Analyses of forest loss and protected areas suggest that 36 to 57% of Amazonian tree flora may qualify as “globally threatened.”
Keywords: Amazonia, Conservation, Deforestation, Protected areas, Indigenous areas, Tree species
Abstract
Estimates of extinction risk for Amazonian plant and animal species are rare and not often incorporated into land-use policy and conservation planning. We overlay spatial distribution models with historical and projected deforestation to show that at least 36% and up to 57% of all Amazonian tree species are likely to qualify as globally threatened under International Union for Conservation of Nature (IUCN) Red List criteria. If confirmed, these results would increase the number of threatened plant species on Earth by 22%. We show that the trends observed in Amazonia apply to trees throughout the tropics, and we predict that most of the world’s >40,000 tropical tree species now qualify as globally threatened. A gap analysis suggests that existing Amazonian protected areas and indigenous territories will protect viable populations of most threatened species if these areas suffer no further degradation, highlighting the key roles that protected areas, indigenous peoples, and improved governance can play in preventing large-scale extinctions in the tropics in this century.
INTRODUCTION
Amazonian forests have lost ~12% of their original extent and are projected to lose another 9 to 28% by 2050 (1, 2). The consequences of ongoing forest loss in Amazonia (here all rainforests of the Amazon basin and Guiana Shield) are relatively well understood at the ecosystem level, where they include soil erosion (3, 4), diminished ecosystem services (5–8), altered climatic patterns (5, 7, 9–11), and habitat degradation. By contrast, little is known about how historical forest loss has affected the population sizes of plant and animal species in the basin and how ongoing deforestation will affect these populations in the future.
As a result, the conservation status of the >15,000 species that compose the Amazonian tree flora—one of the most diverse plant communities on Earth—remains unknown. To date, only a tiny proportion of Amazonian tree species have been formally assessed for the International Union for Conservation of Nature (IUCN) Red List. Two previous studies have attempted to estimate the extinction threat to Amazonian plants using theory, data, and vegetation maps to model reductions in range size, but they disagreed on whether the proportion of threatened plant species in the Amazon is low (5 to 9%) (12) or moderate (20 to 33%) (13).
Here, we build on that work by using a spatially explicit model of tree species abundance (14) based on 1485 forest inventories (fig. S1) to quantify how historical deforestation across Amazonia (1, 2, 15) has reduced the population sizes of 4953 relatively common tree species. We use a separate model to estimate population declines for an additional 10,247 rarer tree species. For both models, we also estimate the population losses expected for 2050 under two deforestation scenarios (1, 2) and ask to what extent projected losses can be prevented by Amazonia’s existing protected area network. In contrast to previous studies, which presented results in the currency of statistical probability of extinction, we interpret our results using the criteria of the IUCN Red List of Threatened Species, the most commonly used yardstick for species conservation status.
RESULTS
Effects of historical forest loss on tree populations
The original lowland forests of Amazonia are estimated to have covered 5.74 million km2 (fig. S2), 11.4% of which had been deforested by 2013 (1, 2) (figs. S3 and S4A and appendix S1). Most of the estimated 3.2 × 1010 individual trees lost to date (appendixes S2 and S3) were in southern and eastern Amazonia (Fig. 1A).
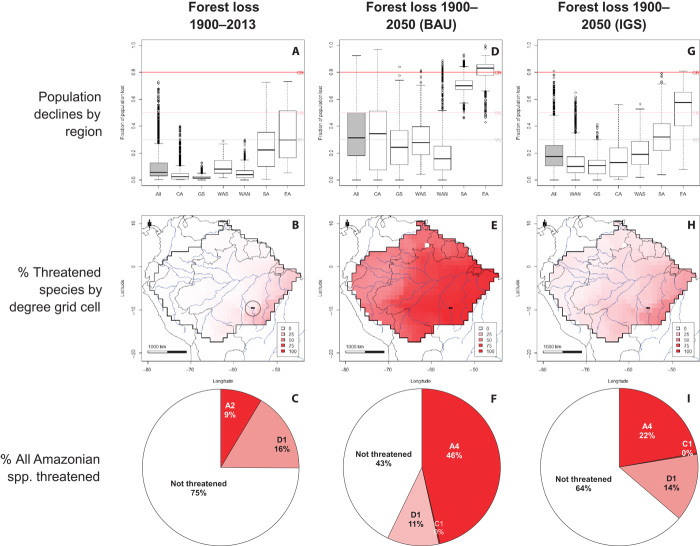
Fig. 1. Estimated population declines and threat status of Amazonian tree species under historical deforestation and two projected deforestation scenarios.
Historical deforestation (A to C). Projected deforestation (D to I). Top row: Percent population loss of 4953 tree species in the entire Amazon and in six Amazonian regions. Middle row: Percent species in a DGC estimated as globally threatened based on projected (including historical) forest loss (IUCN A2 and A4; n = 4953). Bottom row: Proportion of all 15,200 Amazonian tree species estimated to be globally threatened based on four different IUCN threat criteria. BAU: projected (including historical) deforestation through 2050 based on a BAU scenario (1, 2); IGS: projected (including historical) deforestation through 2050 based on an IGS (1, 2). Cristalino State Park is the small black polygon in southeastern Amazonia, encircled in (B). CA, Central Amazonia; GS, Guiana Shield; WAS, Southwestern Amazonia; WAN, Northwestern Amazonia; SA, Southern Amazonia; EA, Eastern Amazonia; CR, critically endangered; EN, endangered; VU, vulnerable.
Overlaying these deforestation data with the output of our spatial model of the distribution and abundance of 4953 relatively common tree species allowed us to estimate the impact of forest loss on the Amazonian populations of these species. Forest loss up to 2013 (figs. S3 and S4A) caused a mean decline of 11% in the number of individuals of tree species across Amazonia (median, 6%) (Fig. 1A and fig. S4D) and mean declines of 2 to 32% in individual Amazonian regions. Of 4953 common species, 342 (7.5%) have lost a large enough proportion of their original populations (≥30%) to qualify as globally threatened under IUCN criterion A2 (Fig. 1A and appendix S2). A separate analysis performed to model the distribution and extinction risk of 10,247 rare tree species in the Amazon suggested that 9% of them (a total of 967 species) have lost enough individuals to qualify as globally threatened under the same criterion (fig. S5A and table S1). Together, these analyses suggest that 9% of all Amazonian tree species likely qualify as threatened as a result of historical forest loss through 2013 (Fig. 1C). Adding the 2579 rare species that may qualify as threatened because they have an estimated <1000 individuals (IUCN criterion D1) increases the proportion of all threatened species to 25% (Table 1).
Table 1. Number of Amazonian tree species estimated to qualify as globally threatened under four IUCN threat status criteria.
Numbers of threatened species are nonoverlapping (that is, species listed for C1 did not qualify for A4). BAU = projected (including historical) deforestation through 2050 based on a BAU scenario (1, 2); IGS = projected (including historical) deforestation through 2050 based on an IGS (1, 2).
|
Forest loss 1900–2013 |
Forest loss 1900–2050 (BAU) |
Forest loss 1900–2050 (IGS) |
|
| Total number of species | 15,200 | 15,200 | 15,200 |
| Number of species with >30% observed population decline to date (IUCN A2) | 1309 | — | — |
| Number of species with >30% projected population decline over three generations (IUCN A4) |
— | 7033 | 3364 |
| Number of species with >10% projected population decline over three generations and <10,000 individuals (IUCN C1) |
— | 38 | 44 |
| Number of species with <1000 individuals (IUCN D1) | 2505 | 1619 | 2107 |
| Total number of threatened species | 3814 | 8690 | 5515 |
| Percentage of all species threatened | 25 | 57 | 36 |
The data in fig. S4 (A and D) suggest a one-to-one relationship between percent historical forest loss and mean percent loss of individuals to date. Consequently, population losses of the common species are highest in regions where deforestation rates are highest, the so-called “Arc of Deforestation” in southern and eastern Amazonia. The same patterns were observed for rare species.
