Abstract
Background
Coronary artery calcium (CAC) measured at a single time point has been associated with an increased risk for atrial fibrillation (AF). It is unknown whether CAC progression over time carries a similar risk.
Methods and Results
This analysis included 5,612 study participants (mean age: 62 ± 10; 52% women; 39% whites; 27% blacks; 20% Hispanics; 12% Chinese-Americans) from the Multi-Ethnic Study of Atherosclerosis. Phantom-adjusted Agatston scores for baseline and follow-up measurements were used to compute change in CAC per year (≤0, 1 to 100, 101 to 300, and >300 units/year). AF was ascertained by review of hospital discharge records and from Medicare claims data through December 31, 2010. Cox regression was used to compute hazard ratios (HR) and 95% confidence intervals (CI) for the association between CAC progression and AF. Over a median follow-up of 5.6 years (25th, 75th percentiles=5.1, 6.8), a total of 203 (3.6%) incident AF cases were detected. Any CAC progression (>0/year) was associated with an increased risk for AF (HR=1.55, 95%CI=1.10, 2.19) and the risk increased with higher levels of CAC progression (≤0/year: HR=1.0 [reference]; 1 to 100/year: HR=1.47, 95%CI=1.03, 2.09; 101 to 300/year: HR=1.92, 95%CI=1.15, 3.20; >300/year: HR=3.23, 95%CI=1.48, 7.05). An interaction was observed by age with the association of CAC progression with AF being stronger for younger (<61 years: HR=3.53, 95%CI=1.29, 9.69) compared with older (≥61 years: HR=1.42, 95%CI=0.99, 2.04) participants (p-interaction=0.037).
Conclusions
CAC progression during an average of 5–6 years of follow-up is associated with an increased risk for AF.
Keywords: coronary calcium, atrial fibrillation, epidemiology
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, affecting nearly 3 million Americans.1 AF represents a substantial burden to the healthcare system with incremental costs projected between $6 billion for AF-related care and $26 billion for AF-related care plus other cardiovascular and non-cardiovascular care.2 Additionally, the prevalence of AF is projected to double by 2050 and this largely is explained by the expected growth in individuals over 65 years of age.1, 3 Due to the rather large burden that will be placed on the health care system to treat AF and finance the care provided, an understanding of risk factors is of paramount importance for the development of targeted preventive interventions.
Recently, we have shown that CAC is associated with an increased risk of AF and the associated risk was found to be stronger for younger compared with older participants.4 These findings suggest that the presence of subclinical atherosclerosis is highly predictive of AF development and implicate vascular age as an important marker of AF risk. It is unclear, however, if the rate of CAC progression over time also will be predictive of AF. Such a finding would suggest that the risk of AF not only depends on the presence of CAC but also on the rate at which the coronary arteries age. Therefore, the purpose of this study was to examine the association of CAC progression with AF in the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS
Study Population
Details of MESA have been reported previously.5 Briefly, between July 2000 and September 2002, a total of 6,814 persons were recruited at 6 field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota). Participants were required to be between 45 and 84 years of age and to have no clinical cardiovascular disease at baseline. All participants provided informed consent and the study protocol was approved by the Institutional Review Boards at each participating institution.
For the purpose of this analysis, we included participants with CAC measurements at baseline (2000–2002) and follow-up (Exam 2: 2002–2004; Exam 3: 2004–2005). Participants were excluded if clinically-recognized AF was present before the follow-up CAC measurement, or if they were missing CAC measurements, baseline characteristics, or follow-up data.
