Modern snakes originated from burrowing ancestors, predicted from the evolution of their inner ear.
Keywords: crown snake origin, HRCT, inner ear, bony labyrinth, virtual model building, geometric morphometrics, functional morphology, ancestral state reconstructions
Abstract
Modern snakes probably originated as habitat specialists, but it controversial unclear whether they were ancestrally terrestrial burrowers or marine swimmers. We used x-ray virtual models of the inner ear to predict the habit of Dinilysia patagonica, a stem snake closely related to the origin of modern snakes. Previous work has shown that modern snakes perceive substrate vibrations via their inner ear. Our data show that D. patagonica and modern burrowing squamates share a unique spherical vestibule in the inner ear, as compared with swimmers and habitat generalists. We built predictive models for snake habit based on their vestibular shape, which estimated D. patagonica and the hypothetical ancestor of crown snakes as burrowers with high probabilities. This study provides an extensive comparative data set to test fossoriality quantitatively in stem snakes, and it shows that burrowing was predominant in the lineages leading to modern crown snakes.
INTRODUCTION
More than 3000 species of extant snakes share a limbless or limb-reduced body plan and distinct sensory systems (1, 2). It is often held that the unique anatomy of snakes evolved through habitat specialization early in the group’s evolution, perhaps associated with their origin from other reptiles (3). Recent studies show conflicting results about whether snakes evolved from marine (4, 5) or terrestrial ancestors (6–9). For stem snakes—fossils that are more basal than all modern species—ecological traits are reconstructed conventionally from depositional environment or qualitative morphological characters (10, 11). Here, we provide quantitative analyses of the inner ear to reconstruct the habit of lineages closely related to the origin of crown snakes, including the Cretaceous fossil Dinilysia patagonica (12, 13) and the hypothetical ancestor of all modern snakes.
We hypothesize that D. patagonica was a burrower and that crown snakes originated from burrowing ancestors. For an ecomorphic indicator for snake habits, we used the shape of the inner ear instead of anatomical features that are not often preserved in fossils. For example, trunk/tail ratio is an ecomorphic indicator for habitat preferences in living species (14), but fossil snakes rarely preserve complete vertebral series.
The inner ear is the hearing and balance organ of all snakes (15). We used x-ray computed tomography (CT) to build three-dimensional models that are virtual endocasts of the bony inner ear labyrinth (Fig. 1, A to D). Our sample includes 34 species of modern and fossil snakes, and 10 species of lizards and amphisbaenians (table S1). The habit of D. patagonica was estimated by comparing its inner ear with modern snakes in three habit groups: aquatic, terrestrial generalists, and burrowing. We also tested the putative burrowing habit of the hypothetical ancestor of crown snakes, as reconstructed from a snake evolutionary tree.
Fig. 1. Modern snakes originated as burrowers, based on their inner ear morphology.
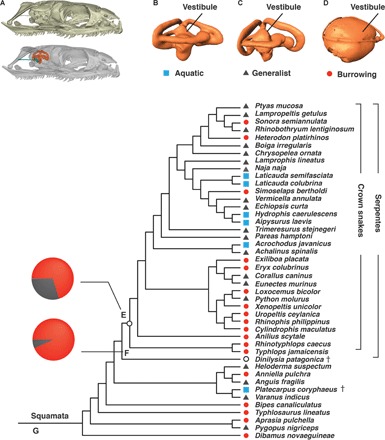
(A) Snake skulls in right lateral view, showing that the inner ear (orange) locates inside the braincase and opens to the stapes (blue) in the middle ear. Ear and skull models are not to scale. (B) Inner ear of Laticauda colubrina, an aquatic species. (C) Ptyas mucosa, terrestrial generalist. (D) Xenopeltis unicolor, a burrowing species. (E) Hypothetical ancestor of crown snakes, predicted as burrowing with 70.1% probability. (F) D. patagonica, predicted as burrowing with 93.4% probability. (G) Phylogeny of all snakes and lizards in this study, adapted from Gauthier et al. (8), Pyron et al. (19), and Yi and Norell (20).