Effects of projected forest loss on tree populations
We repeated the above analyses for two scenarios of projected forest loss (which include historical loss). The business-as-usual (BAU) scenario model (1) estimates that, by 2050, ~40% of the original Amazon forest will be destroyed (figs. S4B and S6 and appendix S1). The improved governance scenario (IGS) model (1) estimates forest loss by 2050 at 21% (figs. S4C and S7 and appendix S1). Under these two scenarios, only 31 to 42% of grid cells maintain >95% forest cover. As is the case for historical deforestation, future deforestation is projected to be most severe in southern and eastern Amazonia (34 to 66% and 42 to 76% forest cover loss, respectively).
For common species, mean population declines under the BAU scenario are estimated to be 35% (median, 32%), and absolute declines range from 0 to 83% (Fig. 1D, fig. S4E, and appendixes S2 and S3). Under the BAU scenario, 2567 (51%) of all common species likely qualify as threatened under IUCN criterion A4 (Fig. 1D). Under IGS, average losses are lower, with a mean of 20% (median, 18%) and a range of 0 to 82% (fig. S4F and appendixes S2 and S3); 774 (16%) of common species likely qualify as threatened (Fig. 1G). Again, the severest threat is found in southern and eastern Amazonia (Fig. 1G and fig. S4D).
Both scenarios also pose severe threats to rare species. Under the BAU scenario, 4466 (43%) of all rare species are predicted to lose ≥30% of their population by 2050 (fig. S5B and table S1), compared to 2590 (25%) of all rare species under IGS (fig. S5C and table S1). Under the BAU scenario, rare species are expected to be most severely hit in southern and eastern Amazonia, where the median population loss is 100% and more than 65 and 86% of the species, respectively, have population losses of more than 80% (table S1).
Combining the analyses of common and rare species suggests that 3364 to 7033 Amazonian tree species likely qualify as globally threatened as a result of a combination of historical and projected forest loss (Fig. 1, F and I). An additional 1657 to 2151 species in the data set are likely to qualify as globally threatened because they have very small population sizes (IUCN criteria C1 and D1). When all criteria are included, we find that 36 to 57% of Amazonian tree species likely qualify as globally threatened (Table 1).
To what degree will protected areas and indigenous territories prevent declines of Amazonian tree populations?
Over the last 50 years, Amazonian countries have formalized a large network of protected areas and indigenous territories (fig. S8 and appendix S1) that now cover 52.2% of the basin: 9% in strict conservation reserves (SCRs) (fig. S9A) and 44.3% in sustainable use and indigenous reserves (SUIRs) (fig. S9B). Our models suggest that all of the 4953 common species are protected to some degree by SCRs and SUIRs (for convenience, we refer to both as protected areas) (fig. S9, C and D). Every common species is estimated to have more than 5500 adult individuals within protected areas, with 23%, on average, of these individuals occurring in SCRs and 77% in SUIRs. However, Performance is poor in some Amazonian regions. For example, the scarcity of SCRs in central and eastern Amazonia means that, on average, only 2% of individuals of common species in these regions are in SCRs (fig. S9, C and D). Our simulation models also suggest that 580 of the 10,247 rare species have more than 70% of their individuals in SCRs (fig. S10A and table S2), compared to 4005 in SUIRs.
Preventing deforestation within protected areas between now and 2050 could significantly reduce the number of threatened Amazonian tree species because both 2050 deforestation scenarios assume significant deforestation within protected areas (figs. S11 to S13): one-third of projected BAU deforestation and 16% of projected IGS deforestation. If the deforestation that is projected to occur within protected areas under the BAU scenario and IGS is not factored in, the number of common species that likely qualify as threatened under IUCN criterion A4 will fall by 29 to 44%. For example, 63% of wild Brazil nut trees (Bertholletia excelsa) are expected to be lost by 2050 under the BAU scenario. Under a modified IGS that allows for no deforestation within protected areas, this percentage drops to 32%, and B. excelsa no longer qualifies as endangered (appendix S2).
DISCUSSION
Our analyses suggest that historical and ongoing forest loss may cause population declines of >30% in one-quarter to one-half of all Amazonian tree species by 2050. These declines affect species in all Amazonian regions, including iconic Amazonian trees such as Brazil nut (B. excelsa), wild populations of major food crops such as cacao (Theobroma cacao; 50% population decline with the BAU scenario) and açai palm (Euterpe oleracea; 72% decline with the BAU scenario), and 167 of the 227 hyperdominant taxa that account for half of all Amazonian trees (14). Although these declines comprise both historical population losses and population losses projected to occur in the future, they could be used to classify these species as threatened now under IUCN criterion A4b.
Thousands of other Amazonian tree species are likely to qualify as globally threatened because they have very small populations (Table 1). Although our methods and results are preliminary (see the Supplementary Materials), the statistical independence that we find between the estimated population size of a species and its fractional decline in numbers (fig. S14) suggests that the primary findings will remain stable as sampling improves.
A 22% increase in the global red list for plants
Our estimates of the threat status of all Amazonian tree species constitute the largest threat assessment ever carried out. In fact, the number of species assessed in our analyses (15,200) is nearly as large as the number of all plant species evaluated by the IUCN over its 50-year history (19,738) [Table 3b in the IUCN Red List (16)]. If the 194 countries that have adopted the Global Strategy for Plant Conservation are to meet target 2 (“A preliminary assessment of the conservation status of all known plant species” by 2020), it will require large scaling-up approaches such as the one described here [see also Miller et al. (17)].
Such approaches are urgently needed for South America’s tropical flora. Over the last 10 years, only 1275 plant species from tropical South America were added to the IUCN Red List, despite strong evidence that the number should be at least an order of magnitude higher (18–21). In general, our results provide strong support to predictions that at least one in four plant species in the South American tropics now deserve listing as globally threatened (20). They also show that most of the species that likely qualify as threatened in the region remain absent from global and national red lists. For example, of the 2567 common species that qualify as threatened under our BAU analysis, only 351 (14%) had previously been assessed using IUCN criteria and only 6% are listed as threatened. Adding all of our threatened Amazonian tree species to the IUCN Red List would increase the number of globally threatened plants on Earth by 22% and the number of globally threatened tree species by 36%.
We are aware, however, that our results are too preliminary to constitute a red list for Amazonian trees. Red-listing these species will require case-by-case assessments by the IUCN/Species Survival Commission Global Tree Specialist Group and country-level teams, taking into account other data sources and threat criteria. What we show here are the size, urgency, and feasibility of this task. A recent Brazilian effort to evaluate the threat status of 4617 plant species in Brazil reported a per-species cost of ~US$50 (19). This suggests that individually assessing the named species that we suspect to be threatened and making their threat status visible to the conservation community would cost <US$1,000,000.
Most tropical tree species may be globally threatened
Despite strong spatial clustering in both deforestation scenarios and species distributions, our analyses reveal a simple rule of thumb that works at both regional and basinwide scales: n% forest loss yields an average of ~n% population loss (Fig. 1 and fig. S4, A and D). This implies that tree species in other forest biomes of tropical South America have lost much larger proportions of their population than in the core closed-canopy Amazonian moist forest: for example, the Atlantic forest (84 to 88% forest loss) (22), the Cerrado (53%) (23), the Caatinga (37%) (23), and dry forests in general (>60%) (24).
