Abstract
Background
Approximately, 20–30% of patients with gastro-esophageal reflux disease (GERD) experience persistent symptoms despite treatment with proton pump inhibitors (PPIs). These patients may have underlying dysmotility; therefore, targeting gastric motor dysfunction in addition to acid inhibition may represent a new therapeutic avenue. The aim of this study was to assess the pharmacodynamic effect of the prokinetic agent revexepride (a 5-HT4 receptor agonist) in patients with GERD who have persistent symptoms despite treatment with a PPI.
Methods
This was a phase II, exploratory, multicenter, randomized, placebo-controlled, double-blind, parallel-group study in patients with GERD who experienced persistent symptoms while taking a stable dose of PPIs (http://ClinicalTrials.gov identifier: NCT01370863). Patients were randomized to either revexepride (0.5 mg, three times daily) or matching placebo for 4 weeks. Reflux events and associated characteristics were assessed by pH/impedance monitoring and disease symptoms were assessed using electronic diaries and questionnaires.
Key Results
In total, 67 patients were enrolled in the study. There were no significant differences between study arms in the number, the mean proximal extent or the bolus clearance times of liquid-containing reflux events. Changes from baseline in the number of heartburn, regurgitation, and other symptom events were minimal for each treatment group and no clear trends were observed.
Conclusions & Inferences
No clear differences were seen in reflux parameters between the placebo and revexepride groups.
Keywords: 5-HT4 receptor agonist, gastro-esophageal reflux disease, prokinetic, reflux esophagitis, revexepride
Key Messages
Between 20% and 30% of patients treated with proton pump inhibitors (PPIs) for gastro-esophageal reflux disease (GERD) experience persistent symptoms of heartburn and/or regurgitation.
This exploratory study assessed the pharmacodynamic effect of the prokinetic agent revexepride (a 5-HT4 receptor agonist) in patients with GERD who have persistent symptoms despite treatment with a PPI.
Reflux events and associated characteristics were assessed by pH/impedance monitoring and disease symptoms were assessed using electronic diaries and questionnaires.
There were no consistent differences in primary or secondary pharmacodynamic endpoints between the group receiving revexepride 0.5 mg three times daily and the group taking placebo.
Overall, revexepride 0.5 mg was well-tolerated and no safety concerns were identified.
No clear differences were seen in reflux parameters between the revexepride and placebo groups. This may reflect the current problems of identifying patients who could potentially benefit from a prokinetic medication.
Introduction
Proton pump inhibitors (PPIs) are widely used to treat patients with gastro-esophageal reflux disease (GERD).1 However, 20–30% of patients treated with PPIs for GERD experience persistent symptoms of heartburn and/or regurgitation.2 Acid exposure tends to be controlled in the majority of these patients, suggesting that their persistent symptoms may be caused by non-acidic or weakly acidic reflux that is not suppressed by PPIs, or by a different mechanism such as esophageal dysmotility or esophageal hypersensitivity.3–6 Persistent reflux may be facilitated by hypotensive esophageal dysmotility or slow gastric emptying.7,8 Consequently, there is potential for prokinetic drugs to improve reflux symptoms by augmenting esophago–gastric junction pressure, by inhibiting transient lower esophageal sphincter (LES) relaxations, and by enhancing esophageal motility and gastric emptying.
Revexepride is a prokinetic 5-HT4 receptor agonist that has been shown to accelerate gastric emptying in animals and healthy humans (unpublished data). It stimulates esophageal and gastrointestinal motility, and accelerates transit by enhancing the physiological release of acetylcholine from myenteric neurons. Elevated local acetylcholine levels increase esophageal peristalsis, accelerate gastric emptying, increase gastro-esophageal barrier function, stimulate gastrointestinal motility, and improve gastroduodenal coordination.9 Other 5-HT4 receptor agonists have also been shown to have stimulating effects on esophageal motility, LES pressure, and gastric emptying, with the potential to improve symptoms in patients with GERD.10–14 However, these drugs have been withdrawn owing to cardiovascular safety concerns, probably related to lack of 5-HT4 receptor selectivity.15
The main aim of this study was to assess the effect of revexepride 0.5 mg on reflux parameters in patients with GERD who had persistent typical reflux symptoms despite treatment with a PPI.
Materials and Methods
Patients
Men and non-pregnant women aged 18–70 years were included in the study if they had at least a 6-month history of reflux symptoms (heartburn and/or regurgitation), and had been on stable treatment with a PPI (a minimum of a labeled dose) for at least the past 6 weeks as determined at the screening visit. Patients were required to have heartburn and/or regurgitation, of at least moderate severity, at a mean frequency of ≥3 days per week during the run-in period, as assessed by twice-daily completion of a diary. Patients also had to have at least 25 liquid-containing reflux events in 24 h at baseline pH/impedance monitoring, performed while the patient was taking a PPI. The criterion of at least 25 liquid-containing reflux events per 24 h is based on this being the median value previously reported in patients who had persistent reflux symptoms despite treatment with a PPI.16,17 An endoscopy within the past 5 years was required to confirm the absence of reflux esophagitis grade C or D (according to the Los Angeles classification).18
Patients were excluded if they had a history of: long segment (>3 cm) Barrett's esophagus; a large (>3 cm) hiatus hernia; fundoplication; an endoscopic antireflux procedure; previous major gastrointestinal surgery; a severe esophageal motility disorder (e.g., scleroderma, achalasia, nutcracker esophagus); a structural abnormality or disease of the gastrointestinal tract; or vomiting more than once per week. In addition, patients were excluded if they had a body mass index (BMI) ≥35 kg/m2 Prohibited medications were: CYP3A4 inhibitors; drugs known to prolong the QT interval; drugs that affect gastrointestinal motility, including opioids and prokinetic agents other than the study drug; and other agents used to treat GERD, such as H2-receptor antagonists and antacids. Additional exclusion criteria were: a history of cardiovascular disease (including prolonged QT interval based on electrocardiogram at screening); thyrotoxicosis; psychiatric disease; clinically significant abnormalities at screening (by physical examination or in blood hematology or biochemistry tests); or any other disease or abnormalities which, in the opinion of the investigators, would compromise the study or the well-being of the patient.
Patient selection was based on an automated local analysis. All participants provided written consent before the initiation of study-related activities, and the study was carried out in accordance with local ethical and legal requirements and the Declaration of Helsinki.
Trial design
This was a 4-week, phase II, exploratory, multicenter, randomized, placebo-controlled, double-blind, parallel-group study of revexepride in patients who had reflux symptoms for a minimum of 6 months that persisted despite being on a stable PPI regimen for at least 6 weeks (http://ClinicalTrials.gov identifier: NCT01370863). The objectives of the study were to determine: (i) the pharmacodynamic effect of revexepride on parameters derived from pH/multichannel intraluminal impedance monitoring (pH/impedance); (ii) the effect of revexepride on reflux symptoms; and (iii) the safety and tolerability of the drug.
