Abstract
A task-switching paradigm was used to examine differences in attentional control across younger adults, middle-aged adults, healthy older adults, and individuals classified in the earliest detectable stage of Alzheimer's disease (AD). A large sample of participants (570) completed a switching task in which participants were cued to classify the letter (consonant/vowel) or number (odd/even) task-set dimension of a bivalent stimulus (e.g., A 14), respectively. A Pure block consisting of single-task trials and a Switch block consisting of nonswitch and switch trials were completed. Local (switch vs. nonswitch trials) and global (nonswitch vs. pure trials) costs in mean error rates, mean response latencies, underlying reaction time distributions, along with stimulus-response congruency effects were computed. Local costs in errors were group invariant, but global costs in errors systematically increased as a function of age and AD. Response latencies yielded a strong dissociation: Local costs decreased across groups whereas global costs increased across groups. Vincentile distribution analyses revealed that the dissociation of local and global costs primarily occurred in the slowest response latencies. Stimulus-response congruency effects within the Switch block were particularly robust in accuracy in the very mild AD group. We argue that the results are consistent with the notion that the impaired groups show a reduced local cost because the task sets are not as well tuned, and hence produce minimal cost on switch trials. In contrast, global costs increase because of the additional burden on working memory of maintaining two task sets.
Keywords: Aging, Alzheimer Disease, Attention, Task-Switching, Response Latency
Episodic memory loss has long been considered a primary cognitive consequence of Alzheimer Disease (AD; Albert, Moss, Blacker, Tanzi, & McArdle, 2007; Rubin et al., 1998; Storandt, Grant, Miller, & Morris, 2006). There is accumulating evidence that AD is also associated with other aspects of cognition such as working memory and attentional control (Baddeley, Baddeley, Bucks, & Wilcock, 2001; Balota & Faust, 2001; Belleville, Bherer, Lepage, Chertkow, & Gauthier, 2008; Duchek, Balota, Holtzman, Fagan, Tse, & Goate, 2009; Perry & Hodges, 1999; Tse, Balota, Yap, Duchek, & McCabe, 2010), factors causally linked to episodic memory (e.g., Castel, Balota, & McCabe, 2009; Dywan & Jacoby, 1989; Sommers & Huff, 2003). Breakdowns in attentional processes have also been shown to be associated with individuals who are at an increased risk for developing AD (Balota, Tse, Hutchison, Spieler, Duchek, & Morris, 2010; Duchek et al., 2013; Twamley, Ropacki, & Bondi, 2006, for review). Therefore, in order to better understand how early stage AD influences cognition relative to healthy aging, it is particularly useful to examine performance on a task that places a heavy emphasis on controlled attention. In pursuit of this goal, the purpose of the present work is to compare performance on a task-switching paradigm across younger adults, middle-aged adults, healthy older adults, and individuals in the earliest detectable stage of AD.
Although there are multiple task-switching paradigms, the focus of the present study is on paradigms that allow for a comparison of global switch and local switch costs (cf. Clark et al., 2012; Mayr, 2001; Wasylyshyn, Verhaeghen, & Sliwinski, 2011; Wetter et al., 2005). These paradigms typically provide participants with a block of trials composed of both switch and nonswitch trials interleaved within a Switch block, and a separate block of trials containing only single-task sets referred to as a Pure block. Errors and mean reaction times (RTs) generally increase on switch trials relative to nonswitch trials within the Switch block—a difference termed the local switch cost (Belleville et al., 2008; Clark Schiehser, Weissberger, Salmon, Delis, & Bondi, 2012; Kray & Lindenberger, 2000; Reimers & Maylor, 2005; Tse et al., 2010). A common interpretation of local switch costs is that they reflect task-set reconfiguration processes associated with changing task sets across trials (Rogers & Monsell, 1995). The local switch cost can be contrasted to the global switch cost, or the difference in response latency and accuracy between the nonswitch trials within the Switch block and Pure block trials. The global switch cost is thought to reflect the cost associated with maintaining multiple task configurations within the Switch block relative to a single task configuration in the Pure block (Minear & Shah, 2008; Wylie & Allport, 2000).
There is a rich literature examining task-switching components (see Monsell, 2003; Vandierendonck, Liefooghe, & Verbruggen, 2010 for reviews), and a critical contribution of these paradigms is the evaluation of local and global switch costs in both error rates and response latencies. The ability to successfully maintain and respond to changing task demands has been suggested to be a distinct executive process that relies upon an efficient top-down control system. This system has been argued to operate by alternating between activating one task set while simultaneously suppressing the other (Miyake, Friedman, Emerson, Witzki, Howerter, & Wager, 2000) and/or an updating process in which working memory transitions from a maintenance to an updating process in accord with the shifting task set (Mayr, 2001; Mayr, Kuhns, & Hubbard, 2014).
Although task switching has become a central paradigm to investigate attentional control systems, relatively few studies have contrasted both local and global costs to evaluate attentional differences between healthy aging and early stage AD. As an exception, Belleville et al. (2008) had AD and age-matched healthy control participants complete a switch task involving separate blocks of spatial and conceptual task sets. The spatial task set required participants to read a digit presented on the left or right side of a fixation point. The conceptual task required participants to add or subtract the two digits from each other. The Switch blocks pseudo-randomly cued the left/right or addition/subtraction task sets every 5-9 trials. Participants completed both Pure and Switch blocks to calculate local and global switch costs. Relative to age-matched controls, AD individuals produced larger local and global switch costs in both RTs and errors; however, this pattern was restricted to the left/right spatial task. For the addition/subtraction task, only local costs in RTs were greater in AD individuals. The increased left/right global and local costs were attributed to AD-related deficits in reconfiguring and maintaining task sets across Switch block trials, however, equivalent costs of the addition/subtraction task suggests AD individuals may show selective deficits for spatial versus conceptual mathematical tasks.
Tse et al. (2010, also see Duchek et al., 2009) also compared switch and nonswitch trial RTs for very mild AD and healthy control individuals using a bivariant consonant-vowel/odd-even (CVOE; Minear & Shah, 2008) switch task, although the focus of this study was on commonalities across the Stroop, Simon, and Switching tasks. In the CVOE task, a letter-number pair (e.g., C 03) was presented and participants were instructed to classify either the letter as being a consonant/vowel or the number as odd/even based on a cued task set. In contrast to Belleville et al., switch and nonswitch trials varied in a predictable sequence in which task sets switched every two trials (AABBAA), a pattern known as the alternating-runs sequence (see Rogers & Monsell, 1995). Tse et al. found that local costs in RT were lower for very mild AD individuals than healthy controls. Tse et al. suggested that the reduced local cost in very mild AD was due to an attentional control deficit that reduced the likelihood that these participants would suppress one of the task sets across trials, keeping both task sets relatively active as trials switched.
Though not a focus of the present study, we note briefly here that there are several methodological differences that could account for the discrepancies between Belleville et al. (2008) and Tse et al. (2010). First, as noted above, Belleville et al. presented switch and nonswitch trials that changed unpredictably every 5-9 trials which may have increased the demand for controlled processing (Tornay & Milan, 2001) resulting in a larger cost for AD individuals. Second, as Belleville et al. demonstrated by evaluating spatial and conceptual switch tasks, AD individuals may show task-specific deficits that produce exaggerated decrements on some tasks but not others. Thus, AD-related switch cost differences may be driven by task differences rather than more general switching effects. Finally, Belleville et al. had participants complete several practice blocks which may have benefitted healthy controls more than AD individuals by reducing local costs.
In contrast to the Belleville et al study, the task sets used in CVOE switching by Tse et al. (2010) required participants to classify alpha-numeric characters and participants were not provided with extensive practice. Deficits in attentional control in advancing age and early-stage AD may therefore be magnified because these individuals were not provided with additional practice that may offset performance declines. If Tse et al. are correct in suggesting that increased age and presence of AD may lead to both task sets remaining relatively active across trials (thereby producing reduced local costs), one might expect that the maintenance of activation of both task sets would come at an increased global cost for the relatively impaired individuals coupled with a reduced local switch cost. Consistent with this pattern, Duchek et al. (2009) reported an overall increased global cost and a reduced local cost in AD individuals relative to healthy controls and younger adults, though their study focused primarily on intraindividual variability in trial responding across three attentional control tasks, instead of focusing on task-switching. Therefore, to more closely examine the local cost pattern of Tse et al. (2010) and Duchek et al., and to evaluate these costs within the context of global costs, the goal of the present study is to evaluate local and global costs across a broad age range of individuals including younger adults, middle-aged adults, older adults, and individuals with early stage AD, using an attentional switch task consisting of a Switch block using an alternating-runs sequence and Pure blocks. Moreover, to examine the nature of the global and local cost differences across groups, we also evaluated how response differences affect response time distributions, which we will now discuss.