Given that Africa has lost ~55% of its tropical forests and Asia has lost ~35%, mostly since 1900 (25), our analyses suggest that most tree species in the Old World tropics have lost more than 30% of their individuals over the last 150 years and thus qualify as globally threatened under IUCN criterion A4. In turn, because >90% of all tree species on Earth are tropical (26), trees may deserve to join cycads (63%), amphibians (41%), and corals (33%) on the list of groups with the highest proportions of globally threatened species.
Although many tropical tree species have symbiotic relationships with animals and co-occur with thousands of species of nonarboreal plants, high rates of threat cannot be inferred for these organisms in the same way because of their much shorter life spans. Bird et al. (27) compared estimated range maps of Amazonian bird species with maps of projected deforestation across three bird generations and found that only 5.5 to 18.8% of species qualified as threatened under IUCN criterion A4. Three bird generations in their model averaged 14.8 years, compared to 150 years in our tree model.
Linking forest loss, species threat status, and protected areas management in the Amazon
Heavy forest clearing in southern and eastern Amazonia has put an especially high proportion of tree species at risk of extinction (Fig. 1A). In the worst hit areas of the Arc of Deforestation, a third of tree species have already lost >30% of their population to deforestation, and more than half likely qualify as globally threatened based on projected (and historical) forest loss (Fig. 1B).
By linking spatial trends in forest loss to trends in the population sizes of individual Amazonian plant species in this way, models such as ours should soon make it possible to translate remote sensing–based data on Amazonian deforestation into site-specific and species-specific guidance for conservation managers. It will also be possible to model how individual species will be affected by infrastructure projects (28) such as major hydroelectric dams (29), degazetting of protected areas (30), and other drivers of Amazonian forest loss. This could have serious implications for large-scale development projects, which are increasingly required to protect IUCN-listed taxa and their habitat [for example, Performance Standard 6. Biodiversity Conservation and Sustainable Management of Natural Resources (31)].
These models can also generate predictions about which plant species occur in which protected areas and, thus, to what extent these species are protected and where. For example, floristic surveys at Cristalino State Park, in one of Brazil’s most severely deforested regions, have recorded at least 551 tree species (32). Appendix S4 lists another 766 species that have a high probability of occurring at Cristalino State Park according to our model and shows that as many as 1214 of the 1317 species known or expected from Cristalino State Park likely qualify as globally threatened under the BAU scenario. Similar analyses could help ensure that Amazonian protected areas with especially high numbers of globally threatened tree species receive the level of protection and funding they merit.
Many practical and scientific obstacles stand in the way of a stable, comprehensive red list for Amazonian tree species (see the Supplementary Materials). We have shown in this study that such a list will include several thousand species, many of which are now considered common, and will include a very large majority of the tree species occurring in the Amazon’s worst hit regions. As Amazonian forest loss continues, new approaches such as these will be needed to help guide management away from BAU scenarios and ensure a long-term future for the world’s richest tree flora. Indeed, sustaining the recent historical trend of reduced Amazonian deforestation through 2050 will keep as many tree species from becoming critically endangered as there are critically endangered plant species on the IUCN Red List today.
MATERIALS AND METHODS
Amazonian base map
To overlay spatial data on deforestation, protected areas, and tree species distribution and abundance, we first made a base map of Amazonia. The borders of the base map were the same as those in our previous study (14). We gridded this landscape into 0.1-degree grid cells (01DGCs) (33) and eliminated all 01DGCs that were more than 50% water (33), nonforest vegetation such as open wetlands or savannahs (1), or elevations of >500 m (34). This reduced the total area by 17%. We then quantified the area of all individual 01DGCs, which varies with latitude because of distance from the equator (~124 km2 at the equator, ~106 km2 at 14°S, and ~120 km2 at 8°N). The final forest map consists of 46,986 01DGCs or 5.79 million km2 (fig. S1).
Tree density
Our tree inventory data come from the Amazon Tree Diversity Network (ATDN) (14). The methods we used to estimate tree density, abundance, and distribution are similar to those used in our previous study (14) but are based on >20% more tree plots than in that study. The ATDN now comprises 1766 (1-ha) tree inventory plots scattered throughout Amazonia (fig. S1).
The total number of trees in Amazonia with ≥10 cm diameter at breast height was estimated as in our previous study (14) but with a larger subset of plots (1625) and at the 1-degree grid cell (DGC) level. We constructed a locally weighted (loess) regression model for tree density (stems/ha) on the basis of the observed tree density in 1625 plots, with latitude, longitude, and their interaction as independent variables. The span was set at 0.5 to yield a relatively smooth average. The model was used to estimate the average tree density in each DGC (DDGC, stems/ha) (fig. S15). This average density per hectare was then multiplied by the total forested area of each DGC to obtain the total number of trees in the DGC. The total number of trees estimated was 3.2 × 1011. This is 17.9% lower than the estimate in our previous study (14) because this number corrects for the actual lowland forest cover in each DGC.
Modeled population sizes and species distributions: Common species
Analyses of tree species composition were performed with a subset of 1560 plots in which all 775,532 free-standing trees ≥10 cm diameter at breast height had been identified with a valid name at the species (86.0%), genus (97.2%), or family (99.0%) level before our study. Most plots (1282) measured exactly 1 ha, 392 were smaller (0.25 to 0.99), 91 were larger (1.01 to 4), and 4 were plotless samples (point-centered quarter) for which the number of trees was equivalent to that typically found in 0.5 to 1 ha. Most issues of species identification and nomenclature were handled as in our previous study (14), but there were some exceptions. Species with a “cf.” identification were accepted as belonging to the named species, whereas those with “aff.” were tabulated at the genus level. All data associated with names that were clearly wrong (for example, those of small herbs) were disregarded.
Although we assume identification error to be within acceptable limits for common species [see discussion in our previous paper (14)], we retained only plots in which ≥60% of individuals were identified to species (1480 plots) (fig. S16). The number of trees belonging to each species in the DGC was estimated as follows. Abundances of all valid species were converted into relative abundances for each plot: RAi = ni/N, where ni is the number of individuals of species i and N is the total number of trees in the plot (including unidentified trees) (14). For each of the 4953 species with a valid name in the 1485 plots, we constructed an inverse distance weighting (IDW) model for RAi, with a power of 2, a maximum number of plots used for each local estimation of 150, and a maximum distance parameter of 4°. We did not use a LOESS model (14) because this had the undesirable effect of predicting very small occurrences of species far from localities where the species was actually recorded. For a similar reason, we used a cutoff of 4° with IDW modeling because, otherwise, species would have very low densities over the entire Amazon. These adjustments have a significant effect on the ranges of species [that is, ranges here are smaller than in our previous study (14)] but a negligible effect on their total number of individuals. The number of individuals of species i in a given DGC was then simply the total number of trees in the DGC multiplied by the fraction of the species i. Although we used a slightly different approach and a slightly larger data set compared to those in our previous study (14), our results are very similar to the results of that study.
Modeled population sizes and species distributions: Rare species
To estimate the total number of tree species present in Amazonia, we extrapolated the rank-abundance distribution of the 4953 named species as in our previous study (14). This yielded an additional 10,247 species, for a total of 15,200 estimated tree species in Amazonia. For shorthand, in this paper, we refer to the 4953 named species as “common species” and to the 10,247 other taxa as “rare species.”
