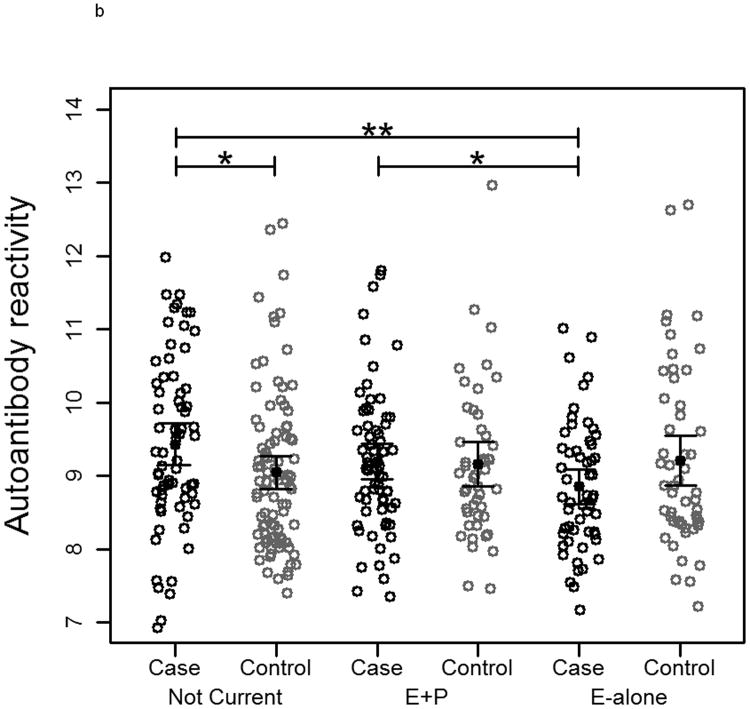
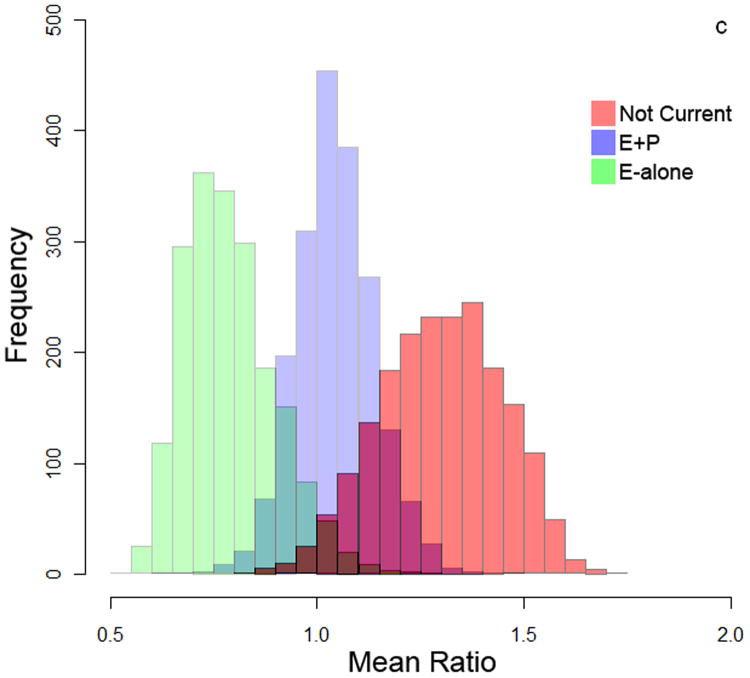
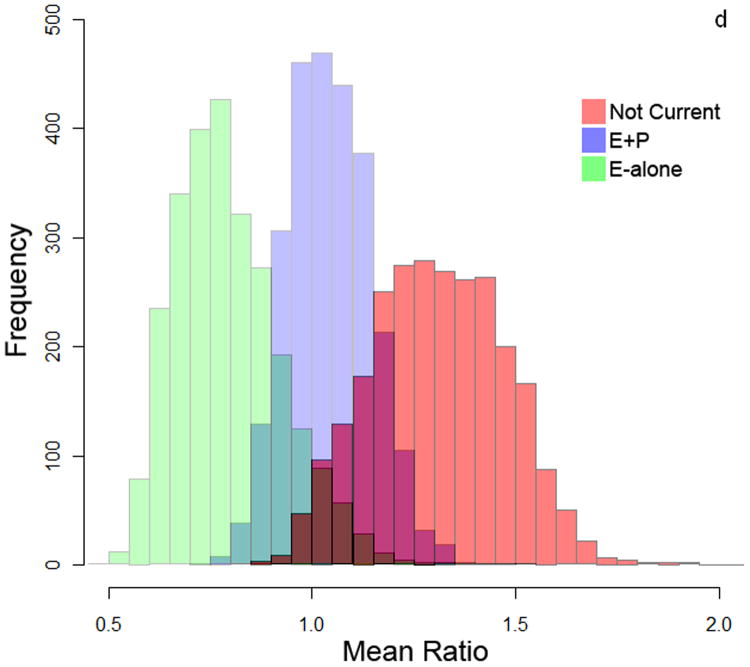
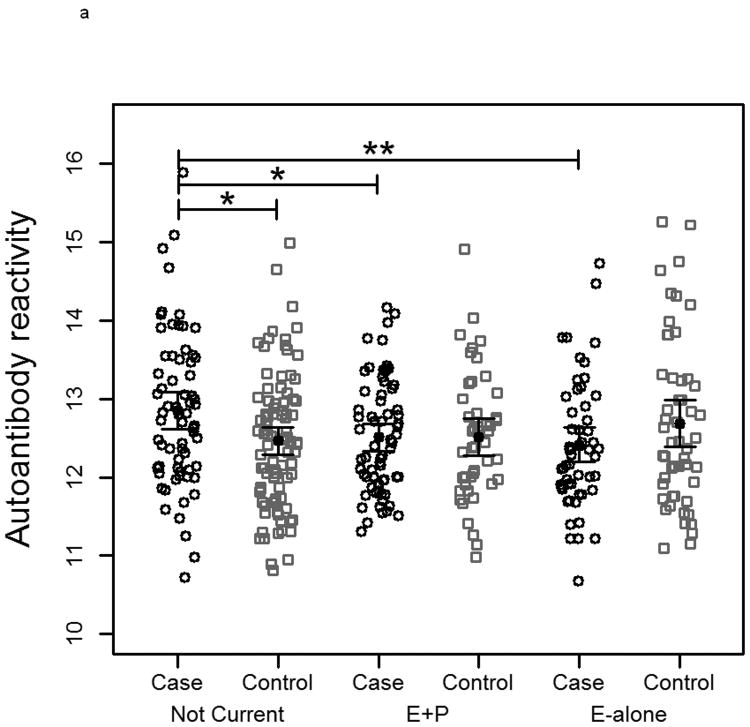
To measure autoantibody response toward breast cancer proteins, protein lysate arrays constructed from 1,950 protein fractions from MCF7 and 2,600 protein fractions from SKBR3 whole cell extracts were probed with pre-diagnostic plasmas from subjects who developed ER+/PR+ breast cancer within 10 months after blood draw and from matched controls who remained breast cancer free during the period of follow-up ranging from 7-12 years. The stability of the platform was assessed using multiple plasma samples. Duplicate samples run on separate arrays showed good correlation (R2>0.91 ; Supplementary Figure 1). The median autoantibody reactivity for each subject was used as an indicator of overall autoantibody response. Autoantibody reactivity is a measure of how much antibody from a subject's sample specifically binds to a protein of interest on the protein lysate array. Significant differences in immunoglobulin reactivity against arrayed proteins were observed between cases in both the E-alone and E+P groups and cases in the Not-Current group for both cell lines (Figure 2a and 2b). Controls in the different groups were not significantly different from each other, indicative of differences in reactivity among E and E+P cases relative to Not-Current cases. Autoantibody reactivity among cases in the Not-Current group was significantly higher than among controls for the Not-Current group, whereas reactivity among E-alone and E+P cases was not significantly different from reactivity in their controls. Ratios of case-over-control reactivity for individual arrayed protein fractions in both cell lines also showed a clear difference among HT groups (Figure 2c and 2d). Not Current users exhibited increased reactivity compared to either the E+P or E-Alone group. E-Alone user cases showed a consistently reduced reactivity compared to controls.
Figure 2.
a-b) Autoantibody reactivity to MCF-7 and SKBR3, respectively, protein lysate arrays represented as sample medians. Reactivities are separated by hormone therapy use and by case /control status. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001. c-d) Histograms of mean case-over-control ratios for autoantibody reactivity to individual fractions on the MCF-7 and SKBR3, respectively, protein lysate array. Samples are separated by hormone therapy use.
Influence of clinical parameters and HT on immune response
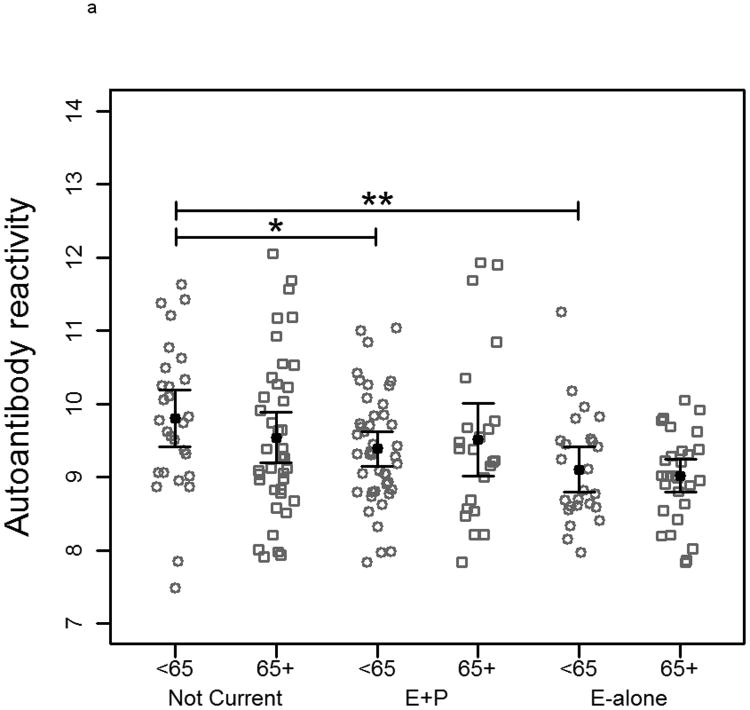