RT Distribution Analyses and Switch Costs
The vast majority of attentional tasks rely upon mean (or median) response latencies and accuracy as the primary dependent measures. However, researchers have also noted that variables can have distinct influences on underlying RT distributions which may differ as a function of age and AD status (Hultsch, MacDonald, & Dixon, 2002; Hultsch, Strauss, Hunter, & MacDonald, 2008; Spieler, Balota, & Faust, 1996; Tse et al., 2010). Therefore, the present study sought to more accurately characterize age- and AD-related switch costs by including a Vincentile analysis. This analysis rank orders all RTs for each trial type within each participant and groups the rank ordered data into bins containing an equal number of trials. For example, to obtain six Vincentiles, trials are ordered from fastest to slowest RTs and the first 16.67% are averaged and placed into the first bin, the second 16.67% of trials averaged into the second bin, and so on.
There is accumulating evidence that characteristics of underlying reaction time distributions can dissociate across young and older adults and individuals with very mild AD (see Balota & Yap, 2011; Castel et al., 2009). In particular, increasing age and the presence of very mild AD is related to an exaggerated slowing in trials found in the last few bins which captures the slowest responses in the distribution (Tse et al., 2010). The slowest RTs show the strongest correlations with critical constructs such as working memory capacity (Schmiedek, Oberauer, Wilhelm, Süβ, & Wittman, 2007; Tse et al. 2010). Variables or individual difference measures that influence the slowest RTs have also been attributed to lapses of attention (West, 1996; West, Murphy, Armilio, Craik, & Stuss, 2002) or increasing demands of memory retrieval processes (Balota, Yap, Cortese, & Watson, 2008). Given the sensitivity of the slow bins of multiple variables and constructs, we expect that advancing age and the presence of early stage AD would show exaggerated effects in the slowest trials in both local and global costs. In this light, the Vincentile analysis is expected to provide a novel characterization of attentional impairment in task-switching.
Although Tse et al. (2010) primarily focused on ex-Gaussian analyses, they also included a Vincentile analysis on overall reaction time in the Switch block, collapsing across switch and nonswitch trials. Indeed, they found group-related differences were exaggerated in the slowest responses, however, they did not compute local switch costs for Vincentile bins. Given local switch costs show age- and AD-related differences, it is important to also determine whether exaggerated differences in the slowest responses also persist when local switch costs are calculated. Further, the absence of a pure block in Tse et al. precluded an analysis of pure trial response distributions and their comparison to nonswitch trials to examine global costs. Our study therefore included pure trial Vincentiles and calculated local and global costs to provide a comprehensive characterization of task-switching processes on reaction time distributions in healthy aging and AD impairment.
Congruency Effects in Errors and Response Latencies
The CVOE task-switching paradigm also affords a comparison of trials in which a correct response to a given task set is made with the same response key (response congruent trials) versus separate keys (response incongruent trials). Specifically, vowel/even and consonant/odd responses are considered congruent trials because both stimulus dimensions mapped onto the same response key (e.g., A 06 and C 03, respectively). In contrast, vowel/odd and consonant/even combinations are considered incongruent trials (e.g., D 06) because the stimulus dimensions were mapped onto two separate response keys. Incongruent trials are likely to create an additional response conflict due to the divergent key mappings of stimulus pairs. Researchers have shown that response conflicts in other attentional tasks such as the Simon and the Stroop-switch paradigms are particularly sensitive to age- and AD-related differences. Specifically, both errors and RTs have been shown to increase when a response conflict occurs on incongruent than congruent trials (Castel, Balota, Hutchison, Logan, & Yap, 2007; Hutchison, Balota, & Duchek, 2010). We therefore expected that errors and RTs would be greater for incongruent than congruent trials for older and very mild AD individuals, providing a third method to characterize attentional differences across groups.
Present Research
The present study included four groups of participants which included three groups of healthy adults (younger, middle-aged, and older adults) and one group of older adults who are at the earliest detectable stage of Alzheimer’s disease. It is noteworthy that these early-stage AD individuals are relatively high functioning, as reflected by their mean Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975) of 26.62, though they are highly likely to display AD pathology at autopsy (Storandt et al., 2006).
All participants completed three blocks of trials: Two Pure blocks of trials (separate consonant/vowel and odd/even blocks), followed by a Switch block that used AABBAA alternating-runs sequencing. Individuals with relatively well-tuned attentional systems (i.e., younger and middle-aged adults) are expected to show reduced global costs as they are better able to maintain two task sets relative to individuals with relatively impaired attentional systems (i.e., older adults and very mild AD individuals). However, a well-tuned system may produce an exaggerated local cost as described above. The analyses of reaction time distributions through Vincentile bins provide greater understanding of the nature of the group differences in local and global switch costs. Finally, by examining the congruency effects, we can further characterize the demands on attentional selection of congruent versus incongruent stimulus-response mappings within a trial during the Switch block.
Method
Participants
A large sample of 570 individuals were recruited to participate in this study with 30 healthy younger adults, 437 non-demented healthy adults (Clinical Dementia Rating; CDR = 0), and 104 very mild AD older adults (CDR = 0.5). Younger adults were recruited from Washington University Psychology Department’s research pool (Mage = 20.23; Range = 18-221) and the remaining participants were recruited from the Charles and Joanne Knight Alzheimer Disease Research Center (Knight ADRC). Healthy adults were further divided into middle-aged adults (age ≤ 67; Mage = 58.08; Range = 30-67; 71% Female) and older adults (age > 68; Mage = 75.92; Range = 68-95; 57% Female). Very mild AD individuals (Mage = 75.15; Range = 51-94; 47% Female) were grouped together regardless of age (see Table 1 for Age, Education, and Psychometric task performance for Knight ADRC participants), but overall matched the healthy older adult sample in mean age. As mentioned above, a subset of these data (30 younger adults, 246 healthy adults and 74 very mild AD older adults) was reported in Tse et al. (2010), but Pure trials were not reported. Importantly, the current set of analyses includes a larger sample separated into three healthy groups and one very mild AD group, and provides contrasts of both global and local switch costs as well as Vincentile analyses of these costs, and congruency effects. By separating our healthy adults into younger, middle-age and older adult groups (as opposed to only young undergraduates versus old in the earlier papers), we can more clearly examine switch costs as a function of age in the present report.
Table 1.
Psychometric Statistics as a Function of Group
| Younger Adults |
Middle-Aged Adults |
Older Adults |
Very Mild AD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | N | SD | M | N | SD | M | N | SD | M | N | SD | |
| Variable/Task | ||||||||||||
| Age (years) | 20.23a | 30 | 1.01 | 58.08b | 217 | 7.35 | 75.92c | 219 | 5.90 | 75.15c | 104 | 7.74 |
| Education (years) | -- | -- | -- | 15.95a | 208 | 2.45 | 15.33b | 214 | 2.80 | 14.95b | 104 | 2.84 |
| Mini Mental State Exam | -- | -- | -- | 29.32a | 208 | 1.04 | 28.66b | 213 | 1.41 | 26.62c | 104 | 3.12 |
| Selective Reminding | -- | -- | -- | 32.68a | 208 | 5.84 | 29.28b | 214 | 5.64 | 20.46c | 96 | 9.00 |
| Animal Fluency | -- | -- | -- | 23.34a | 207 | 5.86 | 19.76b | 214 | 5.71 | 16.23c | 104 | 5.67 |
| Trail Making A | -- | -- | -- | 26.84a | 207 | 9.32 | 34.85b | 214 | 11.69 | 43.93c | 104 | 23.52 |
Note. Very Mild AD = CDR 0.5 status. Values within each row with different subscripts differ significantly (p < .05, two-tailed).
Knight ADRC participants were screened for other forms of cognitive impairment (e.g., depression, hypertension, etc.) to be consistent with the criteria for “probable AD” as determined by the National Institute of Neurological and Communication Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association (McKhann, Drachman, Folstein, Katzman, Price, & Stadlan, 1984). Dementia ratings were assessed using the Washington University CDR scale (Morris, 1993), which assesses dementia severity with CDR 0, 0.5, 1, 2, and 3, corresponding to no dementia, very mild, mild, moderate, and severe dementia, respectively. The CDR assessment encompasses a 90-min clinical interview that assesses participant functioning and gathers information from a close collateral source (e.g., family member). The CDR assesses changes in cognition and function in such domains as memory, orientation, problem solving, community involvement, and personal care. The assessment methods used permit the diagnosis of AD in individuals who may be characterized as having mild cognitive impairment elsewhere (Berg et al., 1998; Morris et al., 2001). Importantly, the reliability and validity of the diagnosis at autopsy have been quite high (93% accuracy), even for those with very mild AD (Storandt et al., 2006).