Because our tree plot data cannot tell us how these very rare species are distributed, we carried out a separate modeling exercise to estimate the degree to which their ranges overlap with deforestation or protected areas. In doing this, we relied on two simplifying assumptions: (i) these rare species have small circular geographic ranges whose sizes are correlated to their population sizes (13) and (ii) these species are not randomly distributed across the Amazon but instead are more likely to occur in DGCs with higher overall tree diversity. This stratification is consistent with the theoretical notion that there is a one-to-one relationship between Fisher’s α at large sample sizes and rare species (in large samples, the number of singletons actually equals Fisher’s α, the number of doubletons equals ~α/2, and the number of tripletons equals ~α/3…) (35). To estimate how many rare species occur in each DGC, we made an updated map of tree diversity (Fisher’s α) in Amazonia (36) at 0.1° resolution and used this map to stratify the position of rare species. For each rare species, a DGC was chosen randomly, with a probability proportional to the DGC’s Fisher’s α. Range size was calculated for all 10,247 species as in the study of Hubbell et al. (13). Each circular range was overlain on deforestation and protected area maps (pixels at 0.1° resolution). The fraction of the population intersecting these maps was then calculated as the number of pixels of deforestation (or protected area) divided by the total number of pixels of forest within that circular section. This was repeated 500 times to provide the mean expectation and confidence limits.
Protected areas and deforestation
Spatial data and categories of Amazonian protected areas were gathered from the World Database of Protected Areas (37) and updated with individual country park service sources (for example, http://geo.sernanp.gob.pe/geoserver) and—for indigenous territories of Guyana, Peru, and Bolivia—with data from Red Amazónica de Información Socioambiental Georeferenciada (http://raisg.socioambiental.org/). We did not include indigenous territories from Suriname, Venezuela, and Ecuador because these areas are not yet officially designated. Protected areas were classified as SCRs (IUCN categories Ia to IV) or SUIRs (IUCN categories V to VII and all other types) (table S3). Where the data indicated an overlap between SCRs and SUIRs, the overlap was designated as SCR.
Historical deforestation up to 2013 was based on data from Soares-Filho et al. (1, 2) and Hansen et al. (15). To estimate projected deforestation in 2050 (including historical deforestation), we used both BAU scenario and IGS based on the work of Soares-Filho et al. (1, 2). Every 01DGC of the Amazonian base map was classified as protected or unprotected and as forested or deforested, depending on whether >50% of the 01DGC was occupied by a protected area or deforestation.
For common species, we estimated the number of individuals of a given species that fell within areas of deforestation or protection by first multiplying the population size in each DGC by the proportion of its 01DGCs that were classified as deforested or protected. This analysis assumes that the individuals of a species are homogeneously distributed within each DGC. We then summed the results for all DGCs to yield the total number of individuals of each species that were lost to deforestation or occurred within a protected area.
For rare species, the proportion of the number of individuals of a given rare species lost in a given DGC was quantified as the proportion of that DGC classified as deforested. Rare species in heavily deforested DGCs thus show a much higher loss than those in less disturbed DGCs, and those in intact DGCs had zero losses. The degree to which rare species’ distributions overlap with protected areas was estimated in the same fashion. All analyses were carried out with R software (38).
Acknowledgments
This report is the result of the work of hundreds of different scientists and research institutions in the Amazon over the last 80 years. Without their hard work, this analysis would have been impossible. Funding: This work was supported by Alberta Mennega Stichting; ALCOA Suriname; Amazon Conservation Association; Banco de la República; CELOS Suriname; CAPES (PNPG); Conselho Nacional de Desenvovimento Científico e Tecnológico of Brazil (CNPq) Projects CENBAM, PELD (558069/2009-6), PRONEX-FAPEAM (1600/2006), Áreas Úmidas, MAUA; PELD (403792/2012-6), PPBio, PVE 004/2012, Universal (479599/2008-4), and Universal 307807-2009-6; FAPEAM projects DCR/2006, Hidroveg with FAPESP, and PRONEX with CNPq; FAPESP; Colciencias; CONICIT; Duke University; Ecopetrol; FEPIM 044/2003; The Field Museum; Conservation International/DC (TEAM/Instituto Nacional de Pesquisas da Amazônia Manaus), Gordon and Betty Moore Foundation; Guyana Forestry Commission; Investissement d’Avenir grant of the French ANR (CEBA: ANR-10-LABX-0025); IVIC; Margaret Mee Amazon Trust; Miquel fonds; MCTI–Museu Paraense Emílio Goeldi–Proc. 407232/2013-3–PVE-MEC/MCTI/CAPES/CNPq; National Geographic Society (7754-04 and 8047-06 to P.M.J.; 6679-99, 7435-03, and 8481-08 to T.W.H.); NSF-0726797 to K.R.Y.; NSF Dissertation Improvement; Netherlands Foundation for the Advancement of Tropical Research WOTRO (grants WB85-335 and W84-581); Primate Conservation Inc.; Programme Ecosystèmes Tropicaux (French Ministry of Ecology and Sustainable Development); Shell Prospecting and Development Peru; Smithsonian Institution’s Biological Diversity of the Guiana Shield Program; Stichting het van Eeden-fonds; The Body Shop; The Ministry of the Environment of Ecuador; TROBIT; Tropenbos International; U.S. National Science Foundation (NSF-0743457 and NSF-0101775 to P.M.J.; NSF-0918591 to T.W.H.); USAID; Variety Woods Guyana; Wenner-Gren Foundation; WWF-Brazil; WWF-Guianas; XIIéme Contrat de Plan Etat Région-Guyane (French Government and European Union) and grants to RAINFOR from the European Union, UK Natural Environment Research Council, and the Gordon and Betty Moore Foundation. We thank D. Zappi for providing the Cristalino State Park checklist. O.L.P. was supported by a European Research Council Advanced Grant and a Royal Society Wolfson Research Merit Award. Author contributions: H.t.S. and N.C.A.P. conceived the study and designed the analyses. H.t.S. carried out most analyses. H.t.S., N.C.A.P., T.J.K., W.F.L., C.A.P., and J.E.G. wrote the manuscript. All of the other authors contributed data, discussed further analyses, and commented on various versions of the manuscript. This is contribution 679 of the technical series of the BDFFP (INPA/STRI). Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials in appendix S1 and S5. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/10/e1500936/DC1
Use of the IUCN threat criteria
Caveats regarding deforestation scenarios
Caveats regarding population models
Caveats regarding the interaction between tree species populations and forest loss
Fig. S1. Map of Amazonia showing the location of the 1485 ATDN plots that contributed data to this report.
Fig. S2. Map of lowland forests in the Amazon.
Fig. S3. Total deforestation of the Amazon by 2013.
Fig. S4. Deforestation and tree population declines in the Amazon.
Fig. S5. Deforestation and tree population declines of rare species in the Amazon.
Table S1. Deforestation and tree population declines of rare species in the Amazon.
Fig. S6. Projected (including historical) deforestation in the Amazon by 2050 in the BAU scenario.
Fig. S7. Projected (including historical) deforestation in the Amazon by 2050 in the IGS.
Fig. S8. Protected areas and indigenous territories in the Amazon.
Fig. S9. How much of the Amazon is protected and how many individual trees do protected areas protect?
Fig. S10. Rare species in protected areas and indigenous territories.
Table S2. Rare species in protected areas and indigenous territories.
Fig. S11. Protected areas and indigenous territories in the Amazon with deforestation according to BAU scenario 2050.
Fig. S12. Protected areas and indigenous territories in the Amazon with deforestation according to IGS 2050.
Fig. S13. How much forest loss has taken place and will take place in Amazonian protected areas?