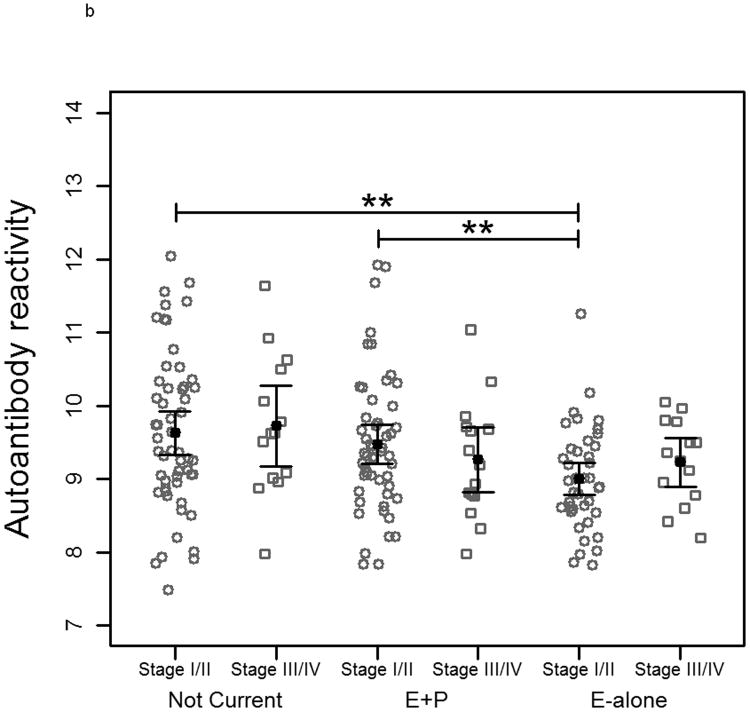
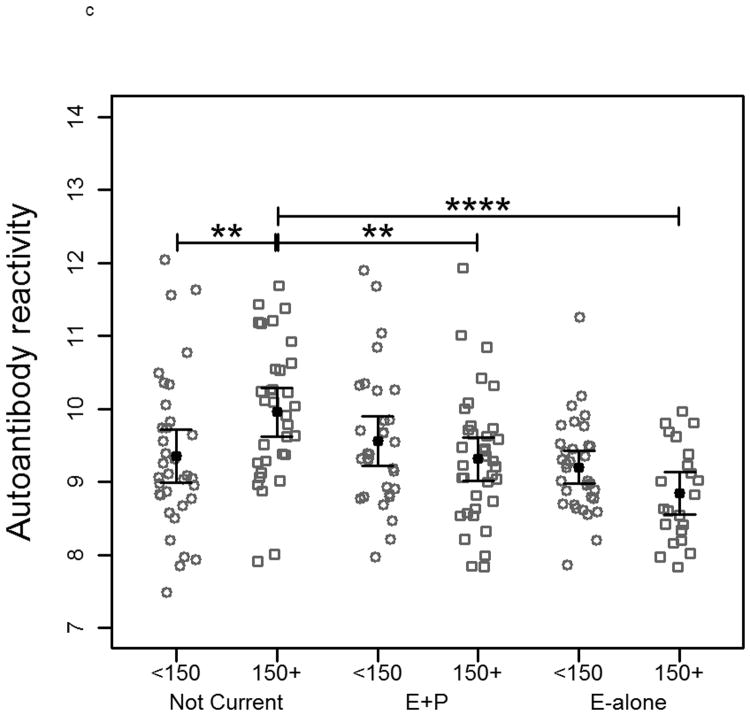
When subjects were separated by age into two groups based on a median age of 65 there was no significant difference between controls within each HT status group on MCF-7-arrayed fractions. Reduced reactivity remained significant between cases in the E-alone and Not-Current groups for both age groups (Figure 3a). A similar trend was observed when cases were grouped according to tumor stage at time of diagnosis (Figure 3b). E-alone patients with stage 2 breast cancer exhibited a significant reduction in immunoglobulin reactivity compared to patients who were not currently using HT. A concordant, though not statistically significant trend was observed for subjects diagnosed with stages 3 or 4 breast cancer. Grouping cancer patient samples by time-to-diagnosis elicited a similar trend as observed when patients were grouped by tumor stage. In patients diagnosed more than 150 days after blood draw, the same significant trend corresponding to hormone use was observed (Figure 3c). Patients diagnosed less than 150 days following blood draw showed no significant differences that correlated with HT. There was no statistically significant difference between controls who had been hysterectomized compared to those who had not within and across all HT groups (data not shown). Cases within the Not Current user group were also not statistically different. Within the E-alone group, cases with hysterectomy were significantly higher (p<0.05) than cases without. Results for sample reactivity to SKBR3-arrayed fractions were concordant with those observed on MCF-7-arrayed fractions for all clinical parameter analyses (Supplementary Figure 2).
Figure 3.
Plots of autoantibody reactivity medians against MCF-7-arrayed proteins from individual cancer samples separated by clinical characteristics: a) subject age, b) tumor stage at time of diagnosis and c) time of blood draw prior to diagnosis of breast cancer. Samples are separated by hormone therapy use and further separated based on the median value of the examined clinical characteristic. The median subject age was 65. The median time of blood draw prior to diagnosis was 150 days. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, **** indicates p<0.0001.
HT effects on plasma cytokine, chemokine and growth factor levels