Psychometric testing
ADRC participants completed one of two different psychometric batteries depending on which project they were enrolled in. These batteries were completed during a separate session in which the experimenter was blind to the participant’s CDR status. Included in both batteries were the MMSE (Folstein et al., 1975), Animal Naming (Goodglass & Kaplan, 1983), Selective Reminding (Buschke, 1973), and Trial Making A (Goodglass & Kaplan) tasks. Group comparisons of these psychometric tasks are displayed in Table 1 and task performance is in the expected direction across age groups and the presence of AD.
Procedures
The CVOE switching task included two sets of task instructions across trials, which differed in the required response to a letter/number stimulus pair. On each trial, a letter-number stimulus (e.g., D 06) was displayed at the center of the computer screen and participants were instructed to classify whether the letter was a consonant or vowel (CV) or if the number was odd or even (OE). Letters used were always either A, D, E, H, I, J, O, P, S, or U, in which 5 were consonants and 5 were vowels. The numbers used were randomly selected between 1 and 99 such that half of the numbers were odd and the other half were even. Letters and numbers did repeat within the Pure and Switch blocks with the specification that repeats did not occur on consecutive trials. Trials required participants to respond either to the letter or number and the response type was specified by a cue (either the words consonant/vowel or odd/even). Depending on the cued response type, the words consonant/vowel or odd/even were presented at the top left and right corners of the computer screen, respectively, to serve as a reminder. Participants were instructed to press the d key when responding either consonant or odd, and the k key when responding either vowel or even. Trials were such that correct responses were distributed equally between the d key and the k key. Stimuli were presented in 24-point Courier New font. Trials were presented without an inter-trial delay.
Participants first completed 10 practice trials with feedback and then completed three blocks of trials. The blocks were always ordered such that the first two blocks were Pure blocks and the third block was a Switch block (modified from Minear & Shah, 2008). Each Pure block contained 48 trials and participants were only required to make responses to either the letter or the number with one Pure block providing cues to respond to the letter and the other Pure block providing cues to respond to the number. Pure blocks were always presented with CV trials first followed by OE trials. The Switch block contained 60 trials that were presented in an alternating-runs sequence in which cues for one trial were presented successively and then switched to the other trial type successively and so forth (e.g., CV, CV, OE, OE, CV, CV). This sequencing resulted in 29 switch trials (e.g., an OE trial preceded by a CV trial) and 31 nonswitch trials (e.g., an OE trial preceded by an OE trial). Congruent and incongruent trials were presented in a pseudo-random order that was not correlated with ordering of CV/OE trials. Both the response cue and the stimulus were presented simultaneously and the stimulus remained on the computer screen until participants made a response. Participants were instructed to respond as quickly as possible without compromising accuracy. Additionally, on the Switch block, participants were instructed before the block about the order of the trials (i.e., the alternating-runs sequencing). The task was presented using E-Prime software (Schneider, Eschman, & Zuccolotto, 2002) on a CRT-VGA monitor and participants were tested individually. No participants reported issues with being able to see the CV/OE stimuli.
Results and Discussion
For all results reported, statistical significance was set at p < .05 two-tailed, unless otherwise noted. Effect sizes of ηp2 are reported for significant F-tests and Cohen’s d for significant t-tests. For all RT analyses, only correct trials were utilized. In order to avoid the analyses being unduly influenced by extreme scores, RT outliers were defined as RTs less than 200 ms (likely anticipation RTs), greater than 10,000 ms (likely off-task RTs), and those above or below 3 standard deviations of each participant's mean. The RT trimming procedure eliminated 1% or less of trials within Pure blocks across groups, 2.0%, 2.7%, 4.7%, and 7.5% of nonswitch trials for younger, middle-aged, older, and very mild AD individuals, respectively, and 7.5%, 4.9%, 4.9%, and 6.1% of switch trials from those same groups.
In the following analyses, we first examine the errors, mean RTs, and z-transformed RTs (to control for group-related slowing, see Faust, Balota, Ferraro, & Spieler, 1999) as a function of group and trial type followed by an age comparison of global and local switch costs. As noted, local costs were calculated by subtracting nonswitch trial responses from switch trial responses within the Switch block. Global switch costs were calculated by subtracting pure trial responses from nonswitch trial responses within the Switch block. Mean Vincentiles for each group of participants were then plotted to produce the RT distribution profile for each participant group as a function of condition. Finally, congruency effects are reported which reflect either congruent or incongruent cross-trial response mapping in the Switch block.
Errors
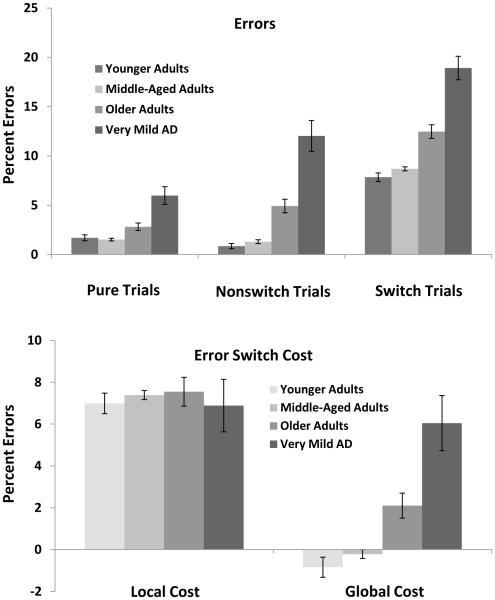
Error percentages as a function of trial type and group are displayed in Figure 1. Error rates were greatest in the switch (12.0%) relative to nonswitch trials (4.8%), which in turn, were greater than the pure trials (3.0%). In addition, errors increased across the young (3.5%), middle (3.8%), older (6.7%), and very mild AD (12.3%) groups. These patterns were confirmed by significant main effects of Trial Type, F(2, 1132) = 193.67, MSE = 35.6, ηp2 = .26, and Group, F(3, 566) = 45.64, MSE = 117.46, ηp2 = .20. These main effects were qualified by a significant interaction, F(6, 1132) = 8.89, MSE = 35.61, ηp2 = .05, which was due to a larger increase in errors on nonswitch and switch trials for older and very mild AD groups than younger and middle-aged adults. Specifically, post hoc comparisons revealed that for younger and middle-aged adults, errors on pure and nonswitch trials were equivalent, ts < 1.83, ps > .08, but increased across nonswitch and switch trials, ts > 12.97, ds > 3.25. For older and very mild AD individuals, errors increased sharply across pure, nonswitch, and switch trials, all ts > 3.52, ds > 0.26.
Figure 1.
Mean percentage errors as a function of pure, nonswitch, and switch trials for each group (top) and local and global costs as a function of group (bottom). Local cost is calculated as the error percentage on switch trials minus the error percentage nonswitch trials. Global cost is calculated as the error percentage on nonswitch trials minus the error percentage on pure trials. Error bars indicate standard errors of the mean.
Local and Global switch costs in errors
As shown in the bottom panel of Figure 1, no differences occurred in local costs in errors rates across groups, F <1. However, a group effect was found in global costs, F(3, 566) = 14.77, MSE = 67.21, ηp2 = .07. Post hoc comparisons revealed that global costs were equivalent among younger adults (−0.8%), middle-aged adults (−0.2%), and older adults (2.1%), all ts < 1.81, ps > .07. However, global costs were greater for very mild AD individuals (6.1%), relative to all other groups, ts > 2.78, ds > 0.48.