Fig. S14. Decline in relative population size shows no relationship with original population size in (A) BAU scenario and (B) IGS.
Fig. S15. Interpolated stem density for the Amazon.
Fig. S16. Interpolated identification level of plots in the Amazon.
Fig. S17. Projected and observed deforestation in Amazonia from 2002 to 2013.
Table S3. IUCN categories, designations, and conversion into SCRs (1) and SUIRs (2).
Appendix S1. Data by DGC.
Appendix S2. Data by species.
Appendix S3. Data of individuals by region.
Appendix S4. Tree species estimated to occur in Cristalino State Park in Brazil but not yet recorded there (32) and their estimated threat status according to historical and projected deforestation.
Appendix S5. Plot metadata.
REFERENCES AND NOTES
- 1.B. S. Soares-Filho, D. C. Nepstad, L. M. Curran, E. Voll, G. C. Cerqueira, R. A. Garcia, C. A. Ramos, A. McDonald, P. Lefebvre, P. Schlesinger, Eds., LBA-ECO LC-14 Modeled Deforestation Scenarios, Amazon Basin: 2002-2050 (Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, TN, 2013); http://daac.ornl.gov//LBA/guides/LC14_Amazon_Scenarios.html
- 2.Soares-Filho B. S., Nepstad D. C., Curran L. M., Cerqueira G. C., Garcia R. A., Ramos C. A., Voll E., McDonald A., Lefebvre P., Schlesinger P., Modelling conservation in the Amazon basin. Nature 440, 520–523 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Fearnside P. M., Deforestation in Brazilian Amazonia: History, rates, and consequences. Conserv. Biol. 19, 680–688 (2005). [Google Scholar]
- 4.Smith N. J. H., Alvim P., Homma A., Falesi I., Serrão A., Environmental impacts of resource exploitation in Amazonia. Glob. Environ. Change 1, 313–320 (1991). [Google Scholar]
- 5.Davidson E. A., de Araújo A. C., Artaxo P., Balch J. K., Brown I. F., Bustamante M. M. C., Coe M. T., DeFries R. S., Keller M., Longo M., Munger J. W., Schroeder W., Soares-Filho B. S., Souza C. M., Wofsy S. C., The Amazon basin in transition. Nature 481, 321–328 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Aragão L. E. O. C., Malhi Y., Roman-Cuesta R. M., Saatchi S., Anderson L. O., Shimabukuro Y. E., Spatial patterns and fire response of recent Amazonian droughts. Geophys. Res. Lett. 34, L07701 (2007). [Google Scholar]
- 7.Malhi Y., Roberts J. T., Betts R. A., Killeen T. J., Li W., Nobre C. A., Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Nepstad D., McGrath D., Stickler C., Alencar A., Azevedo A., Swette B., Bezerra T., DiGiano M., Shimada J., Seroa da Motta R., Armijo E., Castello L., Brando P., Hansen M. C., McGrath-Horn M., Carvalho O., Hess L., Slowing Amazon deforestation through public policy and interventions in beef and soy supply chains. Science 344, 1118–1123 (2014). [DOI] [PubMed] [Google Scholar]
- 9.D’Almeida C., Vörösmarty C. J., Hurtt G. C., Marengo J. A., Dingman S. L., Keim B. D., The effects of deforestation on the hydrological cycle in Amazonia: A review on scale and resolution. Int. J. Climatol. 27, 633–647 (2007). [Google Scholar]
- 10.Aragão L. E. O. C., Environmental science: The rainforest’s water pump. Nature 489, 217–218 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Spracklen D. V., Arnold S. R., Taylor C. M., Observations of increased tropical rainfall preceded by air passage over forests. Nature 489, 282–285 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Feeley K. J., Silman M. R., Extinction risks of Amazonian plant species. Proc. Natl. Acad. Sci. U.S.A. 106, 12382–12387 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubbell S. P., He F., Condit R., Borda-de-Água L., Kellner J., ter Steege H., How many tree species are there in the Amazon and how many of them will go extinct? Proc. Natl. Acad. Sci. U.S.A. 105, 11498–11504 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ter Steege H., Pitman N. C. A., Sabatier D., Baraloto C., Salomão R. P., Guevara J. E., Phillips O. L., Castilho C. V., Magnusson W. E., Molino J.-F., Monteagudo A., Vargas P. N., Montero J. C., Feldpausch T. R., Honorio Coronado E. N., Killeen T. J., Mostacedo B., Vasquez R., Assis R. L., Terborgh J., Wittmann F., Andrade A., Laurance W. F., Laurance S. G. W., Marimon B. S., Marimon B.-H. Jr, Vieira I. C. G., Amaral I. L., Brienen R., Castellanos H., López D. C., Duivenvoorden J. F., Mogollón H. F., de Almeida Matos F. D., Dávila N., García-Villacorta R., Diaz P. R. S., Costa F., Emilio T., Levis C., Schietti J., Souza P., Alonso A., Dallmeier F., Montoya A. J. D., Piedade M. T. F., Araujo-Murakami A., Arroyo L., Gribel R., Fine P. V. A., Peres C. A., Toledo M., Aymard C. G. A., Baker T. R., Cerón C., Engel J., Henkel T. W., Maas P., Petronelli P., Stropp J., Zartman C. E., Daly D., Neill D., Silveira M., Paredes M. R., Chave J., de Andrade Lima Filho D., Jørgensen P. M., Fuentes A., Schöngart J., Valverde F. C., Di Fiore A., Jimenez E. M., Mora M. C. P., Phillips J. F., Rivas G., van Andel T. R., von Hildebrand P., Hoffman B., Zent E. L., Malhi Y., Prieto A., Rudas A., Ruschell A. R., Silva N., Vos V., Zent S., Oliveira A. A., Schutz A. C., Gonzales T., Nascimento M. T., Ramirez-Angulo H., Sierra R., Tirado M., Medina M. N. U., van der Heijden G., Vela C. I. A., Torre E. V., Vriesendorp C., Wang O., Young K. R., Baider C., Balslev H., Ferreira C., Mesones I., Torres-Lezama A., Giraldo L. E. U., Zagt R., Alexiades M. N., Hernandez L., Huamantupa-Chuquimaco I., Milliken W., Cuenca W. P., Pauletto D., Sandoval E. V., Gamarra L. V., Dexter K. G., Feeley K., Lopez-Gonzalez G., Silman M. R., Hyperdominance in the Amazonian tree flora. Science 342, 1243092 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Hansen M., Potapov P. V., Moore R., Hancher M., Turubanova S. A., Tyukavina A., Thau D., Stehman S. V., Goetz S. J., Loveland T. R., Kommareddy A., Egorov A., Chini L., Justice C. O., Townshend J. R. G., High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 16.IUCN, IUCN Red List (2014); http://cmsdocs.s3.amazonaws.com/summarystats/2014_3_Summary_Stats_Page_Documents/2014_3_RL_Stats_Table_3b.pdf
- 17.Miller J. S., Krupnick G. A., Stevens H., Porter-Morgan H., Boom B., Acevedo-Rodríguez P., Ackerman J., Kolterman D., Santiago E., Torres C., Velez J., Toward target 2 of the Global Strategy for Plant Conservation: An expert analysis of the Puerto Rican flora to validate new streamlined methods for assessing conservation status. Ann. Mo. Bot. Gard. 99, 199–205 (2013). [Google Scholar]
- 18.León B., Roque J., Ulloa Ulloa C., Pitman N., Jørgensen P. M., Cano A., Libro rojo de las plantas endémicas del Perú. Rev. Peruana Biol. 13, 1–976 (2006). [Google Scholar]