Circulating levels of a set of 27 cytokines, chemokines and growth factors were assayed in a subset of the WHI plasma samples due to sample volume limitations. Of the 27 assayed proteins, 3 were below the limit of detection of the assay. Comparisons between HT groups revealed significant differences for many of the proteins tested. 17 of the 24 proteins were significantly (p<0.05) elevated in the Not-Current group compared to samples from E-alone users. RANTES was the only assayed protein that was significantly higher in E-alone compared to Not-Current. Thirteen of these 16 proteins were also significantly higher in the Not-Current group compared to the E+P group. Levels of IL-1Rα, FGF Basic, GM-CSF and VEGF were not significantly different between the E+P and Not-Current groups. RANTES was significantly elevated in the E+P group compared to the Not-Current group. Ten proteins were significantly elevated in E+P samples compared to E-alone samples. IL-1Rα, IL-7, IL-13, IL-17, FGF Basic, GM-CSF, MIP-1α and VEGF were significantly elevated in both Not-Current and E+P groups compared to E-alone.
Separate analyses of cases and controls across HT groups yielded similar results to the above analysis. In a comparison of the Not-Current and E-alone groups, G-CSF was not significantly elevated in Not-Current cases, though it was significantly elevated in Not-Current controls, while IL-1Rα, IL-9, GM-CSF and IFN-γ were significantly elevated in Not Current cases, but not in controls. IL-6, IL-9 and MIP-1α were significantly elevated in the Not-Current group compared to E+P cases. MIP-1α was also significantly elevated in controls from the Not-Current group compared to E+P, while IL-6 and IL-9 were not significantly elevated in Not-Current group controls. IL-5 and MIP-1α were significantly higher in E+P cases compared to E-alone cases, and RANTES and TNF-α were significantly lower in E+P cases compared to E-alone cases. IL-5 and RANTES were not significantly different in E+P controls and E-alone controls.
A comparison of cases to controls within each HT group showed few significant (p<0.1) differences. In the Not-Current group, 10 of the assayed proteins (IL-1β, IL-5, IL-6, IL-9, IL-12 (p70), Eotaxin, G-CSF, IP-10, MCP-1 and TNF-α) were elevated greater than 1.2 fold in cases compared to controls, though only GM-CSF (p=0.0025) and IL-9 (p=0.0929) reached significance. In the E+P group, IL-9, Eotaxin and G-CSF were elevated greater than 1.2 fold in cases compared to controls. FGF Basic (p=0.0941) was significantly higher in case than control, though its median ratio was only 1.05. No proteins were significantly higher in E-alone cases compared to controls, though IL-9, Eotaxin and RANTES were elevated greater than 1.2 fold.