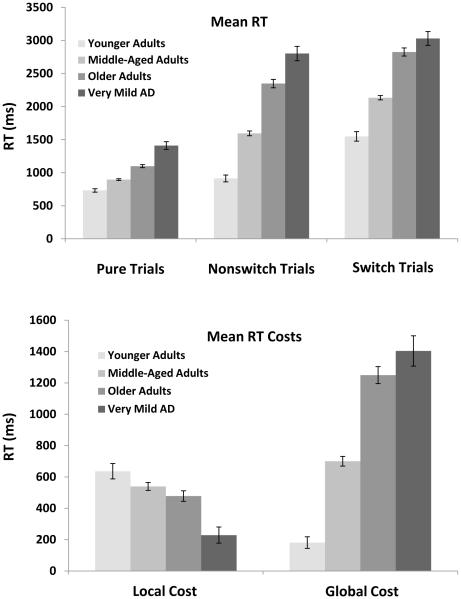
Mean RT
As displayed in the top panel of Figure 2, participants were faster to respond to pure trials followed by nonswitch and switch trials. As expected, there were also group differences in overall response latencies, with the very mild AD individuals producing the slowest responses. These patterns were confirmed by main effects of Trial Type, F(2, 1128) = 741.09, MSE = 195252, ηp2 = .57, and Group, F(3, 564) = 80.39, MSE = 1038148, ηp2 = .30. Also a significant interaction was found, F(6, 1128) = 33.96, MSE = 6629876, due to an exaggerated increase in mean RTs for older and very mild AD individuals compared to young and middle-aged adults, for trials in the Switch block relative to the Pure block, all ts > 4.42, ds > 0.21.
Figure 2.
Mean reaction time (RT) for pure, nonswitch and switch trials from each group (top) and mean RT local and global costs as a function of group (bottom). Local cost is calculated as the RT for switch trials minus the RT for nonswitch trials. Global cost is calculated as the RT for nonswitch trials minus the RT for pure trials. Error bars indicate standard errors of the mean.
Local and Global switch costs in mean RT
As shown in the bottom panel of Figure 2, local switch costs appeared to be greatest for younger adults, followed by middle-aged, older, and very mild AD individuals. This decrease was confirmed by a significant one-way ANOVA, F(3, 564) = 13.00, MSE = 2617589, ηp2 = .07. Post-hoc comparisons revealed that local costs were only numerically greater for younger adults than middle-aged adults, t(245) = 1.38, SEM = 37.24, p = .17, and marginally greater for younger adults than older adults, t(246) = 1.70, SEM = 41.49, p = .09, d = 0.22. Local costs were similarly equivalent between middle-aged and older adults, t(433) = 1.46, SEM = 29.42, p = .15, however, local costs in these three groups were all greater than the very mild AD individuals, ts > 4.12, ds > 0.46.
Turning to global switch costs—the response difference between nonswitch trials in the switch block and pure trials—the opposite pattern was found. Younger adults actually showed the smallest global switch cost which increased across middle-aged, older, and very mild AD individuals, F(3, 564) = 46.33, MSE = 23321652, ηp2 = .20. Global costs increased significantly across younger, middle-aged, and older adults, ts > 6.24, ds > 0.79, but were similarly equivalent between older and very mild AD individuals, t(319) = 1.49, SEM = 72.81, p = .14.
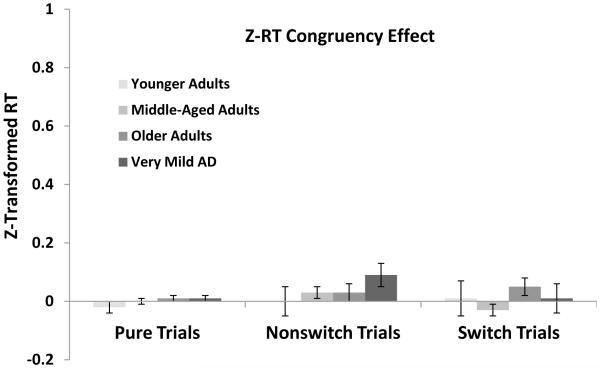
Standardized Response Latencies
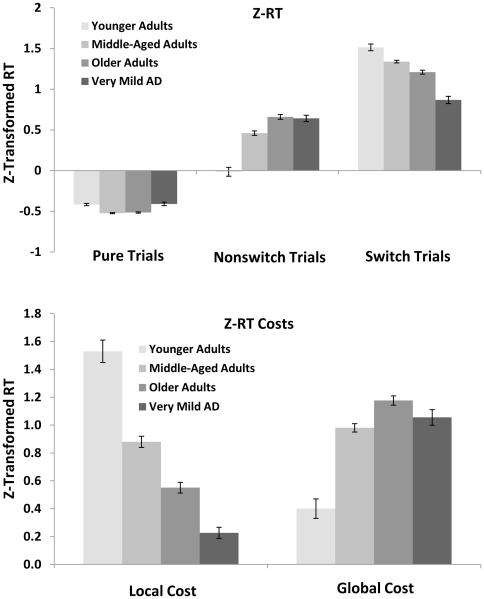
One needs to be cautious in interpreting the effects of variables when mean response latency varies dramatically across groups. Hence, we also report a z-transformation on raw RTs to control for group-related processing speed differences (see Faust et al., 1999) in which response latencies were converted to z-scores separately for each individual based on their mean and standard deviation across trial types. Figure 3 (top panel) summarizes participants’ z-transformed RTs for each of the three trial types for each of the groups. Responses were fastest on pure trials followed by nonswitch and switch trials, a pattern confirmed by a main effect of Trial Type, F(2, 1128) = 1888.84, MSE = .12, ηp2 = .77. An effect of Group was also found, F(3, 564) = 9.77, MSE = .06, ηp2 = .05, revealing a quadratic pattern in which z-scores increased from younger adults to middle-aged adults and older adults, then decreased for very mild AD. The Group × Trial Type interaction was highly significant, F(6, 1128) = 46.05, MSE = .12, ηp2 = .20, showing that for pure trials, z-scores decreased across younger adults, middle-aged adults, and older adults, but increased for very mild AD. Confirming this pattern, pure trial responses for younger adults were equivalent to very mild AD, t < 1, as were middle-aged adults and older adults, t < 1, and all other comparisons differed reliably, ts > 4.50, ds > 0.57. For nonswitch trials, z-scores showed a linear increase across groups, though older adults were equivalent to very mild AD, t < 1, all other ts > 3.80, ds > 0.42. For switch trials however, z-scores were greatest for younger adults and showed a linear decrease across the remaining groups, all ts > 3.75, ds > 0.47.
Figure 3.
Z-transformed reaction time (RT) for pure, nonswitch and switch trials from each group (top) and mean RT local and global costs as a function of group (bottom). Local cost is calculated as the RT for switch trials minus the RT for nonswitch trials. Global cost is calculated as the RT for nonswitch trials minus the RT for pure trials. Error bars indicate standard errors of the mean.
Local and Global Switch Costs in z-scores
Local and global switch costs in z-scores for each of the groups are displayed in Figure 3 (bottom panel). Consistent with the mean RT costs described above, local costs were largest for younger adults and decreased across middle-aged, older and very mild AD groups. This pattern was confirmed by a one-way ANOVA, F(3, 566) = 61.28, MSE = .30, ηp2 = .25, and corresponding post hoc comparisons, all ts > 5.17, ds > 0.57.
Turning to the global costs, the pattern was in the opposite direction: Response costs were smallest for younger adults, increased for middle-aged and older adults, then decreased slightly for very mild AD individuals—a pattern confirmed by a separate one-way ANOVA, F(3, 566) = 21.86, MSE = .25, ηp2 = .10. Post-hoc comparisons confirmed that global costs increased between younger adults and middle-aged adults, t(245) = 6.30, SEM = .05, d = 0.80, between middle-aged adults and older adults, t(433) = 4.09, SEM = .03, d = 0.39, but decreased marginally between older adults and very mild AD individuals, t(319) = 1.95, SEM = .04, p = .05, d = 0.22. Global costs were equivalent between middle-aged adults and very mild AD individuals, t(318) = 1.15, SEM = .05, p = .25. Thus, after controlling for general slowing, there is a large and systematic decrease in local costs across groups, however global costs increased primarily between the younger, middle-aged, and older adults with a slight decrease in global costs for very mild AD individuals relative to older adults. Of course, this slight decrease in global costs for the very mild AD in z-transformed response latencies comes with a considerable increased global cost in error rates as noted above.
