Abstract
Matrix metalloproteinases (MMP)-2 and -9 (also referred to gelatinases-A and -B, respectively) are upregulated in the inflamed gut of mice and men. We recently demonstrated that synthetic gelatinase blockage reduced large intestinal pro-inflammatory immune responses and apoptosis following murine Campylobacter (C.) jejuni infection. In order to address which gelatinase mediates C. jejuni-induced immune responses, gnotobiotic MMP-2–/–, MMP-9–/–, and wildtype (WT) mice were generated by broadspectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176. The pathogen stably colonized the murine intestinal tract irrespective of the genotype but did not translocate to extra-intestinal compartments. At days 8 and 14 postinfection (p.i.), less pronounced colonic histopathological changes were observed in infected MMP-2–/– mice, less distinct epithelial apoptosis, but more epithelial proliferation in both MMP-2–/– and MMP-9–/– mice, as compared to WT controls. Reduced immune responses in gelatinase-deficient mice were characterized by lower numbers of effector as well as innate and adaptive immune cells within the colonic mucosa and lamina propria. The expression of IL-22, IL-18, IL-17A, and IL-1β mRNA was higher in the colon of MMP-2–/– as compared to WT mice. In conclusion, both MMP-2 and MMP-9 are differentially involved in mediating C. jejuni-induced intestinal immunopathology.
Keywords: Campylobacter jejuni, in vivo infection model, gnotobiotic mice, matrix metalloproteinases, gelatinases, pro-inflammatory immune responses, translocation, IL-22, IL-18, apoptosis
Introduction
Human cases of bacterial gastroenteritis caused by the gram-negative bacterium Campylobacter (C.) jejuni are worldwide on the rise [1, 2]. Being part of the commensal gut microbiota in many wild and domestic animal species, zoonotic transmission to humans takes place from livestock animals via consumption of contaminated water or meat products [3–5]. Infected patients present symptoms of considerable variability ranging from rather mild, non-inflammatory, watery diarrhea to severe, inflammatory, bloody diarrhea, and abdominal pain lasting for up to a few weeks [6]. In most patients, the disease is self-limiting. In some cases, however, infected patients develop postinfectious sequelae such as reactive arthritis and peripheral neuropathies including the Guillain–Barré and Miller–Fisher syndromes later on [6, 7]. In intestinal tissues of infected patients histological changes such as apoptosis, crypt abscesses, ulcerations, and pronounced influx of pro-inflammatory immune cell populations including lymphocytes and neutrophils into the intestinal mucosa and lamina propria are observed [8, 9]. Although human campylobacteriosis is of global importance, our understanding of the molecular mechanisms underlying C. jejuni infection has been hampered for a long time due to the scarcity of appropriate vertebrate models. Newborn piglets, weanling ferrets, chicken, gnotobiotic canine pups, and primates have been more or less successfully used for studying C. jejuni– host interactions [6, 10]. Our group has recently shown that gnotobiotic mice lacking the commensal intestinal microbiota following broad-spectrum antibiotic treatment can be readily colonized by C. jejuni [11]. Subsequently, mice display C. jejuni induced pro-inflammatory immune responses and epithelial apoptosis in the large intestinal mucosa, hence, mimicking key feature of human campylobacteriosis [11]. Thus, gnotobiotic mice are very well suited as C. jejuni infection model to study host–pathogen interactions in vivo.