The study was carried out at 14 sites across six European countries: Belgium, France, Germany, Great Britain, The Netherlands, and Switzerland. After an initial screening visit, participants underwent a run-in phase of 14 days to establish their symptom frequency and adherence to PPI treatment. For participants who had been taking prohibited medications that were stopped at screening, there was an additional 7-day washout period before the run-in period.
At baseline, patients eligible for inclusion in the study were randomized 1 : 1 to receive either revexepride 0.5 mg or placebo to be taken three times daily for 4 weeks. The revexepride dose corresponds well to the optimal dose derived from available preclinical animal data (unpublished data). In addition, patients continued on their stable PPI treatment regimen (maintained the same dose and type of PPI during the study). PPI compliance was verified by patients' daily e-diary records. Revexepride was taken 30 min before breakfast, lunch, and dinner. Administration in relation to meals is beneficial because meals provoke transient LES relaxations, which causes most reflux episodes.19 The randomization was generated using a central interactive web-based/voice response system that applied a minimization algorithm to ensure an approximate 1 : 1 balance between the two study arms. Participants were allowed to take antacid rescue medication (Rennie®, Bayer, Bladel, the Netherlands) up to three times daily, except during the pH/impedance monitoring periods. Assessments were performed at the baseline visit and after 2 and 4 weeks of treatment. The presence of Helicobacter pylori was assessed at baseline based on medical history. The presence and grade of reflux esophagitis was determined by evaluation of endoscopic reports from the 5 years before randomization or, if unavailable, by an endoscopy.
Revexepride 0.5 mg and placebo tablets were visually indistinguishable and provided in identical medication boxes. Implementation of a central randomization system ensured that the double-blind treatment was maintained.
Assessments
pH/impedance monitoring
Twenty-four-hour pH/impedance moni-toring was performed at baseline and at day 28 while patients were on a PPI. A single-use pH/impedance catheter (ComforTec® Z/pH or Sleuth Multichannel Intraluminal Impedance [MII] ambulatory system, Sandhill Scientific, Highlands Ranch, CO, USA, or Ohmega ambulatory system, Medical Measurement Systems [MMS], Enschede, The Netherlands, depending on the study center) was passed transnasally under topical anesthesia and the esophageal pH electrode positioned 5 cm above the LES. The same monitoring system and catheter were used for the same individual at both baseline and day 28. System-specific standard precalibration procedures were conducted accordingly. For the duration of the two 24-h ambulatory pH/impedance monitoring periods, each patient was instructed to eat similar meals at regular times between the two pH/impedance assessment visits, not to eat between meals, not to consume acidic drinks or excessive amounts of alcohol- or quinine-containing beverages, and not to lie down, except at bedtime. Participants were instructed not to eat or drink from 22:00 hours the night before each pH/impedance monitoring period. During both of the pH/impedance monitoring periods, no strenuous activities were allowed and patients were asked to record the onset of heartburn, regurgitation, or other symptoms. These records were used to assess symptom association with reflux events. The pH/impedance measurements were analyzed by 24-h period, and by recumbent (nocturnal) and upright (diurnal) periods, as well as by the postprandial (defined as 4 h after eating any meal) and postprandial breakfast (defined as 4 h after eating breakfast) periods. In each center, investigators analyzed impedance tracings to confirm patient's inclusion either manually or by using automatic analysis software. This was the standard automated analysis available on the local pH-impedance software at each study site. A central reader was employed to standardize the evaluation of the pH/impedance data.
The three primary pharmacodynamic endpoints of the study, assessed by prespecified post hoc central reading of the 24-h pH/impedance monitoring records, were: (i) the number of liquid-containing reflux events; (ii) the mean proximal extent of all liquid-containing reflux events; and (iii) the mean bolus clearance time of all liquid-containing reflux events. In addition, 24-h pH/impedance monitoring allowed the evaluation of the following secondary endpoints: the number and percentage of reflux events categorized by acidity (acidic [pH <4], weakly acidic [pH 4–7], or weakly alkaline [pH >7])20; composition of the reflux event (liquid, mixed, or gas); proximal extent >15 cm; acid clearance time; and impedance baseline levels.
Symptoms
Frequency and severity of heartburn and regurgitation were assessed using electronic diaries (e-diaries), which were completed twice daily (in the morning and evening), from screening until the final visit. In the morning, questions were asked relating to sleep disturbances due to reflux events and to heartburn and/or regurgitation symptoms when lying down. In the evening, heartburn and/or reflux symptoms that were experienced throughout the day were assessed. In addition, patients were asked about their drug intake (investigational product and PPI use) to estimate exposure and compliance.
Symptoms and health-related quality of life (HRQoL) were also evaluated at baseline, week 2, and week 4 using the Patient Assessment of Upper Gastrointestinal Symptom Severity index (PAGI-SYM) and Patient Assessment of Upper Gastrointestinal Quality of Life (PAGI-QOL) questionnaires, both of which have a 2-week recall period. The PAGI-SYM and PAGI-QOL instruments have shown good validity in assessing symptoms and HRQoL in patients with upper gastrointestinal disorders.21,22 The PAGI-SYM consists of 20 questions about heartburn/regurgitation, fullness/early satiety, nausea/vomiting, bloating, upper abdominal pain, and lower abdominal pain; each item is graded on a 6-point Likert scale (none, very mild, mild, moderate, severe, very severe).21 The PAGI-QOL includes 30 items graded on a 6-point scale, in the following domains: daily activities, diet and food habits, psychological well-being and distress, clothing, and relationships.22 Symptom assessments were also performed during pH/impedance monitoring and the symptom association probability (SAP) was estimated at baseline and at day 28. The SAP indicates the statistical probability (by Fisher's exact test) of the association between symptoms and reflux episodes; a positive association is defined as a SAP greater than 95%.
Adverse events and safety
Adverse events (AEs) were recorded from signing of the informed consent form to the final visit. At baseline and final visit, vital signs (blood pressure and pulse) were measured and electrocardiography and clinical laboratory assessments (hematology, biochemistry, and urinalysis) were performed.