- 19.G. Martinelli, M. Avila Moraes, in Livro vermelho da flora do Brasil (Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro, Brazil, 2013). [Google Scholar]
- 20.Pitman N. C. A., Jørgensen P. M., Estimating the size of the world’s threatened flora. Science 298, 989 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Pitman N. C. A., Jørgensen P. M., Williams R. S. R., León-Yánez S., Valencia R., Extinction-rate estimates for a modern neotropical flora. Conserv. Biol. 16, 1427–1431 (2002). [Google Scholar]
- 22.Ribeiro M. C., Metzger J. P., Martensen A. C., Ponzoni F. J., Hirota M. M., The Brazilian Atlantic forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153 (2009). [Google Scholar]
- 23.Beuchle R., Grecchia R. C., Shimabukuro Y. E., Seligerc R., Eva H. D., Sano E., Achard F., Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Appl. Geogr. 58, 116–127 (2015). [Google Scholar]
- 24.Portillo-Quintero C. A., Sánchez-Azofeifa G. A., Extent and conservation of tropical dry forests in the Americas. Biol. Conserv. 143, 144–155 (2010). [Google Scholar]
- 25.Sodhi N. S., Posa M. R. C., Lee T. M., Bickford D., Koh L. P., Brook B. W., The state and conservation of Southeast Asian biodiversity. Biodivers. Conserv. 19, 317–328 (2010). [Google Scholar]
- 26.Fine P. V. A., An evaluation of the geographic area hypothesis using the latitudinal gradient in North American tree diversity. Evol. Ecol. Res. 3, 413–428 (2001). [Google Scholar]
- 27.Bird J. P., Buchanan G. M., Lees A. C., Clay R. P., Develey P. F., Yépez I., Butchart S. H. M., Integrating spatially explicit habitat projections into extinction risk assessments: A reassessment of Amazonian avifauna incorporating projected deforestation. Divers. Distrib. 18, 273–281 (2012). [Google Scholar]
- 28.Fearnside P., Laurance W. F., Cochrane M. A., Bergen S., Sampaio P., Barber C., D’Angelo S., Fernandes T., The future of Amazonia: Models to predict the consequences of future infrastructure in Brazil’s multi-annual plans. Novos Cad. 15, 25–52 (2012). [Google Scholar]
- 29.Benchimol M., Peres C. A., Edge-mediated compositional and functional decay of tree assemblages in Amazonian forest islands after 26 years of isolation. J. Ecol. 103, 408–420 (2015). [Google Scholar]
- 30.Bernard E., Penna L. A. O., Araújo E., Downgrading, downsizing, degazettement, and reclassification of protected areas in Brazil. Conserv. Biol. 28, 939–950 (2014). [DOI] [PubMed] [Google Scholar]
- 31.International Finance Corporation, Performance Standard 6. Biodiversity Conservation and Sustainable Management of Natural Resources (International Finance Corporation, Washington, DC, 2012). [Google Scholar]
- 32.Zappi D. C., Sasaki D., Milliken W., Iva J., Henicka G. S., Biggs N., Frisby S., Plantas vasculares da região do Parque Estadual Cristalino, norte de Mato Grosso, Brasil. Acta Amazonica 41, 29–38 (2011). [Google Scholar]
- 33.Environmental Systems Research Institute, ESRI Data & Maps 1999. An ESRI White Paper (Environmental Systems Research Institute, Redlands, CA, 1999). [Google Scholar]
- 34.Jet Propulsion Laboratory, (NASA, 2009), vol. 2008; www2.jpl.nasa.gov/srtm/
- 35.Fisher R. A., Corbet A. S., Williams C. B., The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 12, 42–58 (1943). [Google Scholar]
- 36.ter Steege H., Pitman N., Sabatier D., Castellanos H., Van Der Hout P., Daly D. C., Silveira M., Phillips O., Vasquez R., Van Andel T., Duivenvoorden J., De Oliveira A. A., Ek R., Lilwah R., Thomas R., Van Essen J., Baider C., Maas P., Mori S., Terborgh J., Vargas P. N., Mogollón H., Morawetz W., A spatial model of tree α-diversity and tree density for the Amazon. Biodivers. Conserv. 12, 2255–2277 (2003). [Google Scholar]
- 37.protectedplanet.net, World Database of Protected Areas (UNEP, WCMC, IUCN, WCPA, 2010), vol. 2014; www.protectedplanet.net
- 38.R Development Core Team (R Foundation for Statistical Computing, Vienna, Austria, 2011).
- 39.IUCN, IUCN Red List Categories and Criteria. Version 3.1 (IUCN, Gland, Switzerland, ed. 2, 2012).
- 40.Baker T. R., Pennington R. T., Magallon S., Gloor E., Laurence W. F., Alexiades M., Alvarez E., Araujo A., Arets E. J. M. M., Aymard G., de Oliveira A. A., Amaral I., Arroyo L., Bonal D., Brienen R. J. W., Chave J., Dexter K. G., Di Fiore A., Eler E., Feldpausch T. R., Ferreira L., Lopez-Gonzales G., van der Heijden G., Higuchi N., Honorio E., Huamantupa I., Killeen T. J., Laurance S., Leaño C., Lewis S. L., Malhi Y., Marimon B. S., Hur Marimon B. Jr, Mendoza A. M., Neill D., Peñuela-Mora M. C., Pitman N., Preito A., Quesada C. A., Ramírez F., Ramírez Angulo H., Rudas A., Ruschel A. R., Salomão R. P., de Andrade A. S., Silva J. N. M., Silveira M., Simon M. F., Spironello W., ter Steege H., Terborgh J., Toledo M., Torres-Lezama A., Vasquez R., Guimaraes Vieira I. C., Vilanova E., Vos V. A., Phillips O. L., Fast demographic traits promote high diversification rates of Amazonian trees. Ecol. Lett. 17, 527–536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slik J. W. F., Arroyo-Rodríguez V., Aiba S.-I., Alvarez-Loayza P., Alves L. F., Ashton P., Balvanera P., Bastian M. L., Bellingham P. J., van den Berg E., Bernacci L., da Conceição Bispo P., Blanc L., Böhning-Gaese K., Boeckx P., Bongers F., Boyle B., Bradford M., Brearley F. Q., Breuer-Ndoundou Hockemba M., Bunyavejchewin S., Matos D. C. L., Castillo-Santiago M., Catharino E. L. M., Chai S.-L., Chen Y., Colwell R. K., Chazdon R. L., Clark C., Clark D. B., Clark D. A., Culmsee H., Damas K., Dattaraja H. S., Dauby G., Davidar P., DeWalt S. J., Doucet J.-L., Duque A., Durigan G., Eichhorn K. A. O., Eisenlohr P. V., Eler E., Ewango C., Farwig N., Feeley K. J., Ferreira L., Field R., de Oliveira Filho A. T., Fletche C., Forshed O., Franco G., Fredriksson G., Gillespie T., Gillet J.-F., Amarnath G., Griffith D. M., Grogan J., Gunatilleke N., Harris D., Harrison R., Hector A., Homeier J., Imai N., Itoh A., Janse P. A., Joly C. A., de Jong B. H. J., Kartawinata K., Kearsley E., Kelly D. L., Kenfack D., Kessler M., Kitayama K., Kooyman R., Larney E., Laumonier Y., Laurance S., Laurance W. F., Lawes M. J., do Amaral I. L., Letche S. G., Lindsell J., Lu X., Mansor A., Marjokorpi A., Martin E. H., Meilb H., Melo F. P. L., Metcalfe D. J., Medjibe V. P., Metzger J. P., Millet J., Mohandass D., Montero J. C., de Morisson Valeriano M., Mugerwa B., Nagamasu H., Nilus R., Ochoa-Gaonau S., Onrizal O., Page N., Parolin P., Parre M., Parthasarathy N., Paudel E., Permana A., Piedade M. T. F., Pitman N. C. A., Poorter L., Poulsen A. D., Poulsen J., Powers J., Prasad R. C., Puyravaud J.