Matrix metalloproteinases (MMPs) comprise a heterogenous family of zinc- and calcium-dependent matrix-degrading endopeptidases under tight control of endogenous inhibitors, namely, tissue inhibitors of matrix metalloproteinases (TIMPs) [12–14]. With respect to their substrate specificity, MMPs can be categorized into collagenases (MMP-1, -8, -13, -18), gelatinases (MMP-2, -9), stromelysins (MMP-3, -7, -10, -11), elastase (MMP-12), and membrane-type matrix metalloproteinases (MT-MMP-1 through -5) [15]. MMPs are crucial for embryonic development, differentiation, proliferation, and regeneration of tissues [13, 14]. A dysbalance between MMP activating and inhibiting factors, however, results in a plethora of diseases including intestinal inflammation, arthritis, atherosclerosis, or cancer [16, 17]. Importantly, expression levels of the gelatinases-A and -B (MMP-2 and MMP-9, respectively) are upregulated in intestinal inflammation models [18–20] and in patients suffering from human inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis [21–23]. To date, however, data regarding the impact of MMPs, particularly the gelatinases, in intestinal infection caused by bacteria including C. jejuni are limited. Distinct MMPs have been shown to be upregulated during infection with gram-negative bacteria [24]. Whereas increased MMP-3 transcripts could be detected in Peyer’s patches following Salmonella and Yersinia infection [25], MMP-2 and MMP-9 were upregulated in Helicobacter pylori infected mice [26]. Furthermore, human and murine Helicobacter-induced gastritis were associated with increased MMP-9 levels derived from macrophages [27]. Interestingly, distinct serine proteases derived from E. coli have been shown to act as specific pro-MMP-2 activators [28].
Recently, we could show that selective gelatinase blockage by the synthetic compound RO28-2653 (blocking both MMP-2 and MMP-9) reduced large intestinal apoptosis and pro-inflammatory immune cell responses in C. jejuni-infected gnotobiotic IL-10–/– mice [29]. This prompted us to investigate in more detail which gelatinase is involved in C. jejuni-induced disease. To address this, we infected gnotobiotic MMP-2–/–, MMP-9–/–, and corresponding wildtype (WT) control mice perorally with C. je-juni strain 81-176. We then investigated 1) the gastrointestinal colonization and translocation properties of C. jejuni, 2) the clinical course of infection, 3) the histopathological colonic changes including apoptosis in situ, 4) the abundances of immune cell populations in the colonic mucosa and lamina propria in situ, and, furthermore, 5) the large intestinal expression of pro-inflammatory cytokines belonging to the IL-22/IL-18/IL-17 axis at two distinct time points postinfection (p.i.).
Methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration number G0135/10). Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice and C. jejuni infection
Female MMP-2–/– and MMP-9–/– mice (all in C57BL/6j background [20]) as well as age- and sex-matched C57BL/6j WT control mice were bred and maintained within the same specific pathogen free (SPF) unit in the Forschungseinrichtungen für Experimentelle Medizin (FEM, Charité – University Medicine Berlin). In order to confirm absence of MMP-2 or MMP-9 gene expresion, genomic DNA was isolated, and disruption of either gene was confirmed by polymerase chain reaction (PCR) [20]. Gnotobiotic mice (with a virtually depleted gastrointestinal microbiota) were generated by broad-spectrum antibiotic treatment as described earlier [30]. Briefly, mice were transferred to sterile cages and treated by adding ampicillin–sulbactam (1 g/L; Pfizer, Berlin, Germany), vancomycin (500 mg/L; Hexal, Holzkirchen, Germany), ciprofloxacin (200 mg/L; Hexal, Holzkirchen, Germany), imipenem (250 mg/L; Fresenius Kabi, Graz, Austria), and metronidazole (1 g/L; Braun, Melsungen, Germany) to the drinking water ad libitum starting at 8 weeks of age and continued for approximately 10 weeks before the infection experiment [11]. Three days prior infection, the antibiotic cocktail was replaced by sterile tap water (ad libitum). Then, mice were perorally infected with 109 colony forming units (CFU) of viable C. jejuni strain 81-176 in a volume of 0.3 mL phosphate buffered saline (PBS) on two consecutive days (day 0 and day 1) by gavage as described earlier [11].
Clinical score
To assess clinical signs of C. jejuni induced infection on a daily basis, a standardized cumulative clinical score (maximum 12 points), addressing the occurrence of blood in feces (0: no blood; 2: microscopic detection of blood by the Guajac method using Haemoccult, Beckman Coulter/ PCD, Krefeld, Germany; 4: macroscopic blood visible), diarrhea (0: formed feces; 2: pasty feces; 4: liquid feces), and the clinical aspect (0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromized locomotion, pre-final aspect) was used [31].