RESULTS
D. patagonica shares with modern burrowing squamates a large spherical vestibule, large foramen ovale, and slender semicircular canals in the inner ear (Fig. 2, A to C). The vestibule occupies most of the space defined by the three semicircular canals and contacts the lateral semicircular canal in a horizontal plane. Our data show that a spherical vestibule is a morphological signature for burrowing, because it only appears in squamates that actively burrow underground (Fig. 1D and fig. S1, taxa 26 to 28, 32, 34, and 39 to 43). Some terrestrial generalists burrow when disturbed (16). Their vestibule may be expanded but does not have a spherical shape. Nor does it almost entirely occupy the space enclosed by the semicircular canals (fig. S1A, taxon 8, Lampropeltis getulus). No aquatic species have a spherical vestibule contacting the lateral semicircular canal (Fig. 1B and fig. S1A, taxa 1 to 6).
Fig. 2. The braincase and inner ear of D. patagonica (MACN-RN 1014).
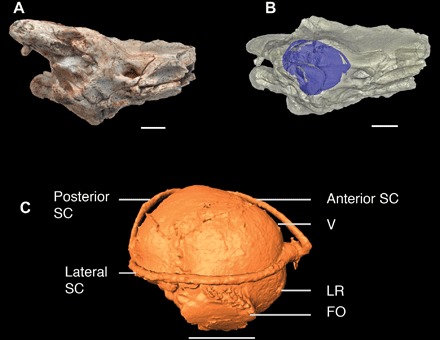
(A) Braincase of D. patagonica, showing the right otic region in lateral view. (B) X-ray CT model of MACN-RN 1014, with the inner ear highlighted in blue. (C) Bony inner ear of D. patagonica. FO, foramen ovale; LR, lagenar recess; SC, semicircular canal; V, vestibule. Scale bars, 5 mm.
We represent the vestibule and lateral semicircular canal with 28 landmarks and semilandmarks placed in horizontal cross sections (fig. S2). The landmark and semilandmark coordinates were standardized using generalized Procrustes analysis to extract shape information independent of specimen size, position, and orientation (17). Multivariate analysis of variance (MANOVA) of Procrustes coordinates found significant difference among the three habit groups (P = 0.01; table S2). On the other hand, phylogenetic signal is insignificant, according to a permutation test of Procrustes coordinates on the cladogram (Fig. 1G) (P = 0.09, iteration = 10). This indicates that habitat, rather than phylogeny, is the overarching driver of inner ear shape differences among living snakes.
Principal components analysis of the Procrustes coordinates demonstrates that aquatic and burrowing species differ in the degree of vestibular expansion (Fig. 3). Principal component 1 explains 47% of total variance. At its positive end, the shape exhibits a reduced vestibule that is well separated from the surrounding semicircular canals, particularly the lateral semicircular canal (Fig. 3A). At the negative end, the shape exhibits an expanded and spherical vestibule that almost entirely occupies the space enclosed by the semicircular canals (Fig. 3B). Burrowing species have an average negative score on principal component 1 (−0.097), whereas aquatic species have an average positive score (0.212). Linear correlation was insignificant between centroid size of Procrustes coordinates and species distributions in principal component 1 (P = 0.28 for a slope differing from zero; fig. S3). This indicates that vestibular shape, rather than size, is represented by the species distribution in the morphospace.
Fig. 3. Principal components analyses of the vestibular shape.
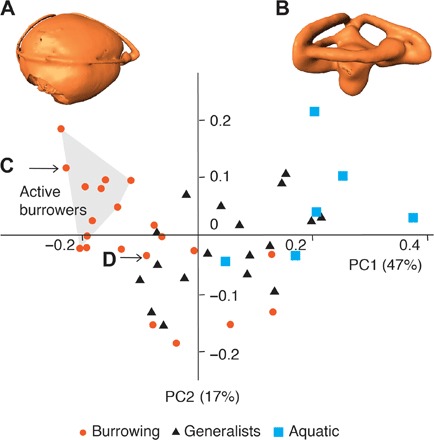
(A and B) The inner ear of X. unicolor (A) represents the shape on the negative side of principal component 1 (PC1), whereas the inner ear of L. colubrina (B) represents the positive side. (C) D. patagonica clusters with modern active burrowers. (D) The hypothetical ancestor of crown snakes clusters with modern burrowers and limbless generalists. We listed the species of each data point in fig. S4. Ear models are not to scale.