-P., Razafimahaimodiso J.-C., Reitsma J., dos Santos J. R., Spironello W. R., Romero-Saltos H., Rovero F., Rozak A. H., Ruokolainen K., Rutishauser E., Saiter F., Saner P., Santos B. A., Santos F., Sarker S. K., Satdichanh M., Schmitt C. B., Schöngart J., Schulze M., Suganuma M. S., Sheil D., da Silva Pinheiro E., Sist P., Stevart T., Sukumar R., Sun I.-F., Sunderland T., Suresh H. S., Suzukic E., Tabarelli M., Tang J., Targhetta N., Theilade I., Thomas D. W., Tchouto P., Hurtado J., Valencia R., van Valkenburg J. L. C. H., Van Do T., Vasquez R., Verbeeck H., Adekunle V., Vieira S. A., Webb C. O., Whitfeld T., Wich S. A., Williams J., Wittmann F., Wöll H., Yang X., Yao C. Y. A., Yap S. L., Yonedac T., Zahawi R. A., Zakaria R., Zang R., de Assis R. L., Luize B. G., Venticinque E. M., An estimate of the number of tropical tree species. Proc. Natl. Acad. Sci. U.S.A. 112, 7472–7477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regalado A., Brazil says rate of deforestation in Amazon continues to plunge. Science 329, 1270–1271 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Nepstad D., Soares-Filho B. S., Merry F., Lima A., Moutinho P., Carter J., Bowman M., Cattaneo A., Rodrigues H., Schwartzman S., McGrath D. G., Stickler C. M., Lubowski R., Piris-Cabezas P., Rivero S., Alencar A., Almeida O., Stella O., The end of deforestation in the Brazilian Amazon. Science 326, 1350–1351 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Finer M., Orta-Martínez M., A second hydrocarbon boom threatens the Peruvian Amazon: Trends, projections, and policy implications. Environ. Res. Lett. 5, 014012 (2010). [Google Scholar]
- 45.Gutiérrez-Vélez V. H., DeFries R., Pinedo-Vásquez M., Uriarte M., Padoch C., Baethgen W., Fernandes K., Lim Y., High-yield oil palm expansion spares land at the expense of forests in the Peruvian Amazon. Environ. Res. Lett. 6, 044029 (2011). [Google Scholar]
- 46.Petherick A., Pipe dream. Nat. Climate Change 3, 859–860 (2013). [Google Scholar]
- 47.Dávalos L. M., Bejarano A. C., Hall M. A., Correa H. L., Corthals A., Espejo O. J., Forests and drugs: Coca-driven deforestation in tropical biodiversity hotspots. Environ. Sci. Technol. 45, 1219–1227 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Müller R., Müller D., Schierhorn F., Gerold G., Pacheco P., Proximate causes of deforestation in the Bolivian lowlands: An analysis of spatial dynamics. Reg. Environ. Change 12, 445–459 (2012). [Google Scholar]
- 49.Asner G. P., Llactayo W., Tupayachi R., Luna E. R., Elevated rates of gold mining in the Amazon revealed through high-resolution monitoring. Proc. Natl. Acad. Sci. U.S.A. 110, 18454–18459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finer M., Jenkins C. N., Pimm S. L., Keane B., Ross C., Oil and gas projects in the Western Amazon: Threats to wilderness, biodiversity, and indigenous peoples. PLOS One 3, e2932 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.G. P. Asner, M. Keller, M. Lentini, F. Merry, C. Souza Jr., in Amazonia and Global Change, M. Keller, M. Bustamante, J. Gash, P. S. Dias, Eds. (John Wiley and Sons, Washington, DC, 2009), pp. 25–42. [Google Scholar]
- 52.Castello L., McGrath D. G., Hess L. L., Coe M. T., Lefebvre P. A., Petry P., Macedo M. N., Renó V. F., Arantes C. C., The vulnerability of Amazon freshwater ecosystems. Conserv. Lett. 6, 217–229 (2013). [Google Scholar]
- 53.Finer M., Jenkins C. N., Proliferation of hydroelectric dams in the Andean Amazon and implications for Andes-Amazon connectivity. PLOS One 7, e35126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aragão L. E. O. C., Shimabukuro Y. E., The incidence of fire in Amazonian forests with implications for REDD. Science 328, 1275–1278 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Lewis S. L., Brando P. M., Phillips O. L., van der Heijden G. M. F., Nepstad D., The 2010 Amazon drought. Science 331, 554 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Phillips O. L., Aragão L. E. O. C., Lewis S. L., Fisher J. B., Lloyd J., López-González G., Malhi Y., Monteagudo A., Peacock J., Quesada C. A., van der Heijden G., Almeida S., Amaral I., Arroyo L., Aymard G., Baker T. R., Bánki O., Blanc L., Bonal D., Brando P., Chave J., de Oliveira Á. C. A., Cardozo N. D., Czimczik C. I., Feldpausch T. R., Freitas M. A., Gloor E., Higuchi N., Jiménez E., Lloyd G., Meir P., Mendoza C., Morel A., Neill D. A., Nepstad D., Patiño S., Peñuela M. C., Prieto A., Ramírez F., Schwarz M., Silva J., Silveira M., Thomas A. S., ter Steege H., Stropp J., Vásquez R., Zelazowski P., Dávila E. A., Andelman S., Andrade A., Chao K.-J., Erwin T., Di Fiore A., Honorio C. E., Keeling H., Killeen T. J., Laurance W. F., Cruz A. P., Pitman N. C. A., Vargas P. N., Ramírez-Angulo H., Rudas A., Salamão R., Silva N., Terborgh J., Torres-Lezama A., Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Brando P. M., Balch J. K., Nepstad D. C., Morton D. C., Putz F. E., Coe M. T., Silvério D., Macedo M. N., Davidson E. A., Nóbrega C. C., Alencar A., Soares-Filho B. S., Abrupt increases in Amazonian tree mortality due to drought–fire interactions. Proc. Natl. Acad. Sci. U.S.A. 111, 6347–6352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brodie J., Post E., Laurance W. F., Climate change and tropical biodiversity: A new focus. Trends Ecol. Evol. 27, 145–150 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Lima A., Silva T. S. F., Oliveira e Cruz de Aragão L. E., de Feitas R. M., Adami M., Formaggio A. R., Shimabukuro Y. E., Land use and land cover changes determine the spatial relationship between fire and deforestation in the Brazilian Amazon. Appl. Geogr. 34, 239–246 (2012). [Google Scholar]
- 60.F. Tierra, Ampliación responsable de la frontera agrícola. Propuestas para Politicas Públicas, No. 1 (Tierra, La Paz, Bolivia, 2014); http://ftierra.org/index.php?option=com_mtree&task=att_download&link_id=53&cf_id=43
- 61.Cambio (Cambio, Periodico de estado plurinacional de Bolivia, 2014); www.cambio.bo/?q=bolivia-proyecta-ampliar-su-frontera-agr%C3%ADcola-10-millones-de-hect%C3%A1reas
- 62.J. L. Dammert B. (Iniciativa para la Conservación en la Amazonía Andina (ICAA), United States Agency for International Development (USAID)/ International Resources Group (IRG), Sociedad Peruana de Derecho Ambiental (SPDA), Corporación de Gestión y Derecho Ambiental (ECOLEX), Social Impact (SI), Patrimonio Natural (PN) y Conservation Strategy Fund (CSF), 2013), vol. 2015; www.amazonia-andina.org/amazonia-activa/biblioteca/publicaciones/cambio-uso-suelos-agricultura-gran-escala-amazonia-andina.