Sampling procedures
Mice were sacrificed at day 8 or day 14 p.i. by isofluran treatment (Abbott, Greifswald, Germany). Cardiac blood and tissue samples from colon, ileum, mesenteric lymphnodes (MLNs), spleen, liver, and kidney were asserved under sterile conditions. Intestinal ex vivo biopsies were collected in parallel for immunohistochemical, microbiological, and immunological analyses. Immunohistopathological changes were assessed in colonic samples that were immediately fixed in 5% formalin and embedded in paraffin. Sections (5 µm) were stained with hematoxylin and eosin (H&E) or respective antibodies for in situ immunohistochemistry as described earlier [32].
Histopathological grading of large intestinal lesions
Histopathological changes were quantitatively assessed in H&E stained large intestinal paraffin sections applying a histopathological scoring system by two independent double-blinded investigators as described previously [33]. In brief:
Colonic histopathology (max. 4 points; according to [34]): 0: no inflammation; 1: single isolated cell infiltrates within the mucosa; no epithelial hyperplasia; 2: mild scattered to diffuse cell infiltrates within the mucosa and sub-mucosa; mild epithelial hyperplasia; starting loss of goblet cells; 3: cell infiltrates within mucosa, submucosa, and sometimes transmural; epithelial hyperplasia; loss of goblet cells; 4: cell infiltrates within mucosa, submucosa, and transmural; severe inflammation; loss of goblet cells, loss of crypts; ulcerations; severe epithelial hyperplasia.
Immunohistochemistry
In situ immunohistochemical analysis of colonic paraffin sections was performed as described previously [35–37]. Primary antibodies against cleaved caspase-3 (Asp175, Cell Signaling, Beverly, MA, USA, 1:200), Ki67 (TEC3, Dako, Denmark, 1:100), myeloperoxidase (MPO-7, no. A0398, Dako, 1:500), F4/80 (no. 14-4801, clone BM8, eBioscience, San Diego, CA, USA, 1:50), CD3 (no. N1580, Dako, 1:10), FOXP3 (FJK-16s, eBioscience, 1:100), and B220 (eBioscience, 1:200) were used. For each animal, the average number of positively stained cells within at least six high power fields (HPF, 0.287 mm2, 400× magnification) were determined microscopically by a double-blinded investigator.
Quantitative analysis of C. jejuni colonization and translocation
Viable C. jejuni were detected in feces over time p.i. or at time of necropsy (day 8 or day 14 p.i.) in luminal samples taken from the the terminal ileum and colon, dissolved in sterile PBS and serial dilutions cultured on Karmali- and Columbia-Agar supplemented with 5% sheep blood (Oxoid) for 2 days at 37 °C under microaerobic conditions using CampyGen gas packs (Oxoid). To quantify bacterial translocation, ex vivo biopsies derived from mesenteric lymph nodes (MLNs), spleen, liver, and kidney were homogenized in 1 mL sterile PBS, whereas cardiac blood (≈100 μL) was directly streaked onto Karmali-Agar and Columbia-Agar supplemented with 5% sheep blood and cultivated accordingly. The respective weights of fecal or tissue samples were determined by the difference of the sample weights before and after asservation. The detection limit of viable pathogens was ≈100 CFU per gram.
Cytokine detection in supernatants of colonic ex vivo biopsies
Ileal and colonic ex vivo biopsies were cut longitudinally and washed in PBS. Strips of approximately 1 cm2 intestinal tissue were placed in 24-flat-bottom well culture plates (Nunc, Wiesbaden, Germany) containing 500 μL serum-free RPMI 1640 medium (Gibco, Life Technologies, Paisley, UK) supplemented with penicillin (100 U/ mL) and streptomycin (100 μg/mL; PAA Laboratories). After 18 h at 37 °C, culture supernatants or serum samples were tested for TNF, IFN-y, MCP-1, IL-6, and IL-10 by the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences) on a BD FACSCanto II flow cytometer (BD Biosciences).