Baseline Characteristics
Participant characteristics were collected during the initial MESA visit. Age, sex, race/ethnicity, income, and education were self-reported. Annual income was categorized as <$20,000 or ≥$20,000 and education was categorized as “high school or less,” or “some college or more.” Smoking was defined as ever (e.g., current or former) versus never smoker. Blood samples were obtained after a 12-hour fast and measurements of total cholesterol, high-density lipoprotein (HDL) cholesterol, and plasma glucose were used. Diabetes was defined as fasting glucose values ≥ 126 mg/dL or a history of diabetes medication use. Blood pressure was measured for each participant after 5 minutes in the seated position. Systolic measurements were recorded 3 separate times and the mean of the last 2 values was used. Aspirin, statin, antihypertensive, and lipid-lowering medication use were self-reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Left ventricular hypertrophy was defined by the Cornell criteria (R wave amplitude AVL plus S wave amplitude V3 ≥ 28 mm males and ≥ 20 mm females) using baseline electrocardiogram data.6
Coronary Artery Calcium
CAC was assessed by cardiac computed tomography (CT) using either cardiac-gated electron-beam CT or multi-detector CT systems, depending on the study site, and the average effective radiation dose per scan in millisieverts (mSv) ranged from 0.6 to 5.6 mSv.7 The CAC score was computed using the phantom-adjusted Agatston score for 2 consecutive scans for each participant and the mean value was used.8 During the CT examinations, the 2 scans were independently analyzed by 2 analysts. Interobserver agreement between different analysts who measured CAC on the same cardiac CT image was excellent (κ-statistic, 0.90). Similarly, intraobserver agreement was excellent when the same analyst measured CAC at separate time periods (κ-statistic, 0.93). Phantom-adjusted Agatston scores for baseline and follow-up measurements were used to compute the change in CAC per year. For those with >0 units of change per year, intervals (e.g., 1 to 100, 101 to 300, and >300 units/year) that have been described previously from MESA were used to compare with participants who had ≤ 0/year change (e.g., no change or reduction in CAC score).9 Participants with any progression (>0/year change) also were compared with those without progression (≤0/year change).
Atrial Fibrillation
Follow-up phone calls to study participants every 9–12 months were used to identify AF events. Medical records, including discharge diagnoses, were obtained for each hospitalization. Additionally, for participants 65 years or older enrolled in fee-for-service Medicare, Medicare claims data were used to identify AF diagnoses in the inpatient setting. Incident AF was defined by International Classification of Disease Ninth Revision codes 427.31 or 427.32.
Statistical analysis
Baseline characteristics were compared by the presence of baseline CAC. Categorical variables were reported as frequency and percentage while continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the chi-square method and for continuous variables using the student’s t-test procedure. Kaplan-Meier estimates were used to compute cumulative incidence of AF by CAC progression and the differences in estimates were compared using the log-rank procedure.10 Follow-up time was defined as the time between the follow-up CT scan until a diagnosis of AF, death, loss to follow-up, or end of follow-up (December 31, 2010). Cox regression was used to compute hazard ratios (HR) and 95% confidence intervals (CI) for the association between CAC progression and AF. Multivariable models were constructed with incremental adjustment as follows: Model 1 adjusted for age, sex, race/ethnicity, education, income, and baseline CAC; Model 2 adjusted for Model 1 covariates plus systolic blood pressure, body mass index, diabetes, smoking, total cholesterol, HDL cholesterol, antihypertensive medications, lipid-lowering therapies, aspirin, and left ventricular hypertrophy. The proportional-hazards assumption was not violated in our analysis. Additionally, we tested for interactions by age (dichotomized at median age), sex, race/ethnicity (white vs. non-white), and the presence of baseline CAC (CAC=0 vs. CAC >0). We examined the graphical dose-response relationship between CAC progression and the multivariable HR for AF using a restricted cubic spline model with incorporated knots at the 5th, 50th, and 95th percentiles.11 Sensitivity analyses were conducted with adjustment for study visit 3 covariates, incident coronary heart disease, and incident heart failure. We also performed a secondary analysis to determine if those with reductions in CAC score (<0/year) had a reduced risk of AF compared with those without change (0/year). Statistical significance was defined as p <0.05. SAS Version 9.4 (Cary, NC) was used for all analyses.
RESULTS
A total of 5,612 study participants (mean age 62 ± 10; 52% women; 39% whites; 27% blacks; 20% Hispanics; 12% Chinese-Americans) were included in the final analysis. At baseline, 2,904 (52%) had no evidence of CAC and 2,708 (48%) had CAC values >0. Participants with baseline CAC were more likely to be older, male, white, and diabetic, and to report smoking, lower educational attainment, and lower income compared with participants without baseline CAC (Table 1). Participants with CAC also were more likely to have higher values for systolic blood pressure and lower values for HDL cholesterol than those without CAC. Antihypertensive medications, aspirin, and lipid-lowering therapies were reported more frequently among those with baseline CAC.
Table 1.