Statistical methods
Safety, demographic, and other baseline patient characteristics were reported for the safety population, which was defined as all individuals randomized into the study who received at least one dose of the investigational product. The pharmacodynamic population was defined as all randomized patients who received at least one dose of the investigational product and who had both a baseline and a postbaseline pharmacodynamic assessment (i.e., 24-h pH/impedance monitoring). All pharmacodynamic and efficacy (symptoms and HRQoL) analyses were based on data from the pharmacodynamic population. The following three endpoints were selected as primary as these were considered to be equally important: number of liquid-containing reflux events, the proximal extent of these reflux events, and the bolus clearance time of these reflux events. The intended sample size for the study was 45 patients per treatment arm. Assuming a SD of 25,23 and a difference of 14.9 in reflux events, this sample size would allow detection of significant differences with a 5% level of significance and with 80% power.24 With this sample size, relevant effects could also be detected for the other two primary endpoints. To have 90 patients completing this study, the aim was to enroll 100 patients. Correction for multiplicity was not necessary; however, if a significant difference for only one of the three endpoints was required, a correction for multiplicity was applied.
Baseline characteristics and exposure data were summarized descriptively. Continuous endpoints were assessed using an ancova on the change from baseline, and included baseline value as a covariate as well as treatment group and country as factors. Least-squares (LS) mean changes from the models were presented. For the PAGI-SYM and PAGI-QOL questionnaires, a change in score of ≥1 point was considered clinically meaningful.25 Inferential statistical analyses of treatment effects were performed using ancova as described above.
Results
Patient characteristics and baseline demographics
This study was initiated in December 2010 and was terminated early, on 23 March 2012, because of recruitment difficulties and the findings of an interim analysis. This analysis showed that the variability in the number of reflux events was much larger than assumed for determination of the study sample size. This would have necessitated a substantial increase in study sample size and therefore the study was terminated. There were no safety concerns. Patients who had enrolled before 23 March 2012 were given the option to complete the study.
The patient disposition is presented in the supplementary online information (Fig. S1). A total of 67 patients were randomized (34 to revexepride, 33 to placebo) before study termination; 65 patients received at least one dose of investigational product and were therefore included in the safety population (34 revexepride, 31 placebo). One randomized patient was withdrawn from the study before receiving a dose of investigational product because not all of the inclusion criteria had been met, and one was withdrawn because of a non-treatment-emergent AE. Of the 65 patients in the safety population, 62 completed the study. One patient in the revexepride group discontinued the study owing to treatment-emergent adverse events (TEAEs; dizziness and headache), and two patients (one from each treatment group) discontinued because they chose not to undergo the second pH/impedance monitoring (Fig. S1). There were 60 patients (31 revexepride, 29 placebo) included in the pharmacodynamic population.
Patient characteristics and baseline demographics for the safety population are presented in Table 1. The mean age was 44.8 years and just over half of participants were female (n = 36; 55.4%). Participants had a mean duration of reflux symptoms of 116.3 months (approximately 9.7 years). Overall, 83.1% of participants stated that PPI therapy provided at least ‘a little bit’ of relief, while 16.9% stated that it provided no relief. In general, patient characteristics and baseline demographics were similar in the placebo and revexepride groups.
Table 1.
Patient characteristics and baseline demographics (safety population)
| Characteristic | Revexepride 0.5 mg t.i.d. (n = 34) | Placebo (n = 31) | Total (n = 65) |
|---|---|---|---|
| Age, years, mean (SD) | 43.8 (16.04) | 45.8 (14.50) | 44.8 (15.24) |
| Sex, n (%) | |||
| Female | 19 (55.9) | 17 (54.8) | 36 (55.4) |
| BMI, kg/cm3, mean (SD) | 25.2 (3.78) | 26.4 (4.36) | 25.8 (4.07) |
| Helicobacter pylori infection*, n (%) | |||
| Yes | 9 (26.5) | 6 (19.4) | 15 (23.1) |
| No | 24 (70.6) | 24 (77.4) | 48 (73.8) |
| Unknown | 1 (2.9) | 1 (3.2) | 2 (3.1) |
| Reflux esophagitis, n (%) | |||
| Grade A | 11 (32.4) | 6 (19.4) | 17 (26.2) |
| Grade B | 3 (8.8) | 3 (9.7) | 6 (9.2) |
| History of GERD symptoms, months, mean (SD) | 128.8 (127.09) | 102.7 (82.24) | 116.3 (108.03) |
| Did PPI therapy provide relief? n (%) | |||
| Not at all | 5 (14.7) | 6 (19.4) | 11 (16.9) |
| A little bit | 11 (32.4) | 18 (58.1) | 29 (44.6) |
| Somewhat | 10 (29.4) | 5 (16.1) | 15 (23.1) |
| Quite a bit | 6 (17.6) | 2 (6.5) | 8 (12.3) |
| Very much | 2 (5.9) | 0 | 2 (3.1) |
Determination of H. pylori infection based on medical history only. BMI, body mass index; GERD, gastro-esophageal reflux disease; PPI, proton pump inhibitor; SD, standard deviation; t.i.d., three times daily.
Investigational product
The majority of patients were exposed to investigational product for 28–30 days. Mean (SD) duration of exposure was comparable in the revexepride (26.9 [3.1] days) and placebo (27.7 [1.2] days) groups. The mean (SD) number of tablets per day was 2.82 (0.30) corresponding to a daily dose of 1.41 mg (0.15) for patients treated with revexepride, and 2.90 (0.14) tablets for those who received placebo, corresponding to a mean (SD) daily dose of 1.45 (0.07) mg (based on the e-diary).
PPI intake
During the study, the overall mean (SD) patient duration of exposure to PPI therapy was 26.4 (2.7) days, which was similar in both study arms (revexepride 0.5 mg, 25.9 [3.24] days; placebo, 26.9 [1.7] days). All patients took their PPI for 21–30 days. The most common PPIs taken (each by >10% of patients) were, in descending order, esomeprazole, omeprazole, and pantoprazole. PPI dosage data were available from 64 patients; 41 (64%) patients received a PPI dose once daily; 23 (36%) received PPIs more than once a day (22 patients received PPIs twice a day and one patient received PPIs three times a day).
Primary pharmacodynamic endpoints
The mean daily number of liquid-containing reflux events at baseline were 92.1 and 82.0 for revexepride and placebo, respectively. The changes from baseline to day 28 (−17.6 for revexepride and −16.3 for placebo) were not statistically significantly different (p = 0.869) in either study arm (Fig.1A). In addition, no statistically significant differences between study arms were recorded for any other periods analyzed (24-h, upright, recumbent, postprandial, and postprandial breakfast). The mean proximal extent of liquid-containing reflux events in a 24-h period changed very little (−0.7) from baseline to day 28 in either treatment group (Fig.1B), and there was no statistically significant difference between the study arms in LS mean changes from baseline (p = 0.992). During the recumbent period, the LS mean change in the mean proximal extent from baseline was +2.06 cm in the revexepride group and −0.25 cm in the placebo group; the difference in LS mean changes was statistically significant (p = 0.020). The mean bolus clearance time of liquid-containing reflux events over a 24-h period at baseline and at day 28 are presented in Fig.1C. There was no statistically significant difference between the study arms in LS mean changes from baseline of the average bolus clearance times of liquid-containing reflux events over a 24-h period; −1.77 for revexepride and −3.77 for placebo (p = 0.715).