Performance of identified tumor antigen across HT groups
Of the 1,950 arrayed fractions, 792 showed significantly elevated reactivity in cases compared to controls. To demonstrate the effect of HT use on individual proteins, we focused on one highly significant reactive cluster for identification and analysis. The identity of a significantly (p<0.05) reactive protein in cases compared to controls in the Not-Current group was determined by mass spectrometry analysis of a digest of the fraction and by one-dimensional separation and Western blotting of the fraction followed by mass spectrometry of a digest of the reactive band (Figure 4). Alpha-enolase (ENO1), a known autoantigen in non-small cell lung cancer [29] was identified as the antigen of interest in Fr_02_45. ENO1 reactivity among cases in the Not-Current group was higher than among cases in the E+P and E-alone groups with p=0.0234 and p=0.0091, respectively.
Figure 4.

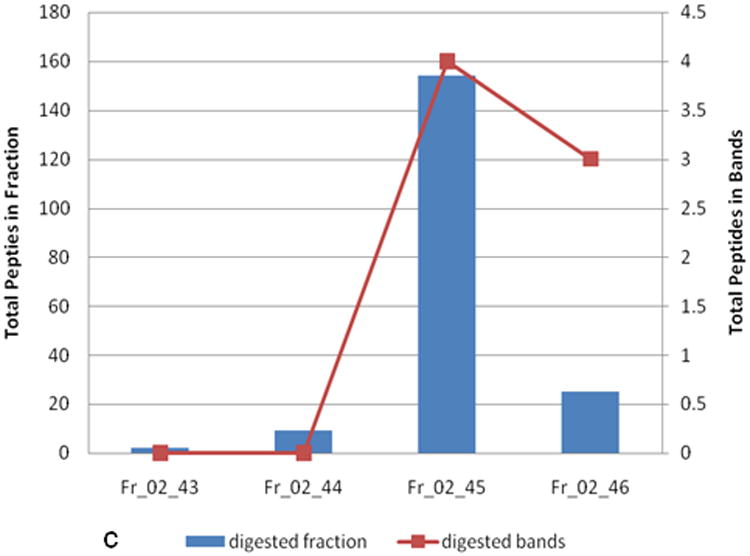
a) Autoantibody reactivity to an individual fraction identified as ENO1. Samples are separated by hormone therapy use and case /control status. b) Western blot reactivity of two individual samples to the ENO1-identified fraction and 3 neighboring anion exchange fractions. “C” is a case sample and “N” is a control sample. c) Mass spectrometry analysis of the same 4 fractions analyzed in (b). Plots show total number of identified peptides in each fraction. Fractions or excised bands from the western blot membranes were trypticly digested prior to mass spectrometry. * indicates p<0.05, ** indicates p<0.01.
4 Discussion
This study shows HT use could have a tremendous impact on study design and results for biomarker discovery and validation studies in women. These studies should be carefully constructed to ensure accurate analysis of adequate samples sizes by adjusting for or stratifying by HT use. HT was associated with a significant decrease in reactivity to breast cancer tumor antigens among breast cancer patients compared to Not Current users. Observed overall reactivity for cases in the Not Current group was significantly higher than in either the E+P or the E-alone groups. The reactivity among healthy controls who received HT was not significantly different from Not Current user controls. Overall, reactivity among E+P cases was significantly higher than among E-alone cases, suggestive of a compensatory effect due to P. These results were concordant across multiple breast cancer cell lines with differing ER/PR statuses, indicating this phenomenon is intrinsic to the patient samples.
The observed effect was further examined among cancer subjects in relation to clinical parameters, including age and tumor stage. When subjects were divided in two groups based on a median age 65 years, the effect of HT was observed in both groups, though statistically significant differences between Not Current and E-Alone cases was only observed in the group of women under 65. It is well known that aging is associated with a decline in immune system function and low efficiency of vaccination [30]. When categorized by tumor stage, subjects with stage 2 tumors exhibited a significant suppression of autoantibody reactivity, compared with subjects with stage 3 tumor. One explanation is a greater tumor antigen load among subjects that were diagnosed with stage 3, potentially neutralizing circulating antigens. The significance of time-to-diagnosis for reactivity was also investigated. Cases from whom blood was obtained farther from diagnosis (time-to-diagnosis >150 days) exhibited reduced reactivity associated with HT. Whereas pre-diagnostic cases with less than 150 days from diagnosis did not. This difference in reactivity also may be related to tumor antigen load. One of the most highly significant fraction clusters containing a previously known lung cancer autoantigen was more closely examined to demonstrate the HT effect on individual proteins. ENO1 was identified in a fraction cluster where reactivity from Not-Current cases was significantly higher than from Not-Current controls. Reactivity patterns by western blot and mass spectrometry (Figure 4) suggest that ENO1 was the immunogenic protein responsible for the observed autoantibody response. HT use significantly decreased the reactivity in cases for both the E+P and E-alone groups but did not affect reactivity of controls. Overexpression of ENO1 has been observed in ductal carcinomas [31] and correlated to poor prognosis in breast cancer [32, 33]. Autoantibodies to ENO1 have previously been identified in breast cancer patients [34], however this is the first report of hormone therapy use affecting autoantibody levels of ENO1.