In the morphospace of the first two principal components, D. patagonica clusters with modern active burrowers, regardless of body size and phylogenetic affinity (Fig. 3). We define active burrowers as species that dig into the substrate (sometimes with modified snouts) instead of using rock cracks or other animals’ burrow (16). These species, in our analysis, range from 40 to 160 cm in adult size (16), but they all have a spherical vestibule.
We predict the habit group of D. patagonica using linear discriminant function analysis because its ecology cannot be inferred using extant phylogenetic bracketing (18). Scores of the first two principal components (table S3) were submitted to linear discriminant function analyses. The first analysis included all samples but D. patagonica, which correctly predicted 67% of predefined habit groups (table S4). This serves as a statistical base for predictions of other samples with unknown habit. Second, D. patagonica was incorporated with no predefined habit group and predicted as a burrower with a 93.4% probability (Fig. 1F and table S5). It is noteworthy that incorrect predictions happen only between the generalist group and two specialist groups; there were no incorrect predictions between burrowing and aquatic groups (table S4).
A virtual model of the hypothetical ancestor of crown snakes was reconstructed using a phylogeny compiled from recent studies (Fig. 1G) (8, 19, 20). The Procrustes shape of the hypothetical ancestor resembles a burrower’s ear with the vestibule expanded in the horizontal plane (fig. S4A). Principal components analysis including the hypothetical ancestor found it clustering with living sand swimming snakes, burrowing lizards, and limbless lizards that hide in leaf litter or rock cracks (fig. S5A). Linear discriminant function analysis predicted the hypothetical ancestor as a burrower with a probability of 70.1%; aquatic habitat was highly unlikely for the hypothetical ancestor with a predicted probability of 0.02% (Fig. 1E and table S5). We also reconstructed the morphology of the ancestral node without D. patagonica (fig. S4B). This gave almost identical results in the principal components analysis and linear discriminant function analysis (fig. S5B and table S6). To further test the robustness of the predictions of habit groups, we mapped the Procrustes coordinates on an alternative phylogeny that has mosasaurs as the sister group to all snakes (fig. S7) (7). Using this alternative inner ear shape reconstruction, we predicted the hypothetical ancestor of crown snakes as a burrower with 64.0% probability (table S7).
DISCUSSION
With a snout-tail length exceeding 1.8 m (measured in MACN-RN 976), D. patagonica is the largest known burrowing snake, living or extinct (Fig. 4). Most modern burrowing snakes are smaller than 1 m (16, 21). This includes scolecophidians, cynlindrophiids, uropeltids, and bur???rowing caenophidians (for example, Simoselaps) (16). The largest burrowers in extant snakes, X. unicolor and L. bicolor, can grow up to 1.6 m (16). D. patagonica probably used the same foraging strategies as X. unicolor and L. bicolor that hunt for buried eggs of other reptiles (22) and small vertebrates aboveground (16).
Fig. 4. Total body size of D. patagonica compared with extant snakes.
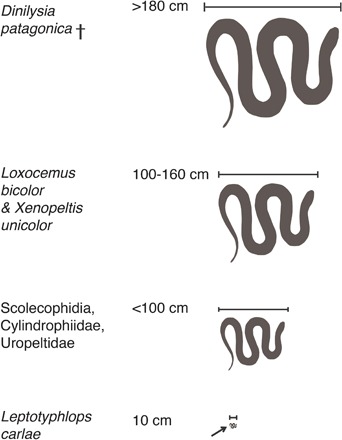
Loxocemus bicolor is the largest extant burrowing snake, growing up to 160 cm (16); Leptotyphlops carlae is the smallest extant snake (21).
The large spherical vestibule of D. patagonica is a morphological signature of burrowing. In modern burrowers, the spherical vestibule usually contains a large saccular otolith (23), or an “ear stone,” that transfers substrate vibrations to the brain. Mathematical modeling has shown that the optimal vibration frequency decreases when the otolith increases in size and mass (24). Previous experimental data have exhibited that species with large otoliths are more sensitive to ground vibrations with lower frequencies than airborne sound (25). The otolith also performs as an accelerometer in the skull to perceive head movements. Species with a large saccular otolith are more sensitive to angular rotations in the vertical plane of the skull, but they can only process a narrow range of constant speed when the animal moves (24). MACN-RN 1014 preserves a cast of a large saccular otolith; this indicates that D. patagonica was sluggish, yet sensitive to ground vibrations.