Figure 1.
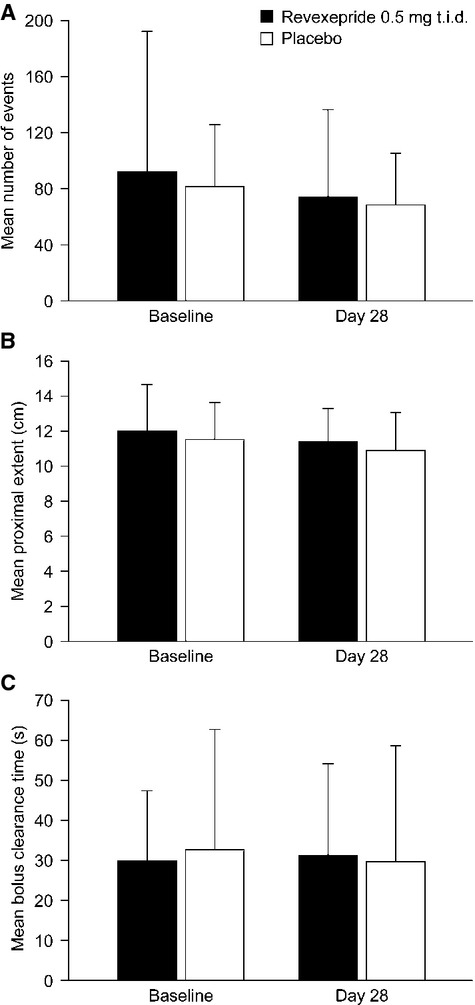
Changes in the primary pharmacodynamic endpoints per 24 h from baseline to day 28 in the revexepride 0.5 mg t.i.d. and placebo groups: (A) mean (SD) number of liquid-containing reflux events; (B) mean (SD) proximal extent of liquid-containing reflux events; and (C) mean (SD) bolus clearance time of liquid-containing reflux events (pharmacodynamic population). Note that differences in the changes from baseline to day 28 between placebo and revexepride groups were not significant. SD, standard deviation; t.i.d., three times daily.
Secondary pharmacodynamic endpoints
pH/impedance monitoring
The secondary pharmacodynamic endpoints derived from pH/impedance monitoring are presented in the supplementary information (Table S1). Acid exposure at baseline was generally within the normal range (placebo: 3.2 [95% CI: 1.17–5.24] and revexepride: 1.78 [95% CI: 0.92–2.65]). The percentage of reflux events that were weakly acidic during the recumbent period significantly decreased from baseline in the revexepride treatment group (LS mean change from baseline: −10.89%) compared with the placebo group (LS mean change: +11.82%; p = 0.023). There were no significant differences in the changes from baseline between study arms for acidic, weakly acidic, and weakly alkaline reflux for the other assessment periods. There was a significant difference between study arms for the LS mean change from baseline in the number of mixed composition reflux events during the postprandial assessment period (revexepride, −2.08 events; placebo, −9.35 events; p = 0.042), but not for any of the other assessment periods. There were no significant differences between treatment groups for liquid or gas reflux events for any assessment period. Acid clearance time changes from baseline and changes from baseline in the percentage of liquid-containing reflux events with a proximal extent greater than 15 cm were not statistically different between placebo and revexepride for any of the assessment periods. Differences between treatment groups in the changes in impedance values from baseline at any of the channels were also not statistically significant (data not presented).
Symptom association
The changes from baseline in the percentage of patients reporting heartburn, regurgitation, and other symptoms during the pH/impedance monitoring by period are presented in the supplementary information (Table S2). No clear trends were in the changes from baseline in the percentage of patients reporting each symptom were observed. The change from baseline in the percentage of patients reporting symptoms was statistically significant in favor of placebo for heartburn and regurgitation (p = 0.043 and 0.049, respectively) during the recumbent period, and for other symptoms while upright (p = 0.049), and during the postprandial period (p = 0.035). By contrast, there was a statistically significant decrease from baseline in the revexepride treatment group compared with the placebo treatment group in percentage of patients reporting regurgitation symptoms for the 24-h (placebo, 16.4%; revexepride, −2.9%), upright (placebo, 15.3%; revexepride, −5.5%), and postprandial (placebo, 9.2%; revexepride, −9.7%) assessment periods (p = 0.011, 0.007 and 0.027, respectively).
The average number of symptom events associated with a reflux event when assessed over 2- and 5-min intervals (for all pH levels) are presented in the supplementary information (Table S3). Overall, the changes in symptom association from baseline were minimal and not statistically significantly different between study arms for any symptom, time interval, or acidity category. The differences between study arms in the changes from baseline in the percentage of patients with a positive SAP were also not statistically significant for any symptom or acidity category.
Symptom e-diaries, PAGI-SYM, and PAGI-QOL
The mean number of daily occurrences of heartburn, regurgitation, and heartburn and/or regurgitation in both placebo and revexepride groups during the run-in period and in the 4-week period are presented in Fig.2. Analysis of the changes from run-in to the treatment period in the number of days with and without symptoms (as recorded by patients in twice-daily e-diaries) is presented in Table 2. There were no statistically significant differences between study arms in the changes from run-in to week 4 in the number of days with heartburn or regurgitation (Table 2). There were also no statistically significant differences between study arms in the changes from run-in to week 4 in the percentage of days without heartburn or regurgitation (Table 2).
Figure 2.
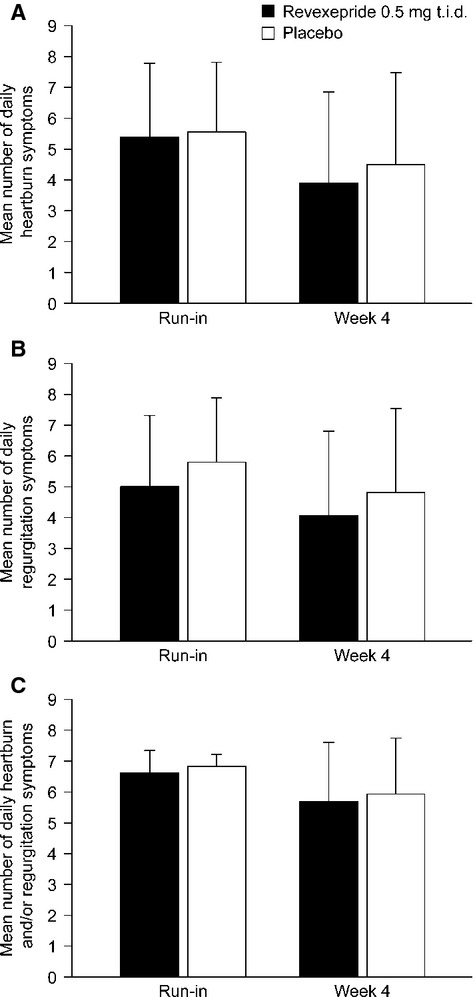
Mean (SD) number of daily occurrences of: (A) heartburn; (B) regurgitation; and (C) heartburn and/or regurgitation, during the run-in and at week 4 as reported in e-diaries. n = 28–31. Note that differences in the changes from baseline to week 4 between placebo and revexepride groups were not significant. SD, standard deviation; t.i.d., three times daily.