It is widely known that estrogen and progesterone may increase tumor cell growth through a variety of mechanisms [35, 36]. Hormone therapy has been shown to affect levels of autoantibodies in individuals free from cancer [16], but this is the first report that immune response to tumor antigens is decreased in HT users compared to those not taking HT. A recent study in cancer-free patients with rheumatoid arthritis suggests that E+P has no adverse effect on circulating immunoglobulin levels or function compared to non-users [37]. E-alone was however not investigated in this study. Cytokine profiling in our work confirms that E+P users do not have suppressed levels of assayed cytokines compared to E-alone users. This is recapitulated in the immune response in women who develop cancer, but not in controls, indicating that the presence of pre-clinical cancer may be altering the effect of hormones on immune activity. The data presented in this work from the WHI observational study did not directly correlate with previous WHI clinical trial studies showing women using E+P had an increased risk of developing breast cancer, but those taking E-alone did not [5, 11]. Therefore, immune response to tumor antigens is not sufficient to explain the previously published risk associations
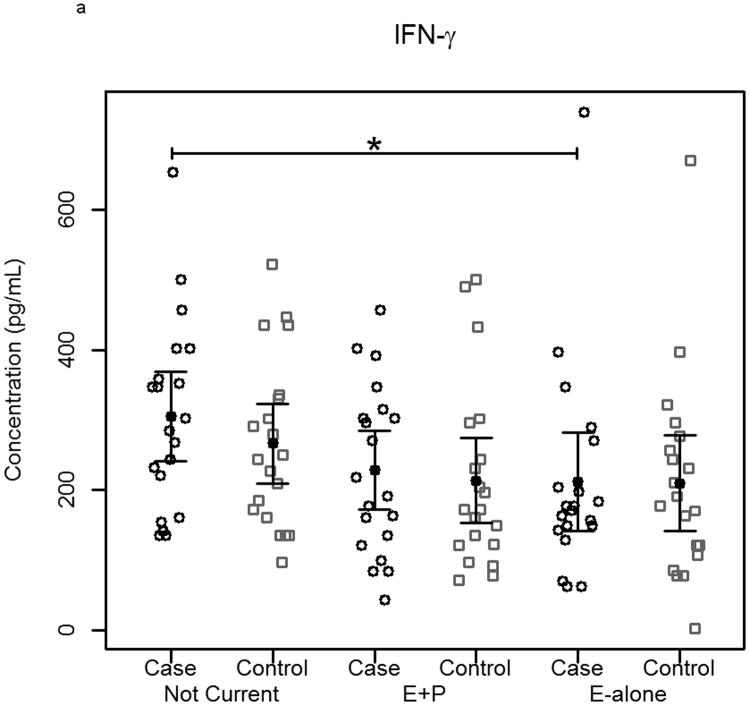
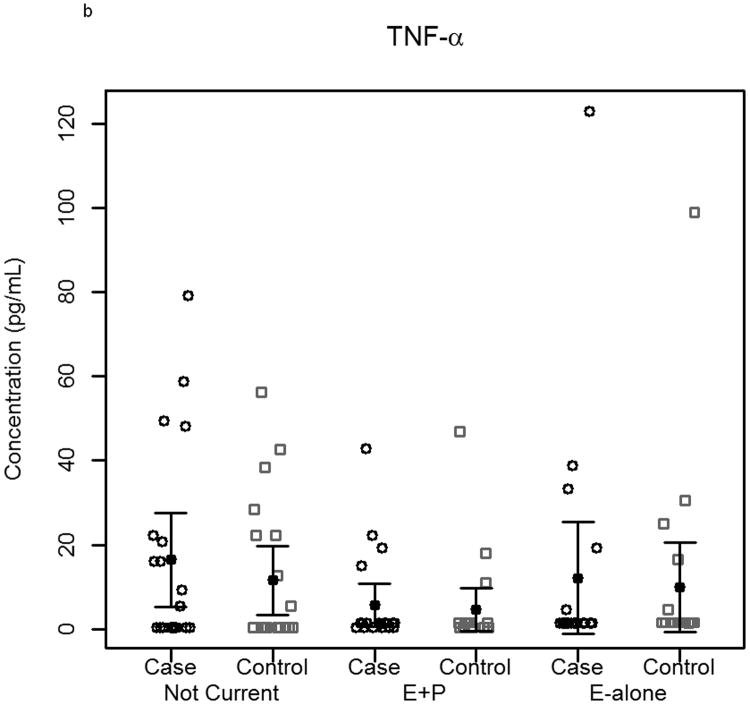
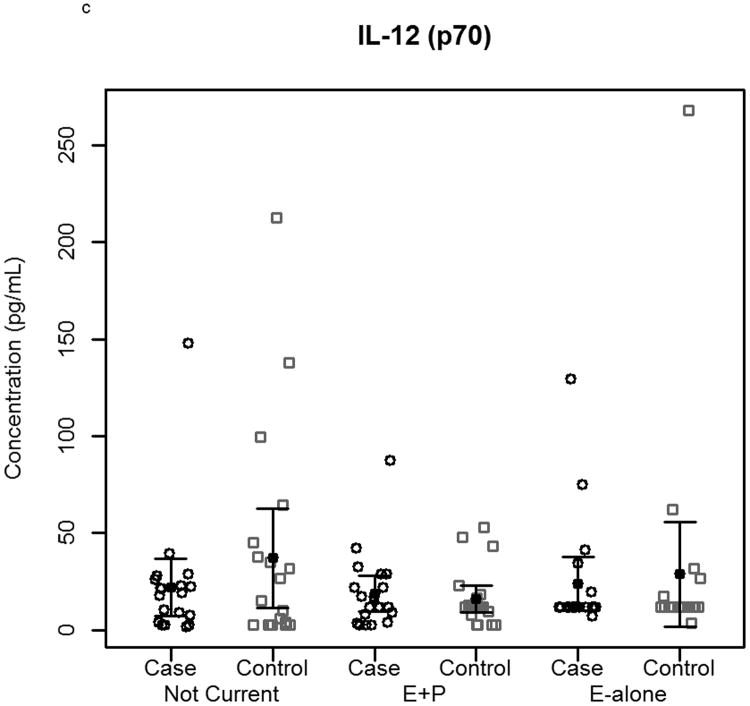
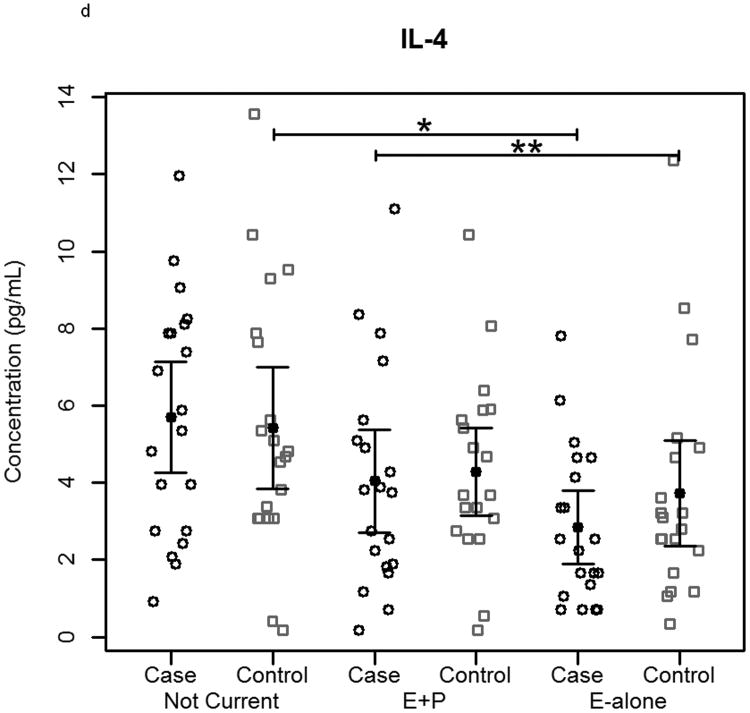
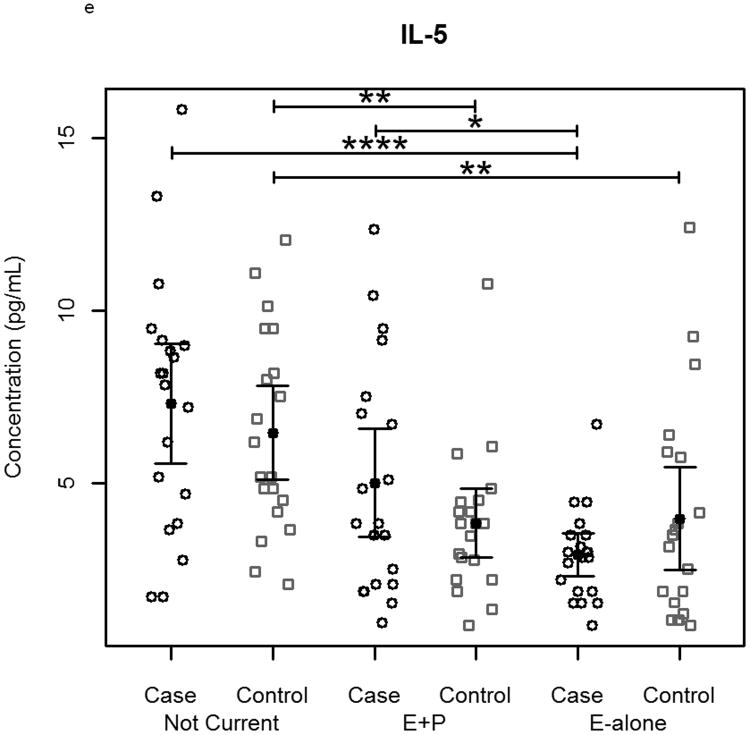
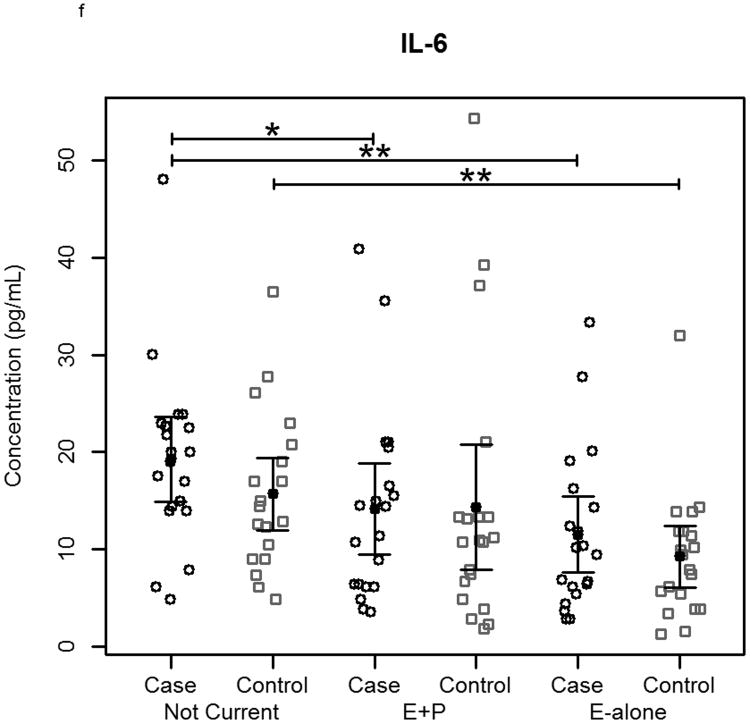
The effect of hormone therapy on the immune system in particular the inflammatory response has been previously investigated. The findings suggest that hormone therapy does have a significant effect on several immune mediators and inflammatory biomarkers including E-selectin, vascular cell adhesion molecule-1, intercellular adhesion molecule-1, monocyte chemoattractant protein-1, tumor necrosis factor-alpha, interleukin-6, transforming growth factor-beta, and C-reactive protein. In our study, plasma levels of most circulating cytokines and chemokines assayed were depressed in women using hormone therapy, with the most noticeable effect in women using E-alone. The suppressed proteins were associated with both