Basal lineages of crown snakes share a spherical vestibule that facilitates fossoriality (fig. S1, taxa 26 to 28, 30, 32, 37, 40, and 41). However, the most basal lineage among living snakes, blind snakes, does not represent the ancestral morphology of the inner ear. Blind snakes have a small vestibule and very stout semicircular canals (fig. S1, taxa 29 and 35). These are lineage-specific apomorphic morphologies.
D. patagonica holds a critical position in the origin of modern snakes. In recent snake phylogenies using large data sets, D. patagonica is recovered as the sister taxon to all crown-group snakes (6, 8, 12), in a basal position within crown-group snakes (5, 26, 27), or as a sister taxon to crown snakes with unresolved relationships among other Mesozoic snakes (28). Regardless of this phylogenetic uncertainty, the hypothetical ancestor of modern snakes—reconstructed with or without D. patagonica in our analyses—is predicted as a burrower in the linear discriminant analysis. Including D. patagonica increases the probability of the hypothetical ancestor being fossorial (59.3 to 70.1%).
CONCLUSIONS
A burrowing life-style predated modern snakes, but it remained as the main, if not exclusive (6), habit for basal lineages among crown snakes. Both D. patagonica and the hypothetical ancestor of crown snakes have a large vestibule that is associated with low-frequency hearing. This suggests that ancestrally, crown snakes were able to detect prey and predator via substrate vibrations.
MATERIALS AND METHODS
Experimental design
We sampled 44 species for inner ear morphology (fig. S1). Digital endocasts of the bony labyrinth is built using x-ray CT data. Virtual models of the inner ear are deposited in MorphoBank (29) Project 2170 in PLY format. We used six type 2 landmarks (17) and 22 semilandmarks placed along the lateral surface of the vestibule and the medial surface of the lateral semicircular canal (fig. S2).
Statistical analysis
The 28 landmarks and semilandmarks are superimposed using generalized Procrustes analysis implemented in the R package Geomorph V 1.1-5 (30). We also performed MANOVA test and principal components analysis in Geomorph V 1.1-5 (30), using Procrustes coordinates as input.
To test between-group shape separation, linear discriminant analysis was performed among the three habit groups using scores of the first two principal components of all extant species. We used standard statistical software (Minitab 17) for the linear discriminant function analysis.
We mapped the shape coordinates on the phylogenies using squared-change parsimony, implemented in MorphoJ (31). For the hypothetical ancestor of crown snakes, shape coordinates of the inner ear were reconstructed with and without D. patagonica. An additional phylogeny (fig. S7) was also used to address the phylogenetic uncertainty of mosasaurs. We listed the details of Materials and Methods in the Supplementary Materials.
Acknowledgments
For specimen access to D. patagonica, we thank D. Pol (CONICET) and colleagues at Museo Argentino de Ciencias Narutales: A. Kramarz, S. Alvarez, and R. Paz. We thank D. Frost, D. Kizirian, R. Pascocello, D. Dickey, M. Arnold, and C. Mahling for collection visits at the American Museum of Natural History (AMNH). M. Ellison provided photos of D. patagonica. M. Hill, H. Towbin, and A. Balanoff taught H. Yi CT techniques. H. Yi discussed with A. Watanabe and J. Chen about geometric morphometrics and ancestral state reconstructions. We thank J. Flynn, J. Meng, G. Bever, W. Harcourt-Smith, and S. Brusatte for comments on earlier versions of the manuscript. We thank N. Longrich and three anonymous reviewers whose comments improved the manuscript. Funding: CT scanning at the AMNH is supported by NSF EAR0959384. CT scans obtained from DigiMorph are supported by NSF EF-0334961 to M. Kearney and O. Rieppel. H.Y.’s travel was funded by Columbia University and the Sino-British fellowship of the Royal Society, London, to H.Y. and the University of Edinburgh. Major support for this project was provided by the Division of Paleontology at the AMNH. Author contributions: H.Y. and M.A.N. designed the study and wrote the paper. H.Y. collected data, performed analytical work, and prepared the figures. M.A.N. contributed CT data. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplementary Materials. Additional data related to this paper may be requested from the authors and virtual models of the inner ear are deposited in MorphoBank (30) Project 2170 in PLY format.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/10/e1500743/DC1
Materials and Methods
Fig. S1. Vestibular shape of all samples.