Table 2.
Analysis of the changes from run-in to week 4 in the number of days with symptoms and percentage of days without symptoms as recorded in patients' e-diaries, using an ancova model (pharmacodynamic population)
| LS mean change from run-in |
||||
|---|---|---|---|---|
| Week 4 results | Revexepride 0.5 mg t.i.d. (n = 31) | Placebo (n = 29) | Between-treatment difference in LS means (95% CI) | p-value |
| Number of days with symptoms | ||||
| Heartburn | ||||
| Night-time | −1.361 | −0.834 | −0.527 (−1.587 to 0.534) | 0.323 |
| Daytime | −0.684 | −0.944 | 0.260 (−0.678 to 1.198) | 0.580 |
| Daily | −1.066 | −1.062 | −0.004 (−1.072 to 1.063) | 0.994 |
| Regurgitation | ||||
| Night-time | −1.420 | −0.376 | −1.043 (−2.285 to 0.198) | 0.097 |
| Daytime | −0.904 | −0.648 | −0.256 (−1.204 to 0.692) | 0.589 |
| Daily | −1.039 | −0.882 | −0.156 (−1.085 to 0.772) | 0.736 |
| Heartburn and/or regurgitation | ||||
| Night-time | −1.506 | −0.952 | −0.555 (−1.759 to 0.649) | 0.359 |
| Daytime | −0.446 | −0.878 | 0.432 (−0.379 to 1.242) | 0.289 |
| Daily | −0.573 | −0.837 | 0.264 (−0.495 to 1.024) | 0.487 |
| Percentage of days without symptoms | ||||
| Heartburn | ||||
| Night-time | 19.436 | 11.914 | 7.522 (−7.622 to 22.666) | 0.323 |
| Daytime | 9.777 | 13.490 | −3.712 (−17.116 to 9.691) | 0.580 |
| Daily | 15.233 | 15.172 | 0.060 (−15.191 to 15.312) | 0.994 |
| Regurgitation | ||||
| Night-time | 20.279 | 5.374 | 14.905 (−2.827 to 32.637) | 0.097 |
| Daytime | 12.910 | 9.250 | 3.660 (−9.887 to 17.206) | 0.589 |
| Daily | 14.836 | 12.603 | 2.233 (−11.028 to 15.494) | 0.736 |
| Heartburn and/or regurgitation | ||||
| Night-time | 21.521 | 13.593 | 7.928 (−9.274 to 25.130) | 0.359 |
| Daytime | 6.373 | 12.538 | −6.165 (−17.746 to 5.416) | 0.289 |
| Daily | 8.181 | 11.958 | −3.777 (−14.628 to 7.075) | 0.487 |
CI, confidence interval; LS, least-squares; t.i.d., three times daily.
The findings from both the PAGI-SYM and PAGI-QOL questionnaires support the results documented in the e-diaries. The mean (SD) PAGI-SYM total scores for symptom severity were similar (very mild to mild) in both study arms at baseline (revexepride, 1.76 [0.96]; placebo, 1.92 [0.86]) and at day 28 (revexepride, 1.39 [0.93]; placebo, 1.55 [0.96]). There was a 0.44-point LS mean decrease in overall score from baseline to day 28 for the revexepride group, and a 0.35-point LS mean decrease from baseline for the placebo group; the difference between these values was not significant (p = 0.673; ancova). The mean (SD) total PAGI-QOL scores were similar in placebo and revexepride groups at baseline (revexepride, 3.95 [0.79]; placebo, 3.74 [0.96]) and at day 28 (revexepride, 4.09 [0.68]; placebo, 3.91 [0.88]). Mean changes from baseline in the total scores in both study arms were minimal (≤0.21). There were no significant differences between study arms in the LS mean changes from baseline to day 14 or to day 28 in the PAGI-QOL total score (p = 0.708 and p = 0.481, respectively).
Adverse events and safety
No patients experienced a serious TEAE, and most TEAEs were mild to moderate. The proportion of patients who experienced at least one TEAE was higher in the revexepride group (73.5%) than in the placebo group (51.6%; Table 3). In the revexepride group, the TEAEs reported by the greatest numbers of patients were transient diarrhea (38.2%), headache (35.3%), abdominal pain (8.8%), nausea (8.8%), and nasopharyngitis (8.8%). One patient in the revexepride group discontinued treatment owing to TEAEs (dizziness and headache).
Table 3.
Summary of patients with TEAEs (safety population)
| Revexepride 0.5 mg t.i.d. (n = 34) n (%) | Placebo (n = 31) n (%) | |
|---|---|---|
| At least one TEAE | 25 (73.5) | 16 (51.6) |
| At least one serious TEAE | 0 | 0 |
| At least one severe TEAE | 4 (11.8) | 1 (3.2) |
| At least one TEAE that led to investigational product permanently discontinued | 1 (2.9) | 0 |
| At least one TEAE that led to death | 0 | 0 |
| At least one TEAE considered treatment-related | 19 (55.9) | 9 (29.0) |
| TEAE severity | ||
| Mild | 13 (38.2) | 7 (22.6) |
| Moderate | 8 (23.5) | 8 (25.8) |
| Severe | 4 (11.8) | 1 (3.2) |
If a patient had events of differing severity, the incidence of greatest severity is presented. If a patient had more than one TEAE of the same preferred term, the worst case was counted. TEAE, treatment-emergent adverse event; t.i.d., three times daily.
Clinical laboratory findings and vital signs (pulse rate, blood pressure, and weight) were similar at screening, predose on day 1, and on day 29 in both study arms. The increase in mean heart rate from baseline to day 29 was numerically greater in the revexepride group (2.3 ± 8.00 beats/min) than in the placebo group (1.4 ± 9.9 beats/min). The number of patients with a numerical heart rate increase at day 29, however, was similar in both study arms.
Discussion
In this study, the pharmacodynamic effect of revexepride 0.5 mg three times daily on reflux parameters were evaluated in patients with GERD who had persistent reflux symptoms despite PPI therapy. In healthy volunteers, a maximum effect on gastric emptying was obtained with a 0.5 mg dose, which corresponds well to the optimal dose derived from available preclinical animal data (unpublished data). In this investigation, however, no consistent effect of revexepride 0.5 mg on the primary or secondary pharmacodynamic endpoints of gastro-esophageal reflux and reflux symptoms was recorded, compared with placebo.