Th2 and Th1 immune response pathways, suggesting E-Alone causes a global immune suppression (Figure 5). IL-4, IL-5, IL-6 and IL-13 are all associated with Th2 immune response, while IL-1β, IL-1ra, IL-8, IL-9, IFN-γ and MIP-1α are associated with Th1 immune response. RANTES affects both Th1 and Th2 immune responses and was seen to be up-regulated in women using E-alone hormone therapy. Additionally, several growth factors (Basic FGF, G-CSF, GM-CSF and VEGF) were significantly suppressed in E-alone compared to Not Current. IL-17, also higher in Not Current compared to E-alone, induces the production of many of the other cytokines and chemokines. It's depression in E-alone is a potential explanation for the lower levels observed for many of the other affected cytokines and chemokines.
Figure 5.
Circulating concentrations of cytokines as measured in individual subject samples. Samples are separated by hormone therapy use and by case /control status. a-c) Cytokines representative of a Th1 response: IFN-γ, TNF-α, and IL-12. d-f) Cytokines representative of a Th2 response: IL-4, IL-5, and IL-6. * indicates p<0.05, ** indicates p<0.01, **** indicates p<0.0001.
IL-6 and other pro-inflammatory cytokines were significantly depressed in women using E-alone therapy regardless of cancer status. Reduced levels among E-alone users who developed breast cancer were ∼2-fold less than observed among healthy controls. Given its role in the immune response higher levels of IL-6 in the Not Current may account for greater autoantibody reactivity observed in this group. IL-6 levels in E-alone and Not Current controls were not significantly different among either women who had a hysterectomy or those that had not. IL-6 levels in cases of women with hysterectomy taking E-alone were significantly (p=0.01) decreased compared to Not Current users, while cases of women without hysterectomy showed an insignificant decrease (p=0.052). IL-6 levels in women without hysterectomy had a positive correlation with autoantibody response to tumor while women who had a hysterectomy showed a slightly negative correlation to autoantibody response. Other studies have shown IL-6 levels were either decreased or were not significantly changed with HT use [38-41].