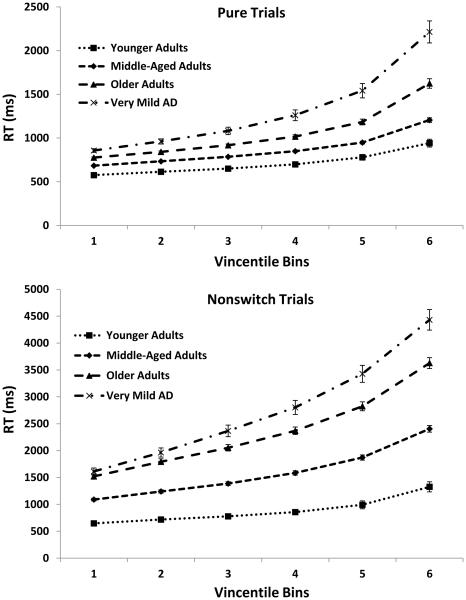
Vincentile Plots
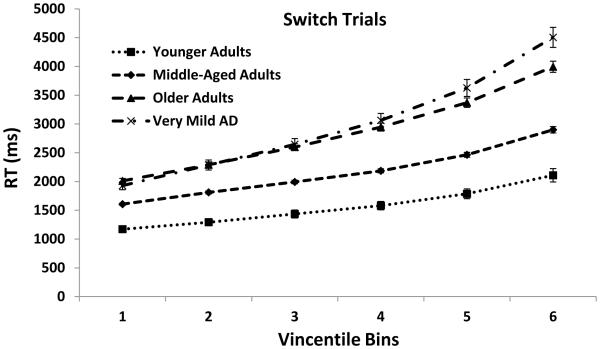
Figure 4 displays the Vincentile plots separated by trial type for the four groups of participants. RTs used to construct the plots were the same RTs used in the mean RT analyses reported above (i.e., the same outliers were removed). As can be seen, RTs increased across bins, were lowest for pure trials followed by nonswitch trials and switch trials, and were lowest for younger adults followed by middle-aged, older, and very mild AD individuals. These patterns were all confirmed by significant effects of Bin, F(5, 5620) = 1069.70, MSE = 60210, ηp2 = .66, Trial Type, F(2, 5620) = 738.01, MSE = 60210, ηp2 = .57 and Group, F(3, 562) = 78.47, MSE = 6132434, ηp2 = .20. In addition, the analyses yielded a reliable three-way Bin × Trial Type × Group interaction, F(30, 5620) = 15.55, MSE = 60210, ηp2 = .08. This interaction revealed that the increase in RT across the distribution was steeper as age increased and with the presence of AD, particularly for nonswitch and switch trials relative to pure trials.
Figure 4.
Mean RT Vincentile bin data points and standard errors for pure, nonswitch, and switch trials.
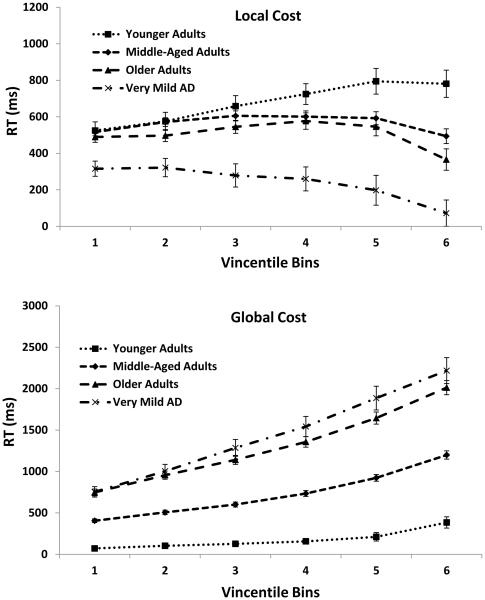
Local and Global Switch Costs
Local and global switch costs for each of the Vincentile bins are presented in Figure 5. An interesting dissociation was found between local and global costs across bins and groups. Beginning with local costs, younger adults showed an increased cost across bins, F(5, 145) = 12.28, MSE = 29687, ηp2 = .30, middle-aged adults showed an increased cost in the first few bins followed by a decrease across remaining bins, F(5, 1080) = 27.26, MSE = 46559, ηp2 = .11, older adults showed a similar increased cost in the first few bins followed by a decrease across in the final bins, F(5, 1075) = 17.79, MSE = 212777, ηp2 = .08, and very mild AD individuals produced a general decreased cost beginning with the second bin, F(5, 510) = 6.19, MSE = 178299, ηp2 = .06. Group differences in these local costs across bins were confirmed by a significant Group × Bin interaction, F(15, 2810) = 4.28, MSE = 98374, ηp2 = .02. As shown, group dissociations were more pronounced in the slowest bins.
Figure 5.
Local and global Vincentile costs for each group in mean raw RTs. Error bars indicate standard errors of the mean.
Turning to global costs, both younger and middle-aged adults showed relatively gradual increases across bins, F(5, 145) = 23.91, MSE = 16021, ηp2 = .46, and F(5, 1080) = 385.97, MSE = 53746, ηp2 = .64, respectively, whereas this increase becomes steeper for older adults, F(5, 1085) = 296.97, MSE = 178444, ηp2 = .58, and very mild AD individuals, F(5, 510) = 116.90, MSE = 294898, ηp2 = .53. The Group × Bin interaction was once again significant, F(15, 2820) = 23.22, MSE = 143397, ηp2 = .11, demonstrating that costs are more pronounced in the slowest Vincentiles—a pattern consistent with local costs.
Taken together, these patterns suggest a clear dissociative effect for local and global costs in response time distributions. Specifically, for the more impaired participants, local costs were more likely to decrease across Vincentiles, whereas, global costs were more likely to increase across Vincentiles. It is important to note that such a dissociative pattern in Vincentiles cannot be due to general slowing since the slower response latencies in the more impaired groups, compared to the less impaired groups, actually produce increasing global costs, but decreased local costs2. In addition, our finding that costs show greater group dissociations in the slowest bins is consistent with other RT distributional studies (e.g., Hultsch et al., 2002; Spieler et al., 1996), that show attentional processes are particularly sensitive to the slowest RTs.
Congruency Effects
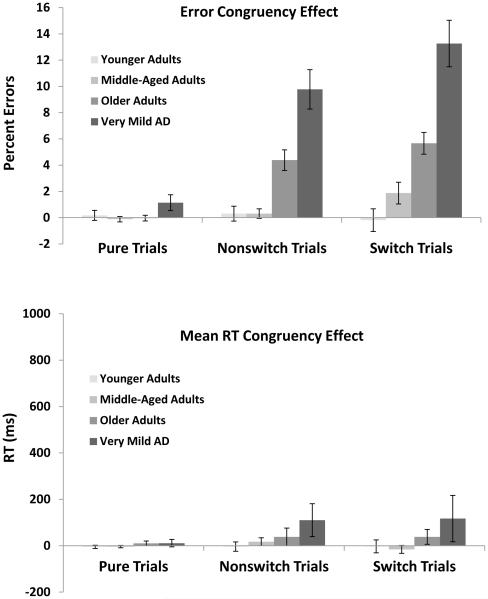
Congruency effects in errors
In order to further evaluate attentional differences across groups, Switch block trials were further divided into congruent (e.g., A 06, vowel/even responses mapped to the same key) and incongruent responses (e.g., D 06, consonant/even responses mapped to separate keys), with the difference between these two trial types reflecting a congruency effect. Congruency effects for both errors and RTs are presented in Figure 6. As shown in the top panel, congruency effects were greater on nonswitch and switch trials and these effects were particularly large in older adults and more so for very mild AD individuals. These patterns were confirmed by significant effects of Trial Type, F(2, 1128) = 30.82, MSE = .01, ηp2 = .05, Group, F(3, 564) = 31.99, MSE = .01, ηp2 = .15, and a significant interaction, F(6, 1128) = 12.52, MSE = .01, ηp2 = .06. Planned comparisons indicated that for younger adults, congruency effects did not differ between trial types, ts < 1, and middle-aged adults showed an increase in switch trials relative to pure and nonswitch trials, ts > 3.50, ds > 0.28, which in turn, did not differ, t < 1. In contrast, older and very mild AD individuals showed an increase across pure, nonswitch, and switch trials, all ts > 2.55, ds > 0.20, with the exception that for older adults, pure and nonswitch trials did not differ, t < 1. Therefore, congruency effects in errors become exaggerated for older adults and very mild AD individuals.
Figure 6.
Congruency effect for errors (top), mean RT (middle), and z-transformed RT (bottom) for pure, nonswitch, and switch trials as a function of group. Congruency effects are calculated as incongruent trials minus congruent trials. Error bars indicate standard errors of the mean.
Congruency effects in mean RTs and z-scores
Congruency effects for RTs were calculated in an identical fashion as errors3. The effects of trial type and group appear to be relatively similar across groups, as reflected by nonsignificant main effects of Congruency, Group, and a nonsignficant interaction between Congruency and Group, all Fs < 1.01. Similar analyses were conducted on z-scores and yielded an identical pattern.