For patients with persistent symptoms on PPI therapy, the relationship between symptoms and gastro-esophageal reflux, both acidic and non-acidic, can be evaluated more thoroughly with pH/impedance monitoring than with conventional pH monitoring.26 In this exploratory study, pH/impedance monitoring did not reveal a clear treatment effect of revexepride on acidic, weakly acidic, or alkaline reflux events of mixed, liquid, or gaseous composition. A high proximal extent of reflux (≥15 cm above the LES) and mixed gas/liquid reflux has been found to be strongly associated with the perception of clinical symptoms during reflux episodes.27,28 No significant effect was detected on the proximal extent or duration of reflux, and no significant changes were observed when evaluating the overall relationship between reflux events and symptoms as assessed during pH/impedance monitoring, although revexepride did reduce the number of regurgitation events more than placebo during pH/impedance monitoring.
Regurgitation can be caused by esophageal dysmotility and volume reflux.27,29,30 In previous studies, a reduction in regurgitation has been attributed to the prokinetic effects of 5-HT4 receptor agonists such as cisapride and tegaserod on esophageal function,11,13 supporting the proposal that prokinetic agents can be complementary to PPIs in regurgitation-predominant patients.31 However, in the present study, while revexepride had an effect on regurgitation, no effect was observed on the putative underlying mechanism (proximal extension of reflux episodes), which suggests that, given the high data variability in this study, pH/impedance assessment may not have been sensitive enough to detect a difference in reflux events. Alternatively, the observed reduction in regurgitation episodes may be attributable to a change in patients' perception of these episodes. In support of such a mode of action, the 5-HT4 receptor agonist tegaserod was reported to decrease esophageal mechanosensitivity in patients with functional heartburn.32
In addition to the pharmacodynamic endpoints, the effect of revexepride 0.5 mg on reflux symptoms was also evaluated through the use of a symptom e-diary that was completed twice daily. There were no significant differences in treatment effects between the revexepride 0.5 mg and placebo groups for any symptom. This was consistent with the findings from the HRQoL and symptom assessments (PAGI-QOL and PAGI-SYM, respectively). These results are also consistent with the findings of previous studies that have demonstrated that other motility drugs such as the 5-HT4 receptor agonist cisapride in patients with functional dyspepsia,29,33 and the reflux inhibitor lesogaberan in patients with GERD who are partially responsive to PPI therapy, have poor efficacy in treating symptoms.34 Although lesogaberan showed a significant effect on transient LES relaxations and reflux variables, these findings did not translate into meaningful symptom relief.
Our findings do not necessarily negate a potential benefit of treatment with revexepride 0.5 mg, particularly when considering the efficacy of the drug in accelerating gastric emptying in healthy volunteers and animal models that has been shown in previous trials (unpublished data). There are several potential study limitations that may have contributed to the lack of clear treatment effect. The relatively small number of patients in this study is a crucial limiting factor. The study was terminated without having reached the initial target of 45 patients per treatment arm because an interim analysis indicated that high variability in reflux events and symptoms would require recruitment of many more patients to demonstrate significant differences between the treatment and placebo groups. Despite attempts to reduce the variability in the pH/impedance data through standardization and the use of a central reader, statistical power remained an issue. Day-to-day variability in the number of reflux events and reflux-related symptoms may genuinely be high, but the picture may also be complicated by high inter- and intra-assessor variation in pH-impedance analyses.35
The lack of a clear treatment effect with revexepride 0.5 mg three times daily compared with placebo may also reflect problems of identifying patients on acid-suppressive therapies with reflux symptoms that are related to persistent, weakly acidic reflux events or dysmotility, who could potentially improve with a prokinetic medication. An estimated 20% of patients with reflux symptoms have functional heartburn36 and this proportion is likely to be higher among those who have a partial response to PPI therapy.17 In an attempt to include patients with GERD in our study, participants were selected on the basis of the outcomes of 24-h pH/impedance monitoring and were required to have a minimum number of 25 liquid-containing reflux events in a 24-h period, together with symptoms of heartburn and/or regurgitation on at least 3 days a week. A positive SAP was not an inclusion criterion in this study. On one hand, this may have led to dilution of the patient population with individuals who have functional or non-reflux-related symptoms that are less likely to respond to antireflux treatment, making the detection of a significant treatment effect less probable.37 On the other hand, given the lack of beneficial effects on reflux parameters, restricting inclusion to patients with a positive SAP most likely would not have changed the overall outcome of the study.
In the present trial, a relatively high proportion of patients (16.9%) reported no response to PPI treatment at baseline, which suggests that they might have had esophageal dysmotility or functional heartburn rather than GERD. In addition, benign physiological reflux events may occur which are generally not associated with pathological signs or symptoms.12 Therefore, the persistent symptoms in the other 83.1% of patients who did at least partially respond to PPI may have been related to acid reflux or other pathology, such as dysmotility or esophageal hypersensitivity. Esophageal hypersensitivity is another factor contributing to persistent symptoms, and prokinetic action seems less likely to be effective for patients when this is a prominent underlying pathophysiological factor.5 In addition, approximately 15% of patients reported that PPIs improved their symptoms ‘very much’ or ‘quite a bit’; therefore, the potential positive effect of revexepride on symptom improvement from baseline in these patients may not have been as obvious if they had reported that PPIs did not improve their symptoms very much. The relatively low symptom severity (indicated by PAGI-SYM scores) at baseline in both placebo and revexepride treatment groups and potential psychosomatic effects may have also hampered the ability to detect a significant treatment effect on symptoms. Furthermore, the investigational dose of 0.5 mg and treatment duration (∼26 days) might also have been suboptimal.
Overall, and consistent with previous clinical studies, revexepride 0.5 mg was well-tolerated. Although the number of AEs was higher than with placebo, most TEAEs were mild or moderate, and no serious TEAEs were observed. There were no clinically significant safety findings; the safety evaluations did not raise any concerns about the dose of revexepride administered in this study. The problems of identifying patients with GERD who have a partial response to PPIs in advance of treatment and the limitations of questionnaires targeting GERD parameters require further attention. Future clinical studies of revexepride and other prokinetics may be better directed at patients with symptoms that are clearly related to persistent weakly acidic reflux, hypotensive esophageal dysmotility, or slow gastric emptying (i.e., symptomatic dysfunction), as determined by appropriate physiological tests.
In conclusion, pH/impedance monitoring and symptom assessments did not reveal a consistent difference in primary or secondary pharmacodynamic endpoints between revexepride 0.5 mg three times daily and placebo in this exploratory study. Overall, revexepride 0.5 mg was well-tolerated and no safety concerns were identified.