This effect of HT was also evident for other cytokines and chemokines that were assayed, suggesting that HT alters levels of a wide range of cytokines and chemokines among women that subsequently developed breast cancer compared to healthy controls. RANTES was the only cytokine or chemokine that was significantly up-regulated in women using E-alone hormone therapy compared to Not Current users. RANTES has been previously reported to enhance breast cancer cell proliferation and invasion [42, 43]. The increase in circulating levels of RANTES in HT users may also contribute to the autoantibody reactivity observed in our study.
It is likely that other non-hormonal factors play a role in the immune response to tumor antigens when such antigens and their reactive epitopes are expressed given the varied responses observed among cancer patients. The response may also vary at different stages of tumor development complicating the development of clinical applications based on autoantibody reactivity to tumor antigens. A potential avenue for which proteomics is well suited is to profile the level of circulating free antigen, antigen-antibody complexes, and free antibody to determine their dynamic nature at different stages of tumor initiation and progression to allow development of robust clinical applications of autoantibody biomarkers in cancer.
Supplementary Material
Supplementary Figure 1. Reproducibility of microarrays was assessed by hybridization of samples on multiple arrays. Pearson correlations between replicate spots for two different samples are a) 0.96 and b) 0.91. Data is displayed as the log-2 transform of fluorescent intensity.
Supplementary Figure 2. Plots of autoantibody reactivity medians against SKBR3-arrayed proteins from individual cancer samples separated by clinical characteristics: a) subject age, b) tumor stage at time of diagnosis and c) time of blood draw prior to diagnosis of breast cancer. Samples are separated by hormone therapy use and further separated based on the median value of the examined clinical characteristic. The median subject age was 65. The median time of blood draw prior to diagnosis was 150 days. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, **** indicates p<0.0001.
Footnotes
Authors have no conflict of interest to declare at this time.
References
- 1.Forrester S, Qiu J, Mangold L, Partin A, et al. Ion experimental strategy for quantitative analysis of the humoral immune response to prostate cancer antigens using natural protein microarrays. Proteomics Clinical Applications. 2007;1:494–505. doi: 10.1002/prca.200600802. [DOI] [PubMed] [Google Scholar]
- 2.Katayama H, Paczesny S, Prentice R, Aragaki A, et al. Application of serum proteomics to the Women's Health Initiative conjugated equine estrogens trial reveals a multitude of effects relevant to clinical findings. Genome Med. 2009;1:47. doi: 10.1186/gm47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitteri S, Hanash S, Aragaki A, Amon L, et al. Postmenopausal estrogen and progestin effects on the serum proteome. Genome Medicine. 2009;1:121. doi: 10.1186/gm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitteri SJ, Hanash SM. Confounding effects of hormone replacement therapy in protein biomarker studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:134–139. doi: 10.1158/1055-9965.EPI-10-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson GL, Limacher M, Assaf AR, Bassford T, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Journal of the American Medical Association. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Journal of the American Medical Association. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. Journal of the American Medical Association. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 9.Cauley JA, Robbins J, Chen Z, Cummings SR, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. Journal of the American Medical Association. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 10.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the women's health initiative randomized trial. J Bone Miner Res. 2006;21:817–828. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 11.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. Journal of the American Medical Association. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 12.Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. Journal of the American Medical Association. 2006;295:1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 13.Hall P, Ploner A, Bjohle J, Huang F, et al. Hormone-replacement therapy influences gene expression profiles and is associated with breast-cancer prognosis: a cohort study. BMC Med. 2006;4:16. doi: 10.1186/1741-7015-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgiadou P, Sbarouni E. Effect of hormone replacement therapy on inflammatory biomarkers. Adv Clin Chem. 2009;47:59–93. doi: 10.1016/s0065-2423(09)47003-3. [DOI] [PubMed] [Google Scholar]
- 15.Miller AP, Chen YF, Xing D, Feng W, Oparil S. Hormone replacement therapy and inflammation: interactions in cardiovascular disease. Hypertension. 2003;42:657–663. doi: 10.1161/01.HYP.0000085560.02979.0C. [DOI] [PubMed] [Google Scholar]
- 16.Castanho VS, Gidlund M, Nakamura R, de Faria EC. Post-menopausal hormone therapy reduces autoantibodies to oxidized apolipoprotein B100. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2011;27:800–806. doi: 10.3109/09513590.2010.526660. [DOI] [PubMed] [Google Scholar]
- 17.Desmetz C, Bascoul-Mollevi C, Rochaix P, Lamy PJ, et al. Identification of a New Panel of Serum Autoantibodies Associated with the Presence of In situ Carcinoma of the Breast in Younger Women. Clinical Cancer Research. 2009;15:4733–4741. doi: 10.1158/1078-0432.CCR-08-3307. [DOI] [PubMed] [Google Scholar]
- 18.Goodell V, Disis ML. Human tumor cell lysates as a protein source for the detection of cancer antigen-specific humoral immunity. Journal of Immunological Methods. 2005;299:129–138. doi: 10.1016/j.jim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Madoz-Gurpide J, Kuick R, Wang H, Misek DE, Hanash SM. Integral protein microarrays for the identification of lung cancer antigens in sera that induce a humoral immune response. Molecular & Cellular Proteomics. 2008;7:268–281. doi: 10.1074/mcp.M700366-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Pereira-Faca S, Qiu J, Krasnoselsky A, Newcomb L, Hanash S. Proteomic identification of tumor antigens in lung cancer. Molecular & Cellular Proteomics. 2005;4:S125–S125. [Google Scholar]