Baseline Characteristics by Coronary Artery Calcium (N=5,612)
| Characteristic | Baseline CAC=0 (n=2,904) | Baseline CAC >0 (n=2,708) | P-value* |
|---|---|---|---|
| Age, mean ± SD, years | 58 ± 9.0 | 66 ± 9.5 | <0.0001 |
| Male (%) | 1,079 (37) | 1,587 (59) | <0.0001 |
| Race | |||
| White (%) | 993 (34) | 1,212 (45) | |
| Chinese-American (%) | 346 (12) | 331 (12) | |
| Black (%) | 904 (31) | 623 (23) | |
| Hispanic (%) | 661 (23) | 542 (20) | <0.0001 |
| Education, high school or less (%) | 948 (33) | 984 (36) | 0.0036 |
| Income, <$20,000 (%) | 739 (25) | 853 (32) | <0.0001 |
| Body mass index, mean ± SD, kg/m2 | 28 ± 5.6 | 28 ± 5.2 | 0.89 |
| Ever smoker (%) | 1,176 (41) | 1,400 (52) | <0.0001 |
| Diabetes (%) | 314 (11) | 479 (18) | <0.0001 |
| Systolic blood pressure, mean ± SD, mm Hg | 122 ± 20 | 130 ± 21 | <0.0001 |
| Total cholesterol, mean ± SD, mg/dL | 194 ± 34 | 195 ± 36 | 0.39 |
| HDL cholesterol, mean ± SD, mg/dL | 52 ± 15 | 49 ± 14 | <0.0001 |
| Antihypertensive medications (%) | 813 (28) | 1,199 (44) | <0.0001 |
| Aspirin (%) | 509 (18) | 803 (30) | <0.0001 |
| Lipid-lowering therapies (%) | 306 (11) | 609 (22) | <0.0001 |
| Left ventricular hypertrophy (%) | 107 (3.7) | 88 (3.3) | 0.37 |
| Baseline CAC, median (25th, 75th percentile) | 0 | 83 (21, 283) | <0.0001 |
Statistical significance for continuous data was tested using the student’s t-test and categorical data was tested using the chi-square test.
CAC=coronary artery calcium; HDL=high-density lipoprotein; SD=standard deviation.
The mean time between CT scans for study participants was 2.4 ± 0.84 years. The median change in CAC/year was 0.26 (25th, 75th percentiles=0, 16.9) units. Participants with baseline CAC were more likely to have CAC progression (n=2,357, 87%) compared with participants without baseline CAC (n=466, 16%) (p<0.0001). The median CAC progression for those with baseline CAC was 18 (25th, 75th percentiles=4.4, 53) units per year, and for those without baseline CAC, it was 0 (25th, 75th percentiles=0, 0) units per year (p<0.0001).
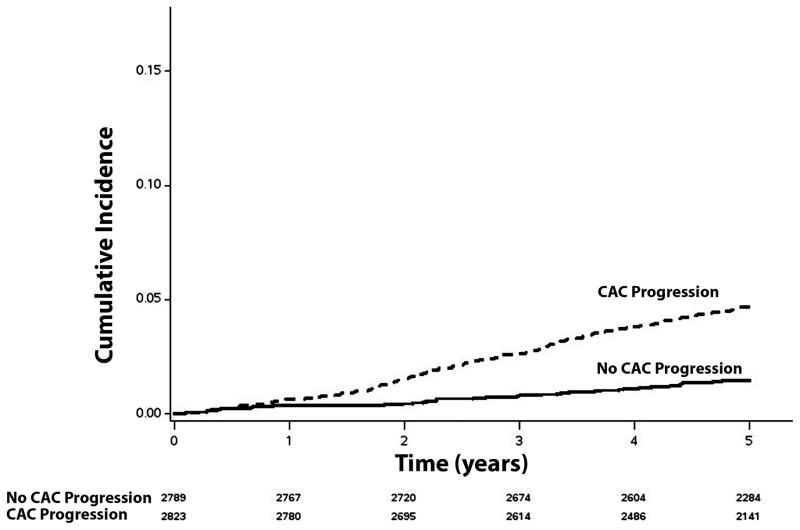
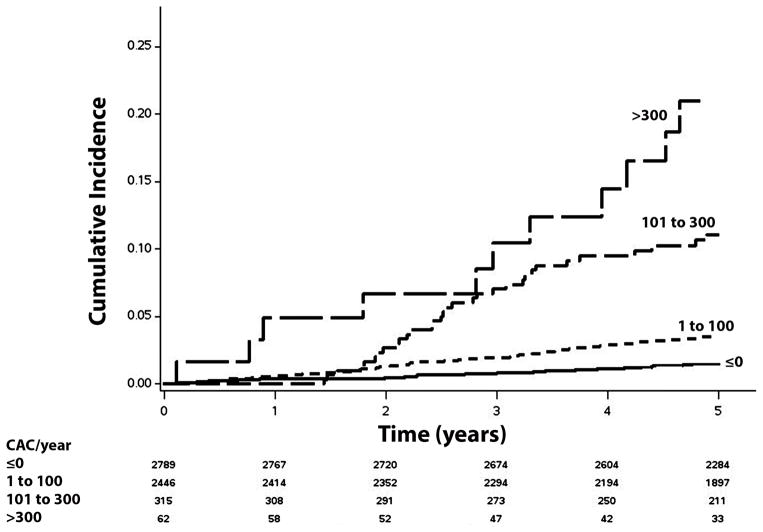
Over a median follow-up of 5.6 years (25th, 75th percentiles=5.1, 6.8), a total of 203 (3.6%) incident AF cases were detected. A higher incidence (per 1000 person-years) of AF was observed in those with CAC (>0/year) progression (incidence rate=10.0, 95%CI=8.6, 11.7) than in those without CAC progression (incidence rate=3.0, 95%CI=2.2, 3.9). The unadjusted cumulative incidence of AF among those with and without CAC progression is shown in Figure 1 (log-rank p<0.0001). Additionally, the incidence rate of AF increased with higher levels of CAC progression (Table 2). The unadjusted cumulative incidence of AF by CAC progression level is shown in Figure 2 (log-rank p<0.0001).