General Discussion
The primary purpose of the present research was to examine local and global switch costs using an attentional-switch task to characterize differences in attentional processes in younger adults, middle-aged adults, healthy older adults, and individuals with very mild AD. Comparisons between local and global costs allowed for an assessment of different hypothesized processes involved in task-switching. Local costs were measured by comparing switch and non-switch trials presented in the Switch block which evaluated performance differences when the task set from the preceding trial changed or remained the same. Global costs were measured by comparing single-task trials in the Pure block to nonswitch trials in the Switch block to evaluate performance when two task sets (vs. one) are maintained.
Our study sought to provide a comprehensive evaluation of global and local switch costs across age groups and very mild AD individuals. We note that Duchek et al. (2009) reported a similar dissociation between local and global costs and Tse et al. (2010) examined underlying RT distribution characteristics in local costs. We depart from these earlier works that focused on intraindividual variability (i.e., Duchek et al.) and RT distribution characteristics in three different tasks (i.e., Tse et al.) by focusing on global- and local-switch costs, their corresponding response distribution profiles, and congruency effects across groups. The data set reported here provided a much larger sample than used in previous studies, which allowed for a cross-sectional analysis of individuals over the lifespan (i.e., separating healthy adults into younger, middle-aged, and older adults). These groups increase our precision of detecting age-related and AD-related task-switching differences given the three healthy adult groups were taken from different age ranges and the very mild AD group was age-matched to the older adult group. Multiple characteristics of performance were measured including error rates, raw and transformed response latencies, and characteristics of underlying RT distributions for each of the trial types which all focused on local and global switch costs. Moreover, we report z-score analyses on the local and global effects on the Vincentile analyses (which were not reported in the previous papers) and hence we can directly evaluate the role of general slowing in modulating local and global response distributions. Finally, we also evaluated response congruency effects which reflect the consistency of the stimulus mapping to the same or different response keys to provide a more complete picture of attentional differences between groups—differences we discuss in turn below.
Global and Local Switch Costs
Our results showed that, across groups, local and global costs produced dissociative effects in mean error rates and response latencies. For errors, local costs were equivalent across groups; however, for global costs, error rates were lowest for younger adults followed by middle-aged adults, older adults, and increased considerably for very mild AD individuals. For RTs, local costs were actually greatest for younger adults and lowest for very mild AD individuals. Global costs in reaction times, however, produced a very different pattern with younger adults showing the lowest cost which then increased across age groups and AD status. Interestingly, z-corrected global costs for very mild AD individuals were lower than those of older adults, though very mild AD individuals also produced an exaggerated global cost in errors.
The dissociation between local and global switch costs across groups is consistent with changes in separate but related processes. Local costs require maintenance of two task sets and also the ability to reconfigure when a new task set comes online. Our use of the alternating-runs paradigm without a preparatory cue-stimulus interval produced a large local cost for individuals with relatively intact attentional systems (younger and middle-aged adults). These individuals are more likely to become well-tuned to a given task set and therefore when the task set changes, inertia from the previous task set slows the reconfiguration needed to respond to the switch trial. For individuals with less well-tuned systems—such as older adults and very mild AD individuals—local switch costs are reduced because both task sets are still relatively active. In contrast, global costs reflect additional demands of maintaining two task sets compared to a single task set, which is most costly for individuals with relatively compromised attentional systems (i.e., older adults and very mild AD individuals).
Consistent with this perspective, Whitson, Karayanidis, and Michie (2012) also evaluated local and global switch costs using a CVOE paradigm in younger and older adults. In their paradigm, Switch block trials were presented in a pseudo-random order instead of a predictable alternating-runs sequence, however a cue-stimulus interval of 150 or 1000 ms was placed between the cue and the target. Whitson et al. argued that individuals would be more likely to recruit additional preparatory processes when sufficient time was provided to prepare for the target response. Consistent with our dissociative patterns, global costs were greater for older than younger adults and local costs were greater for younger than older adults. Of note, although local costs decreased with a longer response interval, younger adults still produced the greatest local cost. Therefore, even when additional time is provided to reconfigure the task set for the upcoming switch trial, the attentional system of younger adults, which becomes well-tuned to a task set with the increasing time, still produced an exaggerated local cost.
It is also important to note that age-related changes in response costs, particularly for local costs, are not consistent in the extent literature and are likely due to specific characteristics of the switch tasks used across studies. Although global switch costs have generally been found to be greater in older adults and in impaired individuals (Belleville et al., 2008; Kray, Li, & Lindenberger, 2002; Kray, 2006; Reimers & Maylor; 2005; Whitson et al., 2012; though see Schmitter-Edgecombe & Sanders, 2009 for exception), local costs have shown an age-related increase (Belleville et al.), a decrease (Whitson et al., and the results from the present study), or no difference across age groups (Kray, Eppinger, & Mecklinger, 2005; Reimers & Maylor). There are several differences across paradigms that are likely to produce the different patterns, including the sequencing of trials presented in the Switch block (alternating-runs versus random trials), the timing of the task cue and stimulus (simultaneous versus delayed interval), amount of practice trials prior to experimental trials, and whether responses are made to bivalent or univalent targets (e.g., Schmitter-Edgecombe & Sanders). Clearly, the alternating-runs sequence appears to systematically decrease local costs across four groups of participants. Possibly, the younger and middle-aged adults may be more likely to proactively benefit from the repeated trial sequence and this may enhance the tuning of a task set which leads to a larger cost when the task set switches.
As the Whitson et al. (2012) study indicated, this age-related difference can also occur with random switch trials when a preparatory cue is provided prior to the presentation of the trial. This preparatory task set account may also accommodate the results from Belleville et al. (2008). Specifically, in their study, switch trials were presented randomly every 5 to 9 trials instead of every other trial in the present study. Possibly, the greater number of repeated trials reinforced the task set sufficiently even for the impaired participants to show an increased local cost. Of course, as mentioned in the Introduction, there are additional design differences that are noteworthy between the present study and Belleville et al., such as the amount of practice and type of switching task, which would need to be parametrically manipulated to identify the locus of the difference. Regardless, the present results (and Whitson et al.) are particularly noteworthy in demonstrating facilitation in switching in participants who have decreased cognitive control4.
It is also worth noting that there are other switch-type tasks that have been used to compare healthy and mild AD individuals that do not compare trial level differences to calculate global and local switch costs. For example, Fine et al. (2008) showed performance differences between individuals who showed signs of cognitive decline consistent with early stage AD within the following year, compared to individuals who did not in a variant of the Stroop task known as the color-word interference test (CWIT; Clark et al., 2005). In the CWIT, participants are presented Stroop trials and complete a Switch block in which participants are cued to either name the color of a presented word or simply read the word. The Switch block is then compared to Pure blocks in which participants only name the color or read the word. The primary measure was the total time to complete all trials within the Switch block as Fine et al. did not separate switch and nonswitch trials to compute local costs. Individuals who were likely to develop early stage AD took longer to complete the Switch block than healthy individuals, which suggests that total time to complete the Switch block may act as another metric for displaying differences associated with cognitive decline. Although the present study does not have a longitudinal component, the overall pattern is similar to the Fine et al. study when comparing the earliest stages of AD to control participants. Specifically, very mild AD individuals took longer to complete nonswitch and switch trials than healthy controls, and made substantially more errors than age-matched controls.
In a separate study, Hutchison et al. (2010) also evaluated a variant of the CWIT paradigm, but importantly, separated switch and nonswitch trial to compute local costs. Switch and nonswitch trials were presented using the alternating-runs sequence. Consistent with our local cost pattern in the CVOE task, very mild AD individuals showed a reduced local cost relative to healthy older adults and younger adults. Thus, the reduced local cost for impaired individuals occurs in other paradigms. In this light, it is worth noting that Hutchison et al. did not include a Pure block of trials and therefore global costs could not be computed.
Given the qualitative differences between local and global costs between healthy older adults and individuals with very mild AD, another interesting prospect is determining how these costs change over time across individuals as attention begins to decline. It should be noted that the current study compared a group of individuals in the earliest detectable stage of AD with older adults who were free of any detectable cognitive impairment. It is possible that the dissociative pattern of global and local costs could hold clinical utility for identifying individuals who are at risk for decline. Specifically, in the present paradigm, one may examine the point in which global costs become greater than local costs within an individual. As shown in the bottom of Figure 2, this global cost begins to exceed local costs in the healthy older adults, and the separation of these costs increases in the very mild AD individuals. Thus, the intersection of these two costs could be an important cognitive marker that may suggest the development of clinical abnormality. In this light, it is noteworthy that the present CVOE switching task is relatively short and is completed in less than 10 min which makes it potentially useful in a clinical setting.