Real-time PCR
RNA was isolated from snap frozen colonic ex vivo biopsies, reverse transcribed, and analyzed as described previously [19]. Murine IL-23p19, IL-22, IL-18, IL-17A, and IL-β mRNA expressions were detected and analyzed using Light Cycler Data Analysis Software (Roche). Expression levels were calculated relative to the HPRT expression and indicated as “Arbitrary Units” (fold expression).
Statistical analysis
Medians and levels of significance were determined using Mann-Whitney test (GraphPad Prism v5, La Jolla, CA, USA) as indicated. Two-sided probability (P) values ≤0.05 were considered significant.
Results
Colonization and translocation properties of C. jejuni in gnotobiotic mice lacking MMP-2 or MMP-9
In the present study, we dissected the role of the gelatinases-A and -B (also referred to MMP-2 and MMP-9, respectively) in murine campylobacteriosis. To address this, gnotobiotic MMP-2–/–, MMP-9–/–, and wildtype (WT) mice were generated in which the intestinal microbiota was virtually depleted by quintuple antibiotic treatment for 10 weeks [11, 29–33, 38]. Following peroral infection with 109 CFU of C. jejuni strain 81-176 on two consecutive days (day 0 and day 1), mice of either genotype harbored the pathogen with loads of 109 per gram fecal sample (Fig. 1). In single cases, however, MMP-9–/– mice had expelled the pathogen as early as 6 days p.i. (Fig. 1B). We next surveyed the effect of MMP-2 and MMP-9 in murine campylobacteriosis at two distinct time points, namely, as early as 8 days and 2 weeks following C. jejuni infection. In small and large intestines of WT and MMP-2–/– mice, C. jejuni loads slightly decreased by approximately 1 to 2 orders of magnitude between days 8 and 14 p.i. (Fig. 2A,B). In the colon, MMP-2–/– and MMP-9–/– mice harbored approximately 1.5 orders of magnitude lower C. jejuni loads as compared to wildtype mice at day 8 p.i. (Fig. 2B). Overall, irrespective of the genotype, gnotobiotic mice could be stably infected with C. jejuni strain 81-176 with relatively high loads over time. We next assessed whether viable C. jejuni were able to translocate from the intestinal tract to extra-intestinal compartments. As early as 8 days p.i., C. jejuni could be detected in the MLNs of all WT and MMP-2–/– mice, but only in 58.3% of MMP-9–/– mice (Fig. 2C). Pathogenic loads were lower in MLNs of MMP-9–/– mice at day 8 p.i. and of MMP-2–/– at day 14 p.i. when compared to WT controls (p < 0.05 and p < 0.01, respectively; Fig. 2C). Between days 8 and 14 p.i., C. jejuni loads decreased in MLNs of WT and MMP-2–/– mice (p < 0.05 and p < 0.001, respectively; Fig. 2C). At day 14 p.i., C. jejuni detection rates in MLNs of WT, MMP-9–/–, and MMP-2–/– mice were 90%, 70%, and 40%, respectively (Fig. 2C). Remarkably, viable C. jejuni did not translocate beyond the intestinal tract since neither in liver, kidney, spleen, and blood viable pathogens could be detected by direct plating (not shown).
Fig. 1.
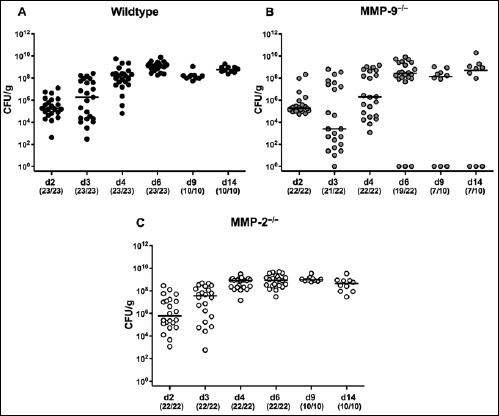
Kinetics of intestinal C. jejuni colonization in perorally infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic (A) wildtype (black circles), (B) MMP-9–/– (grey circles), and (C) MMP-2–/– (white circles) mice were generated following broad-spectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176 by gavage at day 0 and day 1. Pathogenic loads were determined in fecal samples (CFU, colony forming units per gram) at distinct time points (d, day) postinfection (p.i.) as indicated by culture. Numbers of mice harboring C. jejuni strain 81-176 out of the total number of analyzed animals are given in parentheses. Data were pooled from three independent experiments
Fig. 2.