Figure 1. Unadjusted Cumulative Incidence of Atrial Fibrillation by CAC Progression (N=5,612)*.
*Cumulative incidence curves are statistically different (log-rank p <0.0001).
CAC=coronary artery calcium.
Table 2.
Risk of Atrial Fibrillation with CAC Progression (N=5,612)
| Adjusted absolute Δ CAC/year | Events/No. at risk | Incidence Rate per 1000 Person-Years (95%CI) | Model 1* HR (95%CI) | P-value | Model 2† HR (95%CI) | P-value |
|---|---|---|---|---|---|---|
| ≤ 0 | 48/2,789 | 3.0 (2.2, 3.9) | 1.0 | - | 1.0 | - |
| 1 to 100 | 109/2,446 | 8.0 (6.7, 9.7) | 1.52 (1.07, 2.17) | 0.019 | 1.47 (1.03, 2.09) | 0.034 |
| 101 to 300 | 33/315 | 20.3 (14.4, 28.6) | 2.10 (1.27, 3.48) | 0.0041 | 1.92 (1.15, 3.20) | 0.012 |
| >300 | 13/62 | 45.8 (26.6, 78.8) | 3.65 (1.66, 8.02) | 0.0013 | 3.23 (1.48, 7.05) | 0.0032 |
| No progression | 48/2,789 | 3.0 (2.2, 3.9) | 1.0 | - | 1.0 | - |
| Any progression (>0) | 155/2,823 | 10.0 (8.6, 11.7) | 1.62 (1.15, 2.28) | 0.0059 | 1.55 (1.10, 2.19) | 0.013 |
Adjusted for age, sex, race/ethnicity, education, income, and baseline CAC.
Adjusted for Model 1 covariates plus smoking, body mass index, diabetes, systolic blood pressure, total cholesterol, HDL cholesterol, antihypertensive medications, lipid-lowering therapies, aspirin, and left ventricular hypertrophy.
CAC=coronary artery calcium; CI=confidence interval; HDL=high-density lipoprotein; HR=hazard ratio.
Figure 2. Unadjusted Cumulative Incidence of Atrial Fibrillation by CAC Progression Level (N=5,612)*.
*Cumulative incidence curves are statistically different between all groups (log-rank p <0.0001).
CAC=coronary artery calcium.
When we adjusted for age, sex, race/ethnicity, education, income, and baseline CAC, any CAC progression (>0/year) was associated with an increased risk for AF and the risk increased with higher levels of CAC progression (Table 2). Similar results were obtained with further adjustment in Model 2 (Table 2). When the analysis was stratified by sex, race/ethnicity, and the presence of baseline CAC, the association between CAC progression and AF remained similar (Table 3). However, a differential association was observed by age, with the association between CAC progression and AF being stronger for younger (<61 years) compared with older (≥61 years) participants (p-interaction=0.037) (Table 3).
Table 3.