Finally, we note that although we account for global and local switch costs to be influenced by how well an individual's attentional system is tuned to a given task set, we note a separate account of task-switching differences. Specifically, Bryck and Mayr (2008; see too Mayr, Kuhns, & Hubbard, 2014) have suggested that local task-switching effects may not be due to the residual task set from the previous trial as traditionally argued, but instead reflect an updating of working memory to adjust from the previous task setting stored in long-term memory. Evidence for this process has been found using an asymmetrical-switch task such as the Stroop task in which one task set (e.g., word naming) is dominant over the other non-dominant task set (e.g., color naming). Bryck and Mayr have shown a cost for non-dominant trials even when the task set does not change from the preceding trial, suggesting that a task-set change is not necessary to produce a cost. Our use of the CVOE switch task may differ somewhat from that of the Stroop task in that the response asymmetry found between color and word dimensions due to the relatively automatic word-reading process may be larger than CV and OE task sets in our switch task. To examine a possible asymmetry, we split CV and OE Pure block trials on errors and RTs across groups to determine whether task set responses were similar. Although there was some evidence that CV and OE trials were asymmetrical with OE trials (M RT = 1121 ms) being more difficult than CV trials (M RT = 1040 ms) in response latencies (p < .01), this difference did not occur in error rates (OE = 3.1% vs. CV = 2.5%, p = .37). Importantly, there was no hint of an interaction with either response latencies or errors with group (Fs < 1), demonstrating that differences in task asymmetry cannot accommodate the present group differences.
Vincentile Analyses
An intriguing and novel aspect of the present results are the different patterns of local and global switch costs on the reaction time distributions, as reflected by the Vincentile analyses. For the younger adults, there was an increase in the local switch cost across Vincentiles, whereas for the more impaired individuals, there was a remarkable decrease across Vincentiles. Although somewhat muted, these effects also occurred in the z-transformed data. Based on these results, it appears that the cost of local switching affects relatively slower RTs as indicated by later bins in younger adults than in older adults and individuals with early stage AD. These results suggest that there are differences in the degree to which individuals can exert control on the more difficult slower trials. It is possible that the task set is not maintained sufficiently for the difficult slow trials, and as time passes, there is little local switch cost in the older and very mildly demented individuals.
In global costs, the Vincentiles are quite different. Young adults now produce relatively little increase in the global cost across Vincentiles until the final bin and the other groups produce a systematic increase in the global cost across Vincentiles, particularly for older adults and very mild AD individuals. The load of having to maintain two task sets appears to be particularly troublesome for the slower more difficult trials in the most compromised individuals. In this light, Vincentile analyses provide converging evidence of a clear dissociation between the two types of switching costs across these groups of individuals. Finally, these distributional analyses provide further evidence of the utility of this approach in analyzing response latencies. In particular, Figure 5 shows that differences across groups are largest in the slowest Vincentiles, thereby producing the greatest discrimination. This pattern is also consistent with the worse performance rule (Coyle, 2003; Larson & Alderton, 1990), wherein individual differences in fluid intelligence are best captured in the most difficult trials.
Congruency Effects
The results from the congruency analyses were also quite clear. As noted, the congruency effect refers to trials in which mappings of a given stimulus correspond to the same versus different keys. Interestingly, this variable had no effect on accuracy or response latencies in the younger and the middle-aged adults in the nonswitch trials. However, an effect did emerge on the accuracy data on the Switch trials in the middle-aged adults and in both the switch and nonswitch trials in the older adults and very mild AD individuals. This pattern appears reminiscent of the Simon effect in which there is inconsistency in the mapping of the stimulus onto response keys. Notably, in a study with younger adults, older adults, and very mildly demented individuals, Castel et al. found (2007) found a similar pattern in that error rates were most discriminating across participants. This is also consistent with work from other standard attentional selection tasks such as Stroop (e.g., Balota et al., 2010; Hutchison et al., 2010) and Semantic Verification (Aschenbrenner et al., 2015) where accuracy in speeded tasks is most discriminating across healthy older adults and early stage Alzheimer’s disease. Thus, the present congruency results provide converging evidence regarding an age- and AD-related deficit in attentional selection of a response under conditions of multiple competing stimulus-response mappings.
Conclusions
In summary, the results of the present task-switching study show dissociations in local and global switch costs across four different groups of individuals in a large sample of participants. First, our results suggest that maintaining two task sets (vs. one) results in a global response cost for both errors and response latencies, which increase across age groups and the presence of AD (primarily in errors), showing that groups with reduced attentional abilities show exaggerated global costs. Second, actively switching between two task sets produces a task reconfiguration cost that similarly increases errors and response latencies. Importantly, in contrast to the global cost, this local switch cost systematically decreases across the young, middle-aged, older, and very mild AD individuals. We argue that younger adults are highly tuned to the task demands of each trial, and therefore switching to a different task set produces a large local cost. In contrast, more impaired individuals are not as highly tuned and therefore switching to a different task is not as costly. The dissociation between local and global switch costs across groups was also found at the level of reaction time distributions, wherein the effect of these costs across groups are exaggerated in the slower more difficult trials. Thus, the present results provide an intriguing pattern in which more impaired individuals actually benefit from the lack of a highly tuned attentional set, at least as reflected by local switch costs.
Acknowledgments
We thank the participants and the Clinical Core of the Knight Alzheimer Disease Research Center at Washington University for providing their careful clinical evaluations. Funding support was provided by the National Institute on Aging (NIA; P01AG03991, P01AG026276, and T32 AG0000-0-39).
Footnotes
Gender information was not collected from younger adult participants.
To control for general slowing, we also conducted z-transformed analyses on the Vincentile data and the same patterns of main effects were observed. Importantly, the Group × Vincentile Bin interactions for both local [F(15, 2810) = 8.28, MSE = .11, ηp2 = .04] and global costs [F(15, 2810) = 6.32, MSE = .09, ηp2 = .03] revealed the same patterns as the raw RT data reported.
Since the CV Pure block was completed before the OE Pure block, participants would not know of the OE mappings when completing the Pure block. Given this ordering, it is possible that congruency effects may not occur in the CV block though we find this unlikely given that RT congruency effects were not found even when participants were actively switching between two task sets. Therefore, it is unlikely that prior exposure of an inactive task set is inducing a congruency effect on the OE Pure block. To be consistent with other Pure block analyses, we collapse across both Pure blocks.
A second possibility, noted elsewhere (Tse et al., 2010), is that groups may show different strategies on Switch block trials. For instance, individuals with compromised attentional systems may be more likely to look towards the cue on the screen before making their response. In the Switch block, these individuals may be looking up towards the cue similarly on both switch and nonswitch trials which may lead to similar response latencies and thus a diminished local cost. Although we did not record the frequency with which all participants looked toward the cue, Tse et al. did investigate this issue when this task was initially implemented. Specifically, eye movements were monitored online by the experimenter via a mirror placed above the monitor. The results indicated that there were no differences between healthy older adults and very mild AD individuals in the frequency in which they looked at the cue during the Switch block. Of course, eye movements are only an indirect way of measuring the maintenance of the trial sequencing; however, given a recent meta-analysis that has shown similar age-related differences in switch costs using different switch-task variants (Wasylyshyn et al., 2011), we do believe that the present large group dissociations are not likely due to differences in external cue use. However, we do acknowledge that this issue should be more systematically explored in future research.