Intestinal C. jejuni loads in perorally infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and 1. Pathogenic loads (CFU, colony forming units per gram) were determined in luminal samples taken from the (A) terminal ileum or (B) colon or in homogenates of (C) mesenteric lymphnodes at day (d) 8 or 14 postinfection (p.i.) as indicated by culture. Numbers of mice harboring C. jejuni strain 81-176 out of the total number of analyzed animals are given in parentheses. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Macroscopic and microscopic sequelae of C. jejuni infection in gnotobiotic mice lacking MMP-2 or MMP-9
We next assessed potential clinical symptoms of C. jejuni infection (such as bloody diarrhea and wasting) in gelatinase-deficient mice. At day 8 p.i., occurrence of blood could be detected microscopically in 8.7%, 18.2%, and 9.1% of fecal samples derived from WT, MMP-9–/–, and MMP-2–/– mice, respectively, whereas 14 days p.i., no bloody diarrhea could be observed at all (Fig. 3A). Hence, despite pathogenic colonization, infected gnotobiotic mice of either genotype were clinically virtually unaffected.
Fig. 3.

Clinical symptoms and colonic histopathological changes in C. jejuni infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and 1. At day (d) 8 and day 14 postinfection (p.i.), (A) occurrence of bloody diarrhea and (B) histopathological changes in H&E stained colonic paraffin sections were assessed applying a standardized haemoccult and histopathological scoring system, respectively. Naive (N) mice served as uninfected controls. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
We next surveyed histopathological changes in the colonic mucosa in the course of C. jejuni infection. Until day 8 p.i., significant but rather mild histopathological changes such as single isolated cell infiltrates within the mucosa, but no epithelial hyperplasia could be observed in the colonic mucosa of mice irrespective of the genotype (Fig. 3). At day 14 p.i., histopathological changes were even more severe in WT and MMP-9–/–, but not MMP-2–/– mice as compared to those observed at day 8 p.i. (p < 0.001 and p < 0.05, respectively; Fig. 3B). Moreover, at day 14 p.i., histopathological changes were less distinct in the large intestines of MMP-2–/– as compared to WT and MMP-9–/– mice (Fig. 3B). Notably, the variability of histopathological changes in the colon of MMP-9–/– mice at day 14 p.i. was relatively high as indicated by high standard deviations of histopathological scores.
Given that apoptosis is a commonly used diagnostic marker in the histopathological evaluation and grading of intestinal disease including campylobacteriosis [11], we quantitatively assessed numbers of caspase-3+ cells within the colonic epithelium of infected mice. Whereas following infection abundances of apoptotic cells increased in colons of mice of either genotype, this increase was less pronounced in MMP-2–/– as compared to WT mice, both at day 8 and day 14 p.i. (p < 0.001 and p < 0.01, respectively; Fig. 4A). Furthermore, MMP-9–/– mice displayed lower colonic apoptotic cell numbers at day 8, but not day 14 p.i., as compared to WT controls (p < 0.05; Fig. 4A). Given that Ki67 comprises a nuclear protein associated with and necessary for cellular proliferation [39], we stained colonic paraffin sections against Ki67 to determine proliferative measures of the colonic epithelium counteracting apoptosis following C. jejuni infection. Proliferating cells increased in the colonic epithelium of MMP-9–/– and MMP-2–/–, but not WT mice until day 8 p.i. (p < 0.01 and p < 0.001, respectively; Fig. 4B). In the colonic mucosa of MMP-2–/– mice, higher Ki67+ cell numbers could be observed at both day 8 and day 14 p.i. as compared to WT controls (p < 0.01 and p < 0.05, respectively; Fig. 4B), whereas MMP-9–/– mice displayed more proliferating cells in the colon at day 14 p.i. only, when compared to WT animals (p < 0.01; Fig. 4B). Hence, MMP-2–/– mice exhibited less microscopic disease following C. jejuni infection, which was accompanied by more regenerative epithelial measures counteracting the inflammatory sequelae.