- 21.Qiu J, Choi G, Li L, Wang H, et al. Occurrence of Autoantibodies to Annexin I, 14-3-3 Theta and LAMR1 in Prediagnostic Lung Cancer Sera. Journal of Clinical Oncology. 2008;26:5060–5066. doi: 10.1200/JCO.2008.16.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nam MJ, Kee MK, Kuick R, Hanash SM. Identification of defensin alpha 6 as a potential biomarker in colon adenocarcinoma. Journal of Biological Chemistry. 2005;280:8260–8265. doi: 10.1074/jbc.M410054200. [DOI] [PubMed] [Google Scholar]
- 23.Nam MJ, Madoz-Gurpide J, Wang H, Lescure P, et al. Molecular profiling of the immune response in colon cancer using protein microarrays: Occurrence of autoantibodies to ubiquitin C-terminal hydrolase L3. Proteomics. 2003;3:2108–2115. doi: 10.1002/pmic.200300594. [DOI] [PubMed] [Google Scholar]
- 24.Bouwman K, Qiu J, Zhou HP, Schotanus M, et al. Microarrays of tumor cell derived proteins uncover a distinct pattern of prostate cancer serum immunoreactivity. Proteomics. 2003;3:2200–2207. doi: 10.1002/pmic.200300611. [DOI] [PubMed] [Google Scholar]
- 25.Hong SH, Misek DE, Wang H, Puravs E, et al. An autoantibody-mediated immune response to calreticulin isoforms in pancreatic cancer. Cancer Research. 2004;64:5504–5510. doi: 10.1158/0008-5472.CAN-04-0077. [DOI] [PubMed] [Google Scholar]
- 26.Pitteri SJ, JeBailey L, Faca VM, Thorpe JD, et al. Integrated proteomic analysis of human cancer cells and plasma from tumor bearing mice for ovarian cancer biomarker discovery. PLoS One. 2009;4:e7916. doi: 10.1371/journal.pone.0007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J, Hanash S. Autoantibody profiling for cancer detection. Clin Lab Med. 2009;29:31–46. doi: 10.1016/j.cll.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Qiu J, Choi G, Li L, Wang H, et al. Occurrence of autoantibodies to annexin I, 14-3-3 theta and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol. 2008;26:5060–5066. doi: 10.1200/JCO.2008.16.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He P, Naka T, Serada S, Fujimoto M, et al. Proteomics-based identification of alpha-enolase as a tumor antigen in non-small lung cancer. Cancer Sci. 2007;98:1234–1240. doi: 10.1111/j.1349-7006.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 31.Somiari RI, Sullivan A, Russell S, Somiari S, et al. High-throughput proteomic analysis of human infiltrating ductal carcinoma of the breast. Proteomics. 2003;3:1863–1873. doi: 10.1002/pmic.200300560. [DOI] [PubMed] [Google Scholar]
- 32.Tu SH, Chang CC, Chen CS, Tam KW, et al. Increased expression of enolase alpha in human breast cancer confers tamoxifen resistance in human breast cancer cells. Breast cancer research and treatment. 2010;121:539–553. doi: 10.1007/s10549-009-0492-0. [DOI] [PubMed] [Google Scholar]
- 33.Chu PY, Hsu NC, Liao AT, Shih NY, et al. Overexpression of alpha-enolase correlates with poor survival in canine mammary carcinoma. BMC veterinary research. 2011;7:62. doi: 10.1186/1746-6148-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mojtahedi Z, Safaei A, Yousefi Z, Ghaderi A. Immunoproteomics of HER2-positive and HER2-negative breast cancer patients with positive lymph nodes. Omics : a journal of integrative biology. 2011;15:409–418. doi: 10.1089/omi.2010.0131. [DOI] [PubMed] [Google Scholar]
- 35.Kariagina A, Xie J, Leipprandt JR, Haslam SZ. Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers. Hormones & cancer. 2010;1:229–244. doi: 10.1007/s12672-010-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mead MN. Estrogens shield breast cancer cells. Environmental health perspectives. 2007;115:A297. doi: 10.1289/ehp.115-a297a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.d'Elia HF, Carlsten H. The Impact of Hormone Replacement Therapy on Humoral and Cell-mediated Immune Responses In Vivo in Post-menopausal Women with Rheumatoid Arthritis. Scandinavian Journal of Immunology. 2008;68:661–667. doi: 10.1111/j.1365-3083.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- 38.Eilertsen AL, Sandvik L, Steinsvik B, Sandset PM. Differential impact of conventional-dose and low-dose postmenopausal hormone therapy, tibolone and raloxifene on C-reactive protein and other inflammatory markers. Journal of Thrombosis and Haemostasis. 2008;6:928–934. doi: 10.1111/j.1538-7836.2008.02970.x. [DOI] [PubMed] [Google Scholar]
- 39.Kooperberg C, Cushman M, Hsia J, Robinson JG, et al. Can biomarkers identify women at increased stroke risk? The women's health initiative hormone trials. Plos Clinical Trials. 2007;2 doi: 10.1371/journal.pctr.0020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossouw JE, Cushman M, Greenland P, Lloyd-Jones DM, et al. Inflammatory, Lipid, Thrombotic, and Genetic Markers of Coronary Heart Disease Risk in the Women's Health Initiative Trials of Hormone Therapy. Archives of Internal Medicine. 2008;168:2245–2253. doi: 10.1001/archinte.168.20.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straub RH, Hense HW, Andus T, Scholmerich J, et al. Hormone replacement therapy and interrelation between serum interleukin-6 and body mass index in postmenopausal women: A population-based study. Journal of Clinical Endocrinology & Metabolism. 2000;85:1340–1344. doi: 10.1210/jcem.85.3.6355. [DOI] [PubMed] [Google Scholar]
- 42.Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem Biophys Res Commun. 2009;387:381–386. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 43.Pinilla S, Alt E, Abdul Khalek FJ, Jotzu C, et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284:80–85. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Reproducibility of microarrays was assessed by hybridization of samples on multiple arrays. Pearson correlations between replicate spots for two different samples are a) 0.96 and b) 0.91. Data is displayed as the log-2 transform of fluorescent intensity.
Supplementary Figure 2. Plots of autoantibody reactivity medians against SKBR3-arrayed proteins from individual cancer samples separated by clinical characteristics: a) subject age, b) tumor stage at time of diagnosis and c) time of blood draw prior to diagnosis of breast cancer. Samples are separated by hormone therapy use and further separated based on the median value of the examined clinical characteristic. The median subject age was 65. The median time of blood draw prior to diagnosis was 150 days. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, **** indicates p<0.0001.