Risk of Atrial Fibrillation with CAC Progression Stratified by Age, Sex, Race/Ethnicity, and Baseline CAC*
| Subgroup | Events/No. at risk | Model 1† HR (95%CI) | P-value | Model 2‡ HR (95%CI) | P-value | Interaction P-value§ |
|---|---|---|---|---|---|---|
| Age, years || | ||||||
| <61 | 24/2,665 | 4.45 (1.68, 11.75) | 0.0026 | 3.53 (1.29, 9.69) | 0.014 | 0.037 |
| ≥61 | 179/2,947 | 1.48 (1.03, 2.11) | 0.033 | 1.42 (0.99, 2.04) | 0.055 | |
| Sex | ||||||
| Female | 74/2,946 | 1.93 (1.14, 3.29) | 0.015 | 1.86 (1.09, 3.18) | 0.024 | 0.31 |
| Male | 129/2,666 | 1.40 (0.90, 2.19) | 0.14 | 1.34 (0.86, 2.10) | 0.20 | |
| Race/Ethnicity | ||||||
| White | 114/2,205 | 1.39 (0.87, 2.23) | 0.17 | 1.44 (0.90, 2.30) | 0.13 | 0.53 |
| Non-White | 89/3,407 | 1.94 (1.17, 3.19) | 0.0096 | 1.77 (1.07, 2.94) | 0.027 | |
| Baseline CAC | ||||||
| CAC = 0 | 50/2,904 | 2.09 (1.16, 3.77) | 0.015 | 2.10 (1.15, 3.84) | 0.016 | 0.24 |
| CAC >0 | 153/2,708 | 1.28 (0.76, 2.15) | 0.35 | 1.32 (0.78, 2.22) | 0.30 | |
HRs presented are for any progression (>0/year).
Adjusted for age, sex, race/ethnicity, education, income, and baseline CAC.
Adjusted for Model 1 covariates plus smoking, body mass index, diabetes, systolic blood pressure, total cholesterol, HDL cholesterol, antihypertensive medications, lipid-lowering therapies, aspirin, and left ventricular hypertrophy.
Interactions tested using Model 2.
Dichotomized at the median age for study participants.
CAC=coronary artery calcium; CI=confidence interval; HDL=high-density lipoprotein; HR=hazard ratio.
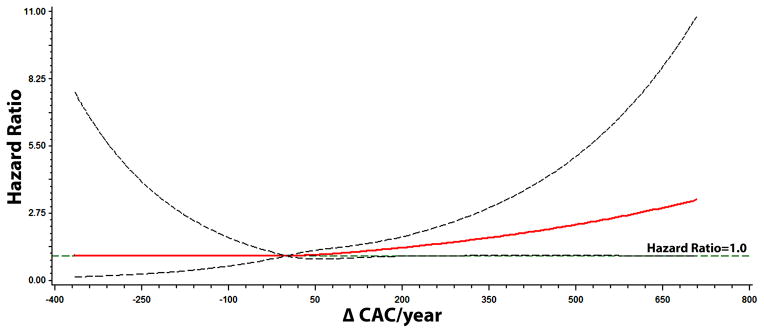
We also observed a dose-response relationship between CAC progression and the risk of AF. Figure 3 shows the association of CAC progression with AF across change in CAC/year computed in Model 2.
Figure 3. Multivariable Risk of Atrial Fibrillation by CAC Progression*.
*Each hazard ratio was computed using a restricted cubic spline model with the median change in CAC/year value of 0.26 as the reference and was adjusted for age, sex, race/ethnicity, education, income, baseline CAC, systolic blood pressure, body mass index, diabetes, smoking, total cholesterol, high-density lipoprotein cholesterol, antihypertensive medications, lipid-lowering therapies, aspirin, and left ventricular hypertrophy. Dotted-lines represent the 95% confidence interval.
CAC=coronary artery calcium.
Any CAC progression (>0/year) was associated with an increased risk for AF when we used time-updated baseline covariates from study visit 3 (HR=1.56, 95%CI=1.09, 2.23), and after adjusting for incident coronary heart disease events (HR=1.54, 95%CI=1.09, 2.17) and incident heart failure events (HR=1.54, 95%CI=1.08, 2.17), separately. The risk of AF was not different in those with reductions (<0/year) in CAC compared with those who had no change (0/year) (HR=1.02, 95%CI=0.48, 2.17).