Fig. 4.

Apoptosis and proliferation in the colonic epithelium of C. jejuni infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and 1. The average number of colonic (A) apoptotic cells (positive for caspase-3, Casp3) and (B) proliferating cells (positive for Ki67) from at least six high power fields (HPF, 400x magnification) per animal was determined microscopically in immunohistochemically stained colonic paraffin sections at day (d) 8 and day 14 postinfection (p.i.). Naive (N) mice served as uninfected controls. Medians (black bars) and level of significance (p-value) determined by Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Immune cell responses in C. jejuni strain 81-176 infected gnotobiotic mice lacking MMP-2 or MMP-9
Given that recruitment of pro-inflammatory immune cells to sites of inflammation is a hallmark of intestinal pathogenic infection including campylobacteriosis [11], we quantitatively assessed the influx of effector cells as well as of innate and adaptive immune cells into the large intestinal mucosa and lamina propria by in situ immunohistochemical staining of colonic paraffin sections. Following C. jejuni infection, numbers of colonic MPO7+ neutrophils, F4/80+ macrophages and monocytes, CD3+ T lymphocytes, FOXP3+ regulatory T cells (Tregs), and B220+ B lymphocytes increased in mice of either genotype and were higher at both day 8 and day 14 p.i. as compared to uninfected control mice (Fig. 5). Increases in neutrophils, Tregs, and B cells were less pronounced in large intestines of MMP-2–/– mice as compared to WT mice at both time points (Fig. 5A, Fig. 6B,C). Moreover, MMP-2–/– mice exhibited lower numbers of macrophages and monocytes as well as of T lymphocytes at day 14 p.i. as compared to WT controls (Fig. 5B, Fig. 6A). For MMP-9–/– mice, lower numbers of colonic neutrophils, macrophages and monocytes, T lymphocytes, and Tregs could be determined at day 14 p.i. when compared to WT mice (Fig. 5, Fig. 6A,B), whereas also at day 8 p.i. lower macrophages and monocytes could be determined in colons of MMP-9–/– versus WT mice (Fig. 5B). Hence, influx of immune cells was overall less pronounced MMP-9–/– mice at day 14 p.i. and in MMP-2–/– at both time points.
Fig. 5.

Innate immune cells in the colon of C. jejuni-infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and day 1. The average number of cells positive for (A) MPO7 (neutrophils) and (B) F4/80 (macrophages and monocytes) from at least six high power fields (HPF, 400× magnification) per animal were determined microscopically in immunohistochemically stained colonic paraffin sections derived from mice at day (d) 8 and day 14 postinfection (p.i.). Naive (N) mice served as uninfected controls. Medians (black bars) and significance levels as determined by the Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Fig. 6.

Adaptive immune cells in the colon of C. jejuni infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and day 1. The average number of cells positive for (A) CD3 (T Lymphocytes), (B) FOXP3 (Regulatory T Cells, Tregs), and (C) B220 (B Lymphocytes) from at least six high power fields (HPF, 400× magnification) per animal were determined microscopically in immunohistochemically stained colonic paraffin sections derived from mice at day (d) 8 and day 14 postinfection (p.i.). Naive (N) mice served as uninfected controls. Medians (black bars) and significance levels as determined by the Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Cytokine secretion in the intestines of C. jejuni strain 81-176 infected gnotobiotic mice lacking MMP-2 or MMP-9
We next determined pro- and anti-inflammatory cytokine secretion in ex vivo biopsies derived from small as well as large intestines of C. jejuni strain 81-176 infected gnotobiotic MMP-2–/– and MMP-9–/– mice. Until day 14 p.i., small intestinal TNF levels increased in mice of either genotype (Fig. 7A), whereas higher ileal IFN-γ concentrations could be determined in WT and MMP-9–/– but not MMP-2–/– mice at day 8 p.i. (Fig. 7B). The anti-inflammatory cytokine IL-10 increased as early as 8 days following C. jejuni infection in ilea of mice, irrespective of their genotype and was still elevated in small intestines of WT mice at day 14 p.i. (Fig. 7D). In the colon, elevated TNF concentrations could be observed at either time point in WT and MMP-9–/– mice and at day 14 p.i. in MMP-2–/– animals as compared to naive controls (Fig. 8A), whereas colonic IFN-γ levels increased as early as 8 days p.i. in WT and MMP-9–/– but not MMP-2–/– mice (Fig. 8B). C. jejuni infection was further accompanied by higher colonic MCP-1 levels at either time point, irrespective of the genotype of mice (Fig. 8C), whereas large intestinal IL-10 was elevated in mice of either genotype at day 8 p.i. only (Fig. 8D). Taken together, pro- and anti-inflammatory cytokines increased in the intestinal tract upon C. jejuni infection, but without significant differences between mice of the respective genotypes.