References
- Albert M, Moss MB, Blacker D, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner AJ, Balota DA, Tse C-S, Fagan AM, Holtzman DM, Benzinger TLS, Morris JC. Alzheimer disease biomarkers, attentional control, and semantic memory retrieval: Synergistic and mediational effects of biomarkers on a sensitive cognitive measure in non-demented older adults. Neuropsychology. 2015;29:368–381. doi: 10.1037/neu0000133. doi: 10.1037/neu0000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain: A Journal of Neurology. 2001;124:1492–1508. doi: 10.1093/brain/124.8.1492. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- Balota DA, Faust ME. Attention and dementia of the Alzheimer’s type. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. 2nd #6. Elsevier Science; 2001. pp. 51–80. [Google Scholar]
- Balota DA, Tse C-S, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: The power of errors in stroop color naming. Psychology and Aging. 2010;25:208–218. doi: 10.1037/a0017474. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Yap MJ. Moving beyond the mean in studies of mental chonometry: The power of response time distributional analyses. Current Directions in Psychological Science. 2011;20:160–166. doi: 10.1177/0963721411408885. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Watson JM. Beyond mean response latency: Response time distributional analyses of semantic priming. Journal of Memory and Language. 2008;59:495–523. doi: 10.1016/j.jml.2007.10.004. [Google Scholar]
- Belleville S, Bherer L, Lepage E, Chertkow H, Gauthier S. Task switching capacities in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychologica. 2008;46:2225–2233. doi: 10.1016/j.neuropsychologia.2008.02.012. doi: 10.1016/j.neuropsychologia.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr., Miller JP, Storandt M, Rubin EH, Morris JC, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: Relation of histologic markers to dementia severity, age, sex and apoliopprotein E genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Bryck RL, Mayr U. Task selection cost asymmetry without task switching. Psychonomic Bulletin & Review. 2008;15:128–134. doi: 10.3758/pbr.15.1.128. doi: 1033758/PBR.15.1.128. [DOI] [PubMed] [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. doi: 10.1016/S0022-5371(73)80034-9. [Google Scholar]
- Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer's disease: Evidence for disproportionate selection breakdowns in the simon task. Neuropsychology. 2007;21:170–182. doi: 10.1037/0894-4105.21.2.170. doi: 10.1037/0894-4105.21.2.170. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, McCabe DP. Memory efficiency and the strategic control of attention at encoding: Impairments of value-directed remembering in Alzheimer’s disease. Neuropsychology. 2009;23:297–306. doi: 10.1037/a0014888. doi: 10.1037/a0014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Schiehser DM, Weissberger GH, Salmon DP, Delis DC, Bondi MW. Specific measures of executive function predict cognitive decline in older adults. Journal of the International Neuropsychological Society. 2012;18:118–127. doi: 10.1017/S1355617711001524. doi: 10.1017/S1355617711001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle TR. A review of the worst performance rule: Evidence, theory, and alternative hypotheses. Intelligence. 2003;31:567–587. doi: 10.1016/S016-2896(03)00054-0. [Google Scholar]
- Duchek JM, Balota DA, Tse C-S, Holtzman DM, Fagan AM, Goate AM. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology. 2009;23:746–758. doi: 10.1037/a0016583. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dywan J, Jacoby L. Effects of aging on source monitoring: Differences in susceptibility to false fame. Psychology and Aging. 1990;5:379–387. doi: 10.1037//0882-7974.5.3.379. doi: 10.1037/0882-7974.5.3.379. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: Implications for group differences in response latency. Psychological Bulletin. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fine EM, Delis DC, Wetter SR, Jacobson MW, Jak AJ, McDonald CR, Braga JC, Bondi MW. Cognitive discrepancies versus APOE genotype as predictors of cognitive decline in normal-functioning elderly individuals: A longitudinal study. The American Journal of Geriatric Psychiatry. 2008;16:366–374. doi: 10.1097/JGP.0b013e3181629957. doi: 10.1097/JGP.0b13e3181629957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2002;57B:101–115. doi: 10.1093/geronb/57.2.p101. doi: 10.1093/geronb/57.2.P101. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Strauss E, Hunter MA, MacDonald SWS. Intraindividual variability, cognition, and aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3rd Psychology Press; New York, NY: 2008. [Google Scholar]
- Hutchison KA, Balota DA, Duchek JM. The utility of Stroop task switching as a marker for early-stage Alzheimer’s disease. Psychology and Aging. 2010;25:545–559. doi: 10.1037/a0018498. doi: 10.1037/a0018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray J. Task-set switching under cue-based versus memory-based switching conditions in younger and older adults. Brain Research. 2006;1105:83–92. doi: 10.1016/j.brainres.2005.11.016. doi: 10.1016/j.brainres.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Kray J, Eppinger B, Mecklinger A. Age differences in attentional control: An event-related potential approach. Psychophysiology. 2005;42:407–416. doi: 10.1111/j.1469-8986.2005.00298.x. doi: 10.111/j/1469-8986.2005.00298.X. [DOI] [PubMed] [Google Scholar]
- Kray J, Li KZH, Lindenberger U. Age-related changes in task-switching components: The role of task uncertainty. Brain and Cognition. 2002;49:363–381. doi: 10.1006/brcg.2001.1505. doi: 10.1006/brcg.2001.1505. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychology and Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. doi: 10.1037/0882-7974.15.126. [DOI] [PubMed] [Google Scholar]
- Larson GE, Alderton DL. Reaction time variability and intelligence: A ‘worst performance’ analysis of individual differences. Intelligence. 1990;14:309–325. doi: 10.1016/0160-2896(90)90021-K. [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Mayr U, Kuhns D, Hubbard J. Long-term memory and the control of attentional control. Cognitive Psychology. 2014;72:1–26. doi: 10.1016/j.cogpsych.2014.02.001. doi: 10.1016/j.cogpsych.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NIN CDS-ADRCD work group under the auspices of the Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Minear M, Shah P. Training and transfer effects in task switching. Memory & Cognition. 2008;36:1470–1483. doi: 10.3758/MC.336.8.1470. doi: 10.3758/MC.336.8.1470. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. doi: 10.1016/S1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Jr., Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early stage Alzheimer’s disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain: A Journal of Neurology. 1999;122:383–404. doi: 10.1093/brain/122.3.383. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the life span: Effects of age on general and specific switch costs. Developmental Psychology. 2005;41:661–671. doi: 10.1037/0012-1649.41.4.661. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. doi: 10.1037/0096-3445.124.2.207. [Google Scholar]
- Rubin EH, Storandt M, Miller P, Kinscherf DA, Grant EA, Morris JC, Berg L. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Sanders C. Task switching in mild cognitive impairment: Switch and nonswitch costs. Journal of the International Neuropsychological Society. 2009;51:103–111. doi: 10.1017/S1355617708090140. doi: 10.1017/S1355617708090140. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime reference guide. Psychology Software Tools; Pittsburg, PA: 2002. [Google Scholar]
- Schmiedek F, Oberauer K, Wilhelm O, Süβ H-M, Wittman WW. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General. 2007;136:414–429. doi: 10.1037/0096-3445.136.3.414. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Huff LM. The effects of age and dementia of the Alzheimer’s type on phonological false memories. Psychology and Aging. 2003;18:791–806. doi: 10.1037/0882-7974.18.4.791. doi: 10.1037/0882-7974.18.4.791. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. doi: 10.1037/0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs. revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. doi: 10.1212/01.wnl.0000228231.2611.6e. [DOI] [PubMed] [Google Scholar]
- Tornay FJ, Milán EG. A more complete task-set reconfiguration in random than predictable task switch. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 2001;54A:785–803. doi: 10.1080/713755984. doi: 10.1080/027724980042000499. [DOI] [PubMed] [Google Scholar]
- Tse C-S, Balota DA, Yap MJ, Duchek JM, McCabe DP. Effects of healthy aging and early stage dementia of the Alzheimer’s Type on components of response time distributions in three attentional tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SAL, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society. 2006;12:707–735. doi: 10.1017/S1355617706060863. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandierendonck A, Liefooghe B, Verbruggen F. Task-switching: Interplay of reconfiguration and interference. Psychological Bulletin. 2010;136:601–626. doi: 10.1037/a0019791. doi: 10.1037/a0019791. [DOI] [PubMed] [Google Scholar]
- Wasylyshyn C, Verhaeghen P, Sliwinski MJ. Aging and task-switching: A meta-analysis. Psychology and Aging. 2011;26:15–20. doi: 10.1037/a0020912. doi: 10.1037/a0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armilio ML, Craik FIM, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain & Cognition. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Wetter SR, Delis DC, Houston WS, Jacobson MW, Lansing A, Cobell K, Salmon DP, Bondi MW. Deficits in inhibition and flexibility are associated with the APOE-E4 allele in nondemented older adults. Journal of Clinical and Experimental Neuropsychology. 2005;27:943–952. doi: 10.1080/13803390490919001. doi: 10.1080/13803390490919001. [DOI] [PubMed] [Google Scholar]
- Whitson LR, Karayanidis F, Michie PT. Task practice differentially modulates task-switching performance across the adult lifespan. Acta Psychologica. 2012;139:124–136. doi: 10.1016/j.actpsy.2011.09.004. doi: 10.1016/j.actpsy.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of ‘switch costs. Psychological Research. 2000;63:212–233. doi: 10.1007/s004269900003. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]