DISCUSSION
In this analysis from MESA, CAC progression was associated with an increased risk for AF. A dose-response relationship was observed suggesting that the AF risk associated with CAC progression depends on the rate at which CAC develops. Additionally, an interaction was observed when the analysis was stratified by age, with the association being stronger in younger compared with older adults.
Recently, we have shown that single CAC measurements predict incident AF in MESA.4 It was clearly shown that the risk of AF increases with the level of CAC burden at one point in time and the lowest risk is observed in individuals who have baseline CAC values of 0. In the current study, the risk associated with CAC progression was observed to increase with higher levels of CAC change per year. In aggregate, the results of our 2 analyses in MESA suggest that the relationship between CAC and AF depends on the baseline level of CAC detected and the extent of CAC progression over time. Additionally, our data provide evidence that CAC progression is an important risk factor for AF development in persons without CAC from a single measurement. These persons may be deemed “low-risk” by single CAC measurements and subsequently reclassified as a population more likely to develop AF if CAC progression occurs.
The association between CAC progression and AF was found to be stronger among participants <61 years compared with those who were ≥ 61 years of age. A similar difference in AF risk by age was detected in our previous work using single CAC measurements.4 Similarly, in the Coronary Artery Disease in Young Adults (CARDIA) study, the presence of CAC in young and middle-aged adults was associated with an increased risk for adverse cardiovascular outcomes, and this risk increased with higher levels of calcium burden.12 These findings suggest that the presence of subclinical atherosclerosis is highly predictive of future coronary events and demonstrates that young individuals with high CAC burden have an increased predisposition for cardiovascular disease (e.g., vascular age hypothesis). Our data extend previous literature to show that young individuals with high CAC burden also have an increased risk for AF. Additionally, the current study suggests that the rate of CAC progression in young persons is important when assessing AF risk instead of relying on single measurements of coronary calcium. Despite reports that have suggested the risk of radiation-induced cancer from whole body CT scanners is lower than the natural risk of cancer,7 future studies should assess the risk associated with repeated radiation exposure before serial measurements of CAC are recommended to identify persons who are at risk for AF development.
The pathophysiologic link between CAC burden and AF remains unclear. Shared risk factors between CAC development and AF possibly link both conditions (e.g., high blood pressure, low HDL, and obesity).13, 14 However, the association between CAC progression and AF was not materially altered with adjustment for several of these factors. It has been suggested that persons with high levels of CAC also have enlarged left atria and pulmonary veins, structures associated with the arrhythmiogenesis of AF.15, 16 Potentially, persons who experience CAC progression are more likely to have enlargement of these structures which provide the necessary substrate to maintain the arrhythmia. Therefore, the detection of CAC possibly identifies individuals who have the necessary abnormal electrophysiologic connections that predispose to AF and also identifies those who will benefit from risk factor modification strategies (e.g., lipid-lowering therapies) to reduce CAC burden and subsequent AF risk.17 Additionally, persons with CAC are more likely to develop coronary heart disease and heart failure,18, 19 and these conditions are associated with an increased risk for AF development.13 Nonetheless, our findings were not substantively altered with adjustment for these potential mediators, suggesting that heart disease and heart failure do not completely explain our findings. In summary, the findings of this study suggest an association between CAC progression and AF and further studies are needed to determine the underlying pathophysiologic link between these conditions.
Our results should be interpreted in the context of several limitations. CAC progression was based on 2 CT scans over an average interval of 2 years. The progression of CAC was presumed to be linear, however it is possible that increases in CAC burden were not linear and the relationship between CAC progression and AF varies with different intervals. Additionally, we were unable to determine if a baseline CAC value exists in which the AF risk is lowest and repeat CAC measurement is unnecessary. Paroxysmal and asymptomatic cases of AF possibly were missed. However, we do not know of any reason to suggest that the resulting bias, if any, would have been differential in nature, rather than merely reducing effect estimates toward the null (e.g., diminishing power to detect a statistically significant result). Incident AF cases were ascertained from hospitalization discharge records and inpatient Medicare claims data using International Classification of Disease codes, which possibly resulted in misclassification. However, these codes have adequate positive predictive value for the identification of inpatient AF events,20 but miss AF cases managed entirely in the outpatient setting. Furthermore, we included several potential confounders in our multivariable models that likely influenced the development of AF but we acknowledge that residual confounding remains a possibility similar to other epidemiologic studies.