- 63.IdHeMAd Amazônia (Instituto do Homem e Meio Ambiente da Amazônia, Brazil, 2013), vol. 2014; http://imazon.org.br/
- 64.Soares-Filho B., Rajão R., Macedo M., Carneiro A., Costa W., Coe M., Rodrigues H., Alencar A., Cracking Brazil’s forest code. Science 344, 363–364 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Soares-Filho B., Moutinho P., Nepstad D., Anderson A., Rodrigues H., Garcia R., Dietzsch L., Merry F., Bowman M., Hissa L., Silvestrini R., Maretti C., Role of Brazilian Amazon protected areas in climate change mitigation. Proc. Natl. Acad. Sci. U.S.A. 107, 10821–10826 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adeney J. M., Christensen N. L. Jr, Pimm S. L., Reserves protect against deforestation fires in the Amazon. PLOS One 4, e5014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson A., Chomitz K. M., Effectiveness of strict vs. multiple use protected areas in reducing tropical forest fires: A global analysis using matching methods. PLOS One 6, e22722 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peres C. A., Terborgh J. W., Amazonian nature reserves: An analysis of the defensibility status of existing conservation units and design criteria for the future. Conserv. Biol. 9, 34–46 (1995). [Google Scholar]
- 69.Ferreira J., Aragão L. E. O. C., Barlow J., Barreto P., Berenguer E., Bustamante M., Gardner T. A., Lees A. C., Lima A., Louzada J., Pardini R., Parry L., Peres C. A., Pompeu P. S., Tabarelli M., Zuanon J., Brazil’s environmental leadership at risk. Science 346, 706–707 (2014). [DOI] [PubMed] [Google Scholar]
- 70.J. Hardner, paper presented at the Conference on Economics and Conservation in the Tropics: A Strategic Dialogue, vol. 31, San Francisco, CA, 2008. [Google Scholar]
- 71.D. P. Kanak, I. Henderson, Closing the REDD+ Gap: The Global Forest Finance Facility (Global Canopy Programme, Oxford, UK, 2012). [Google Scholar]
- 72.Peres C. A., Conservation in sustainable-use tropical forest reserves. Conserv. Biol. 25, 1124–1129 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Morton D. C., Le Page Y., DeFries R., Collatz G. J., Hurtt G. C., Understorey fire frequency and the fate of burned forests in southern Amazonia. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moser P., Oliveira W. L., Medeiros M. B., Pinto J. R., Eisenlohr P. V., Lima I. L., Silva G. P., Simon M. F., Tree species distribution along environmental gradients in an area affected by a hydroelectric dam in Southern Amazonia. Biotropica 46, 367–376 (2014). [Google Scholar]
- 75.ANEEL (SIGEL, Agência Nacional de Elergia Elétrica, 2013), vol. 2014; www.aneel.gov.br/
- 76.Malhi Y., Aragão L. E. O. C., Galbraith D., Huntingford C., Fisher R., Zelazowski P., Sitch S., McSweeney C., Meir P., Exploring the likelihood and mechanism of a climate-change–induced dieback of the Amazon rainforest. Proc. Natl. Acad. Sci. U.S.A. 106, 20610–20615 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pos E., Andino J. E. G., Sabatier D., Molino J.-F., Pitman N., Mogollón H., Neill D., Cerón C., Rivas G., Di Fiore A., Thomas R., Tirado M., Young K. R., Wang O., Sierra R., García-Villacorta R., Zagt R., Palacios W., Aulestia M., ter Steege H., Are all species necessary to reveal ecologically important patterns? Ecol. Evol. 4, 4626–4636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.H. ter Steege, Amazon Tree Diversity Network (Universiteit Utrecht, Utrecht, Netherlands, 2011), vol. 2014; http://web.science.uu.nl/Amazon/ATDN/
- 79.Asner G. P., Knapp D. E., Broadbent E. N., Oliveira P. J. C., Keller M., Silva J. N., Selective logging in the Brazilian Amazon. Science 310, 480–482 (2005). [DOI] [PubMed] [Google Scholar]
- 80.ter Steege H., Welch I., Zagt R. J., Long-term effect of timber harvesting in the Bartica Triangle, Central Guyana. For. Ecol. Manage. 170, 127–144 (2002). [Google Scholar]
- 81.Grogan J., Blundell A. G., Landis R. M., Youatt A., Gullison R. E., Martinez M., Kómetter R., Lentini M., Rice R. E., Over-harvesting driven by consumer demand leads to population decline: Big-leaf mahogany in South America. Conserv. Lett. 3, 12–20 (2010). [Google Scholar]
- 82.J. Mark, A. C. Newton, S. Oldfield, M. Rivers, The International Timber Trade: A Working List of Commercial Timber Tree Species (Botanic Gardens Conservation International, Richmond, UK, 2014). [Google Scholar]
- 83.Laurance W. F., Nascimento H. E. M., Laurance S. G., Andrade A. C., Fearnside P. M., Ribeiro J. E. L., Capretz R. L., Rain forest fragmentation and the proliferation of successional trees. Ecology 87 469–482 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/10/e1500936/DC1
Use of the IUCN threat criteria
Caveats regarding deforestation scenarios
Caveats regarding population models
Caveats regarding the interaction between tree species populations and forest loss
Fig. S1. Map of Amazonia showing the location of the 1485 ATDN plots that contributed data to this report.
Fig. S2. Map of lowland forests in the Amazon.
Fig. S3. Total deforestation of the Amazon by 2013.
Fig. S4. Deforestation and tree population declines in the Amazon.
Fig. S5. Deforestation and tree population declines of rare species in the Amazon.
Table S1. Deforestation and tree population declines of rare species in the Amazon.
Fig. S6. Projected (including historical) deforestation in the Amazon by 2050 in the BAU scenario.
Fig. S7. Projected (including historical) deforestation in the Amazon by 2050 in the IGS.
Fig. S8. Protected areas and indigenous territories in the Amazon.
Fig. S9. How much of the Amazon is protected and how many individual trees do protected areas protect?
Fig. S10. Rare species in protected areas and indigenous territories.
Table S2. Rare species in protected areas and indigenous territories.
Fig. S11. Protected areas and indigenous territories in the Amazon with deforestation according to BAU scenario 2050.
Fig. S12. Protected areas and indigenous territories in the Amazon with deforestation according to IGS 2050.
Fig. S13. How much forest loss has taken place and will take place in Amazonian protected areas?
Fig. S14. Decline in relative population size shows no relationship with original population size in (A) BAU scenario and (B) IGS.
Fig. S15. Interpolated stem density for the Amazon.
Fig. S16. Interpolated identification level of plots in the Amazon.
Fig. S17. Projected and observed deforestation in Amazonia from 2002 to 2013.
Table S3. IUCN categories, designations, and conversion into SCRs (1) and SUIRs (2).
Appendix S1. Data by DGC.
Appendix S2. Data by species.
Appendix S3. Data of individuals by region.
Appendix S4. Tree species estimated to occur in Cristalino State Park in Brazil but not yet recorded there (32) and their estimated threat status according to historical and projected deforestation.
Appendix S5. Plot metadata.