Acknowledgments
Writing support was provided by Vivienne Stein-Rostaing of PharmaGenesis™ London and funded by Shire.
Glossary
- AE
adverse event
- BMI
body mass index
- e-diary
electronic diary
- GERD
gastro-esophageal reflux disease
- HRQoL
health-related quality of life
- LES
lower esophageal sphincter
- LS
least-squares
- PAGI-QOL
patient assessment of upper gastrointestinal quality of life
- PAGI-SYM
patient assessment of upper gastrointestinal symptom severity index
- PPI
proton pump inhibitor
- SAP
symptom association probability
- TEAE
treatment-emergent adverse event
Funding
This study was funded in full by Shire-Movetis NV.
Disclosure
Jan Tack has provided scientific advice to Almirall, AstraZeneca, Cosucra, Danone, GI Dynamics, GlaxoSmithKline, Ironwood, Janssen, Menarini, Novartis, Rhythm, Shire, Takeda, Theravance, Tsumura, Will-Pharma, and Zeria, has received a research grant or support from Abbott, Alpro, Novartis, Sandhill Scientific, and Shire, and has served on speaker bureaus for Abbott, Almirall, AstraZeneca, Danone, Janssen, Menarini, MMS, Novartis, Shire, Takeda, and Zeria. Albert J Bredenoord is supported by the Netherlands Organisation for Scientific Research (NWO), has received research funding from AstraZeneca, EndoStim, Given Imaging, MMS, and Shire, and has received speaker and/or consulting fees from Almirall, MMS, and Shire. Mark Fox has served as speaker, consultant and/or advisory board member for Almirall, AstraZeneca, Given Imaging, Reckitt Benckiser, Sanofi, and Shire Movetis NV. Hubert Louis has received speaker and/or consulting fees from AstraZeneca and Shire. Jan Borovicka has received speaker and/or consulting fees from Allmiral, Abbvie and MSD. François Mion has received speaker and/or consulting fees from Allmiral, Given Imaging, and Shire. Frank Zerbib has served as a speaker, consultant, and advisory board member for Abbott, Addex Pharma SA, Almirall, Cephalon, AstraZeneca, Given Imaging, Janssen Cilag, Norgine, Pfizer, Reckitt Benckiser, Sanofi Aventis, Shire Movetis NV, and Xenoport. Hubert Piessevaux has received speaker and/or consulting fees from Shire. Stanislas Bruley des Varannes has served as a speaker, consultant, and advisory board member for Almirall, Alfa Wassermann, Cephalon, Given Imaging, Janssen, and Shire Movetis. Sophie Dedrie, Mieke Hoppenbrouwers, Ann Meulemans, An Rykx, Leen Thielemans, and Magnus Ruth were employees of Shire at the time of the study. Kathleen Blondeau has nothing to disclose.
Author Contribution
JT, MR, LT, AR, AM, and MH contributed to the concept and design of the study; JT, KB, FZ, SBdV, HP, HL, JB, FM, MF, and AJB were involved in data acquisition; LP performed the data analysis. All authors contributed to data interpretation and critically reviewed the manuscript. All authors approved the final version of the manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher's web site:
Figure S1. Analysis population flowchart. AE, adverse event; PD, pharmocodynamic; pH/impedance, combined pH, and multichannel intraluminal impedance.
Table S1. Secondary endpoints: pH/impedance monitoring (pharmacodynamic population).
Table S2. Symptom association results reported by patients during pH/impedance monitoring (pharmacodynamic population).
Table S3. Changes in symptom reflux association from baseline to day 28 for placebo and revexepride treatment groups (pharmacodynamic population).
References
- 1.Katz PO, Zavala S. Proton pump inhibitors in the management of GERD. J Gastrointest Surg. 2010;14:S62–6. doi: 10.1007/s11605-009-1015-3. [DOI] [PubMed] [Google Scholar]
- 2.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009;58:295–309. doi: 10.1136/gut.2007.145581. [DOI] [PubMed] [Google Scholar]
- 3.Hershcovici T, Fass R. Management of gastroesophageal reflux disease that does not respond well to proton pump inhibitors. Curr Opin Gastroenterol. 2010;26:367–78. doi: 10.1097/MOG.0b013e32833ae2be. [DOI] [PubMed] [Google Scholar]
- 4.Vela MF, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz PO, Castell DO. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599–606. doi: 10.1053/gast.2001.24840. [DOI] [PubMed] [Google Scholar]
- 5.Tack J. Is there a unifying role for visceral hypersensitivity and irritable bowel syndrome in non-erosive reflux disease? Digestion. 2008;78:S42–5. doi: 10.1159/000151254. [DOI] [PubMed] [Google Scholar]
- 6.Sweis R, Anggiansah A, Wong T, Brady G, Fox M. Assessment of esophageal dysfunction and symptoms during and after a standardized test meal: development and clinical validation of a new methodology utilizing high-resolution manometry. Neurogastroenterol Motil. 2014;26:215–28. doi: 10.1111/nmo.12252. [DOI] [PubMed] [Google Scholar]
- 7.Piche T, Galmiche JP. Pharmacological targets in gastro-oesophageal reflux disease. Basic Clin Pharmacol Toxicol. 2005;97:333–41. doi: 10.1111/j.1742-7843.2005.pto_273.x. [DOI] [PubMed] [Google Scholar]
- 8.Emerenziani S, Sifrim D. Gastroesophageal reflux and gastric emptying, revisited. Curr Gastroenterol Rep. 2005;7:190–5. doi: 10.1007/s11894-005-0033-x. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Conklin JL. Neuromuscular control of esophageal peristalsis. Curr Gastroenterol Rep. 1999;1:186–97. doi: 10.1007/s11894-999-0033-3. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman LR, Faulds D. Cisapride: an updated review of its pharmacology and therapeutic efficacy as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1994;47:116–52. doi: 10.2165/00003495-199447010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Staiano A, Clouse RE. The effects of cisapride on the topography of oesophageal peristalsis. Aliment Pharmacol Ther. 1996;10:875–82. doi: 10.1046/j.1365-2036.1996.94266000.x. [DOI] [PubMed] [Google Scholar]
- 12.Kahrilas PJ. GERD pathogenesis, pathophysiology, and clinical manifestations. Cleve Clin J Med. 2003;70:S4–19. doi: 10.3949/ccjm.70.suppl_5.s4. [DOI] [PubMed] [Google Scholar]
- 13.Fox M, Menne D, Stutz B, Fried M, Schwizer W. The effects of tegaserod on oesophageal function and bolus transport in healthy volunteers: studies using concurrent high-resolution manometry and videofluoroscopy. Aliment Pharmacol Ther. 2006;24:1017–27. doi: 10.1111/j.1365-2036.2006.03090.x. [DOI] [PubMed] [Google Scholar]