In this analysis from MESA, CAC progression was associated with an increased risk for AF development. Potentially, persons with low levels of CAC who subsequently have progression of CAC represent an at risk population for AF. Additionally, we likely have identified a population with poor cardiovascular risk factor profiles who have the necessary substrate for AF to propagate. Therefore, targeted preventive programs that reduce the burden of subclinical atherosclerosis possibly will reduce the occurrence of AF.
Clinical Perspective.
Atrial fibrillation is the most common arrhythmia encountered in clinical practice. Recently, increased levels of coronary artery calcium, a marker for subclinical atherosclerosis, obtained from single observations were shown to predict incident atrial fibrillation. It was unclear if the rate of coronary artery calcium progression also was predictive of atrial fibrillation. To address this question, we examined the association between coronary artery calcium progression and atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. Coronary artery calcium progression was computed as the mean change per year in the phantom-adjusted Agatston score between two consecutive computed tomography scans. Incident cases of atrial fibrillation were identified by review of hospital discharge records and from Medicare claims data. In this prospective cohort study, coronary artery calcium progression was associated with an increased risk for atrial fibrillation and a dose-response relationship was observed, suggesting that the atrial fibrillation risk depends on the rate at which coronary calcium develops. Potentially, we have identified a population with poor cardiovascular risk factor profiles who also have the necessary substrate for arrhythmia propagation. Our findings also suggest that targeted preventive programs that reduce the burden of subclinical atherosclerosis will reduce the occurrence of atrial fibrillation.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
Footnotes
Disclosures
Dr. Nazarian is a consultant and principal investigator for research funding awarded to Johns Hopkins University from Biosence Webster Inc.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the united states. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the united states. Am J Med. 2011;124:827–833. doi: 10.1016/j.amjmed.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neal WT, Efird JT, Dawood FZ, Yeboah J, Alonso A, Heckbert SR, Soliman EZ. Coronary artery calcium and risk of atrial fibrillation (from the multi-ethnic study of atherosclerosis) Am J Cardiol. 2014;114:1707–1712. doi: 10.1016/j.amjcard.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Casale PN, Eisenberg RR, Miller DH, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. J Am Coll Cardiol. 1984;3:82–87. doi: 10.1016/s0735-1097(84)80433-7. [DOI] [PubMed] [Google Scholar]
- 7.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in population-based studies: Standardized protocol of multi-ethnic study of atherosclerosis (mesa) and coronary artery risk development in young adults (cardia) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 8.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: Mesa (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray RJ, Tsiatis AA. A linear rank test for use when the main interest is in differences in cure rates. Biometrics. 1989;45:899–904. [PubMed] [Google Scholar]
- 11.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511–517. doi: 10.1016/j.jclinepi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Okwuosa TM, Greenland P, Ning H, Liu K, Lloyd-Jones DM. Yield of screening for coronary artery calcium in early middle-age adults based on the 10-year framingham risk score: The cardia study. JACC Cardiovasc Imaging. 2012;5:923–930. doi: 10.1016/j.jcmg.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 14.Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: The muscatine study. J Am Coll Cardiol. 1996;27:277–284. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 15.Pan NH, Tsao HM, Chang NC, Lee CM, Chen YJ, Chen SA. Dilated left atrium and pulmonary veins in patients with calcified coronary artery: A potential contributor to the genesis of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:153–158. doi: 10.1111/j.1540-8167.2008.01290.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartzman D, Lacomis J, Wigginton WG. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol. 2003;41:1349–1357. doi: 10.1016/s0735-1097(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 17.Burgstahler C, Reimann A, Beck T, Kuettner A, Baumann D, Heuschmid M, Brodoefel H, Claussen CD, Kopp AF, Schroeder S. Influence of a lipid-lowering therapy on calcified and noncalcified coronary plaques monitored by multislice detector computed tomography: Results of the new age ii pilot study. Invest Radiol. 2007;42:189–195. doi: 10.1097/01.rli.0000254408.96355.85. [DOI] [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 19.Leening MJ, Elias-Smale SE, Kavousi M, Felix JF, Deckers JW, Vliegenthart R, Oudkerk M, Hofman A, Steyerberg EW, Stricker BH, Witteman JC. Coronary calcification and the risk of heart failure in the elderly: The rotterdam study. JACC Cardiovasc Imaging. 2012;5:874–880. doi: 10.1016/j.jcmg.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and african-americans: The atherosclerosis risk in communities (aric) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]