Fig. S2. Placement of shape variables.
Fig. S3. Regression of centroid size to scores of principal component 1.
Fig. S4. Distribution of shape variables reconstructed for the hypothetical ancestor of crown snakes.
Fig. S5. Principal components analyses of vestibular shape.
Fig. S6. Missing data in shape variables.
Fig. S7. An alternative phylogeny for all samples.
Table S1. Taxon sampling in the three habitat groups.
Table S2. MANOVA test of Procrustes coordinates among the three habitat groups.
Table S3. Scores of the first two principal components.
Table S4. Accuracy of the linear discriminant function analysis.
Table S5. Habit predictions for D. patagonica and the hypothetical ancestor of modern snakes.
Table S6. Habit predictions for D. patagonica and the hypothetical ancestor of modern snakes.
Table S7. Habit predictions for D. patagonica and the hypothetical ancestor of modern snakes.
Table S8. CT scanning parameters*.
Reference (32)
REFERENCES AND NOTES
- 1.Walls G. L., Ophthalmological implications for the early history of the snakes. Copeia 1940, 1–8 (1940). [Google Scholar]
- 2.Miller M. R., The cochlear duct of lizards and snakes. Am. Zool. 6, 421–429 (1966). [DOI] [PubMed] [Google Scholar]
- 3.Bellairs A. D. A., Underwood G., The origin of snakes. Biol. Rev. 26, 193–237 (1951). [DOI] [PubMed] [Google Scholar]
- 4.Lee M. S. Y., Bell G. L. Jr, Caldwell M. W., The origin of snake feeding. Nature 400, 655–659 (1999). [Google Scholar]
- 5.Reeder T. W., Townsend T. M., Mulcahy D. G., Noonan B. P., Wood P. L. Jr, Sites J. W. Jr, Wiens J. J., Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLOS One 10, e0118199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiang A. Y., Field D. J., Webster T. H., Behlke A. D. B., Davis M. B., Racicot R. A., Gauthier J. A., The origin of snakes: Revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 15, 87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martill D. M., Tischlinger H., Longrich N. R., A four-legged snake from the Early Cretaceous of Gondwana. Science 349, 416–419 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Gauthier J. A., Kearney M., Maisano J. A., Rieppel O., Behlke A. D. B., Assembling the squamate tree of life: Perspectives from the phenotype and the fossil record. Bull. Peabody Mus. Nat. Hist. 53, 3–308 (2012). [Google Scholar]
- 9.Conrad J. L., Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull. Am. Mus. Nat. Hist. 310, 1–182 (2008). [Google Scholar]
- 10.Zaher H., Apesteguía S., Scanferla C. A., The anatomy of the Upper Cretaceous snake Najash rionegrina Apesteguía & Zaher, 2006, and the evolution of limblessness in snakes. Zool. J. Linn. Soc. 156, 801–826 (2009). [Google Scholar]
- 11.Apesteguía S., Zaher H., A Cretaceous terrestrial snake with robust hindlimbs and a sacrum. Nature 440, 1037–1040 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Zaher H., Scanferla C. A., The skull of the Upper Cretaceous snake Dinilysia patagonica Smith-Woodward, 1901, and its phylogenetic position revisited. Zool. J. Linn. Soc. 164, 194–238 (2012). [Google Scholar]
- 13.Woodward A. S., On some extinct reptiles from Patagonia, of the genera Miolania, Dinilysia, and Genyodectes. Proc. Zool. Soc. London 70, 169–184 (1901). [Google Scholar]
- 14.A. M. Lawing, J. J. Head, P. D. Polly, in Paleontology in Ecology and Conservation, J. Louys, Ed. (Springer, Berlin, 2012), pp. 117–146. [Google Scholar]
- 15.E. G. Wever, The Reptile Ear: Its Structure and Function (Princeton Univ. Press, Princeton, NJ, 1978). [Google Scholar]
- 16.H. Greene, Snake: The Evolution of Mystery in Nature (University of California Press, Berkeley, 2000). [Google Scholar]
- 17.F. L. Bookstein, Morphometric Tool for Landmark Data: Geometry and Biology (Cambridge Univ. Press, Cambridge, 1997). [Google Scholar]