- 14.Manzotti ME, Catalano HN, Serrano FA, Di Stilio G, Koch MF, Guyatt G. Prokinetic drug utility in the treatment of gastroesophageal reflux esophagitis: a systematic review of randomized controlled trials. Open Med. 2007;1:171–80. [PMC free article] [PubMed] [Google Scholar]
- 15.Tack J, Becher A, Mulligan C, Johnson DA. Systematic review: the burden of disruptive gastrooesophageal reflux disease on health-related quality of life. Aliment Pharmacol Ther. 2012;35:1257–66. doi: 10.1111/j.1365-2036.2012.05086.x. [DOI] [PubMed] [Google Scholar]
- 16.Mainie I, Tutuian R, Shay S, Vela M, Zhang X, Sifrim D, Castell DO. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut. 2006;55:1398–402. doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerbib F, Roman S, Ropert A, des Varannes SB, Pouderoux P, Chaput U, Mion F, Vérin E, et al. Esophageal pH-impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol. 2006;101:1956–63. doi: 10.1111/j.1572-0241.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 18.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–8019. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holloway RH, Kocyan P, Dent J. Provocation of transient lower esophageal sphincter relaxations by meals in patients with symptomatic gastroesophageal reflux. Dig Dis Sci. 1991;36:1034–9. doi: 10.1007/BF01297443. [DOI] [PubMed] [Google Scholar]
- 20.Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–31. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rentz AM, Kahrilas P, Stanghellini V, Tack J, Talley NJ, de la Loge C, Trudeau E, Dubois D, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–49. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 22.de la Loge C, Trudeau E, Marquis P, Kahrilas P, Stanghellini V, Talley NJ, Tack J, Revicki DA, et al. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: the PAGI-QOL. Qual Life Res. 2004;13:1751–62. doi: 10.1007/s11136-004-8751-3. [DOI] [PubMed] [Google Scholar]
- 23.Boeckxstaens GE, Denison H, Ruth M, Adler J, Silberg DG, Sifrim D. Effect of AZD3355, a novel GABA(B) agonist, on reflux and lower esophageal sphincter function in patients with GERD with symptoms despite proton pump inhibitor treatment. Gut. 2009;58:A-433. [Google Scholar]
- 24.Sakpal TV. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1:67–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Wyrwich KW, Mody R, Larsen LM, Lee M, Harnam N, Revicki DA. Validation of the PAGI-SYM and PAGI-QOL among healing and maintenance of erosive esophagitis clinical trial participants. Qual Life Res. 2010;19:551–64. doi: 10.1007/s11136-010-9620-x. [DOI] [PubMed] [Google Scholar]
- 26.Tutuian R, Vela MF, Shay SS, Castell DO. Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J Clin Gastroenterol. 2003;37:206–15. doi: 10.1097/00004836-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Zerbib F, Duriez A, Roman S, Capdepont M, Mion F. Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut. 2008;57:156–60. doi: 10.1136/gut.2007.133470. [DOI] [PubMed] [Google Scholar]
- 28.Tutuian R, Vela MF, Hill EG, Mainie I, Agrawal A, Castell DO. Characteristics of symptomatic reflux episodes on acid suppressive therapy. Am J Gastroenterol. 2008;103:1090–6. doi: 10.1111/j.1572-0241.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 29.Tutuian R, Mainie I, Allan R, Hargreaves K, Agrawal A, Freeman J, Gale J, Castell DO. Effects of a 5-HT(4) receptor agonist on oesophageal function and gastro-oesophageal reflux: studies using combined impedance-manometry and combined impedance-pH. Aliment Pharmacol Ther. 2006;24:155–62. doi: 10.1111/j.1365-2036.2006.02968.x. [DOI] [PubMed] [Google Scholar]
- 30.Sifrim D, Mittal R, Fass R, Smout A, Castell D, Tack J, Gregersen H. Review article: acidity and volume of the refluxate in the genesis of gastro-oesophageal reflux disease symptoms. Aliment Pharmacol Ther. 2007;25:1003–15. doi: 10.1111/j.1365-2036.2007.03281.x. [DOI] [PubMed] [Google Scholar]
- 31.Kahrilas P, Howden C, Jonsson A, Denison H, Wernersson B, Hughes N. Responsiveness of regurgitation, dimensionalized by the Reflux Disease Questionnaire, to potent acid-suppressing therapy. Gastroenterology. 2011;140:S-189. [Google Scholar]
- 32.Rodriguez-Stanley S, Zubaidi S, Proskin HM, Kralstein JR, Shetzline MA, Miner PB., Jr Effect of tegaserod on esophageal pain threshold, regurgitation, and symptom relief in patients with functional heartburn and mechanical sensitivity. Clin Gastroenterol Hepatol. 2006;4:442–50. doi: 10.1016/j.cgh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Champion MC, MacCannell KL, Thomson AB, Tanton R, Eberhard S, Sullivan SN, Archambault A. A double-blind randomized study of cisapride in the treatment of nonulcer dyspepsia. The Canadian Cisapride NUD Study Group. Can J Gastroenterol. 1997;11:127–34. doi: 10.1155/1997/314839. [DOI] [PubMed] [Google Scholar]
- 34.Shaheen NJ, Denison H, Björck K, Karlsson M, Silberg DG. Efficacy and safety of lesogaberan in gastroesophageal reflux disease: a randomized controlled trial. Gut. 2013;62:1248–55. doi: 10.1136/gutjnl-2012-302737. [DOI] [PubMed] [Google Scholar]
- 35.Loots CM, van Wijk MP, Blondeau K, Tanton R, Eberhard S, Sullivan SN, Archambault A. Interobserver and intraobserver variability in pH-impedance analysis between 10 experts and automated analysis. J Pediatr. 2012;160:441–6. doi: 10.1016/j.jpeds.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Quigley EM. Non-erosive reflux disease, functional heartburn and gastroesophageal reflux disease; insights into pathophysiology and clinical presentation. Chin J Dig Dis. 2006;7:186–90. doi: 10.1111/j.1443-9573.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 37.Weijenborg PW, Cremonini F, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012;24:747–57. doi: 10.1111/j.1365-2982.2012.01888.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analysis population flowchart. AE, adverse event; PD, pharmocodynamic; pH/impedance, combined pH, and multichannel intraluminal impedance.
Table S1. Secondary endpoints: pH/impedance monitoring (pharmacodynamic population).
Table S2. Symptom association results reported by patients during pH/impedance monitoring (pharmacodynamic population).
Table S3. Changes in symptom reflux association from baseline to day 28 for placebo and revexepride treatment groups (pharmacodynamic population).