- 18.L. M. Witmer, in Functional Morphology in Vertebrate Paleontology, J. Thomason, Ed. (Cambridge Univ. Press, New York, 1995). [Google Scholar]
- 19.Pyron R. A., Burbrink F. T., Wiens J. J., A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi H. -Y., Norell M. A., New materials of Estesia mongoliensis (Squamata: Anguimorpha) and the evolution of venom grooves in lizards. Am. Mus. Novit. 3767, 1–31 (2013). [Google Scholar]
- 21.Hedges S. B., At the lower size limit in snakes: Two new species of threadsnakes (Squamata: Leptotyphlopidae: Leptotyphlops) from the Lesser Antilles. Zootaxa 1841, 1–30 (2008). [Google Scholar]
- 22.Mora J. M., Robinson D. C., Predation of sea turtle eggs (Lepidochelys) by the snake Loxocemus bicolor Cope. Rev. Biol. Trop. 32, 161–162 (1984). [Google Scholar]
- 23.H. Yi, thesis, Columbia University (2014). [Google Scholar]
- 24.Lychakov D. V., Rebane Y. T., Otolith regularities. Hear. Res. 143, 83–102 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Christensen C. B., Christensen-Dalsgaard J., Brandt C., Madsen P. T., Hearing with an atympanic ear: Good vibration and poor sound-pressure detection in the royal python, Python regius. J. Exp. Biol. 215, 331–342 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Longrich N. R., Bhullar B.-A. S., Gauthier J. A., A transitional snake from the Late Cretaceous period of North America. Nature 488, 205–208 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Wilson J. A., Mohabey D. M., Peters S. E., Head J. J., Predation upon hatchling dinosaurs by a new snake from the Late Cretaceous of India. PLOS Biol. 8, e1000322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caldwell M. W., Nydam R. L., Palci A., Apesteguía S., The oldest known snakes from the Middle Jurassic-Lower Cretaceous provide insights on snake evolution. Nat. Commun. 6, 5996 (2015). [DOI] [PubMed] [Google Scholar]
- 29.M. A. O’Leary, S. G. Kaufman, MorphoBank 3.0: Web application for morphological phylogenetics and taxonomy (2012); www.morphobank.org.
- 30.Adams D. C., Otárola-Castillo E., Geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 4, 393–399 (2013). [Google Scholar]
- 31.Klingenberg C. P., MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11, 353–357 (2011). [DOI] [PubMed] [Google Scholar]
- 32.D. F. Wiley N. Amenta, D. A. Alcantara, D. Ghosh, Y. J. Kil, Eric Delson, W. Harcourt-Smith, F. J. Rohlf, K. St. John, B. Hamann, R. Motani, S. Frost, A. L. Rosenberger, L. Tallman, T. Disotell, R. O’Neill, Evolutionary morphing, in Proceedings of IEEE Visualization 2005, Minneapolis, MN, 23 to 28 October 2005, pp. 431–438. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/10/e1500743/DC1
Materials and Methods
Fig. S1. Vestibular shape of all samples.
Fig. S2. Placement of shape variables.
Fig. S3. Regression of centroid size to scores of principal component 1.
Fig. S4. Distribution of shape variables reconstructed for the hypothetical ancestor of crown snakes.
Fig. S5. Principal components analyses of vestibular shape.
Fig. S6. Missing data in shape variables.
Fig. S7. An alternative phylogeny for all samples.
Table S1. Taxon sampling in the three habitat groups.
Table S2. MANOVA test of Procrustes coordinates among the three habitat groups.
Table S3. Scores of the first two principal components.
Table S4. Accuracy of the linear discriminant function analysis.
Table S5. Habit predictions for D. patagonica and the hypothetical ancestor of modern snakes.
Table S6. Habit predictions for D. patagonica and the hypothetical ancestor of modern snakes.
Table S7. Habit predictions for D. patagonica and the hypothetical ancestor of modern snakes.
Table S8. CT scanning parameters*.
Reference (32)