Fig. 7.

Cytokine concentrations in the ileum of C. jejuni-infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and 1. (A) TNF, (B) IFN-γ, (C) MCP1, and (D) IL-10 concentrations were determined in supernatants of ileal ex vivo biopsies derived at day (d) 8 and day 14 postinfection (p.i.). Naive (N) mice served as uninfected controls. Medians (black bars) and significance levels as determined by the Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Fig. 8.

Cytokine concentrations in the colon of C. jejuni-infected gnotobiotic mice lacking lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and 1. (A) TNF, (B) IFN-γ, (C) MCP1, and (D) IL-10 concentrations were determined in supernatants of colonic ex vivo biopsies derived at day (d) 8 and day 14 postinfection (p.i.). Naive (N) mice served as uninfected controls. Medians (black bars) and significance levels as determined by the Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Colonic mRNA expression of pro- and anti-inflammatory cytokines in C. jejuni strain 81-176 infected gnotobiotic mice lacking MMP-2 or MMP-9
We next determined colonic mRNA expression levels of pro- and anti-inflammatory mediators of the IL-22/IL-18/ IL-17 axis during C. jejuni infection of gelatinase-deficient mice. In WT and MMP-2–/– mice, colonic IL-23p19 mRNA levels were elevated at either time point and in the large intestines of MMP-9–/– mice at day 14 p.i. (Fig. 9A). In MMP-9–/– mice, however, colonic IL-23p19 mRNA concentrations were lower as compared to WT mice at day 8 and at day 14 p.i. (p < 0.001 and p < 0.01, respectively; Fig. 9A). Following C. jejuni infection, IL-22 mRNA increased in colons of either gelatinase deficient mice as early as day 8 p.i. and until day 14 p.i. in WT mice (Fig. 9B). Interestingly, large intestinal IL-22 mRNA levels were higher in MMP-2–/– mice at day 8 p.i. (p < 0.001), but lower in MMP-9–/– mice at day 14 p.i. (p < 0.05), when compared to WT control animals at the respective time points (Fig. 9B). C. jejuni-infection induced increases in colonic IL-18 mRNA concentrations were less distinct in MMP-9–/– mice at day 8 p.i. (p < 0.05), but even more pronounced in MMP-2–/– mice at either time point (p < 0.05) as compared to WT controls (Fig. 9C). Irrespective of the genotype, colonic IL-17A was upregulated upon C. jejuni infection. In MMP-2–/– mice, however, IL-17A mRNA expression levels were higher at day 8 p.i. (p < 0.01), but lower at day 14 p.i. (p < 0.001), when compared to WT animals (Fig. 9D). Notably, this held also true for colonic IL-1β mRNA expression levels in both MMP-9–/– and MMP-2–/– as compared to WT mice (Fig. 9E). Moreover, upon C. jejuni infection, colonic IL-1β expression was upregulated at day 14 p.i. in WT mice, at day 8 p.i. in MMP-2–/– mice and at both time points in MMP-9–/– mice (Fig. 9E). Hence, mRNA expression of pro- and anti-inflammatory cytokines belonging to the IL-22/ IL-18/IL-17 axis are differentially regulated in C. jejuni-infected gnotobiotic MMP-2–/– and MMP-9–/– mice.
Fig. 9.
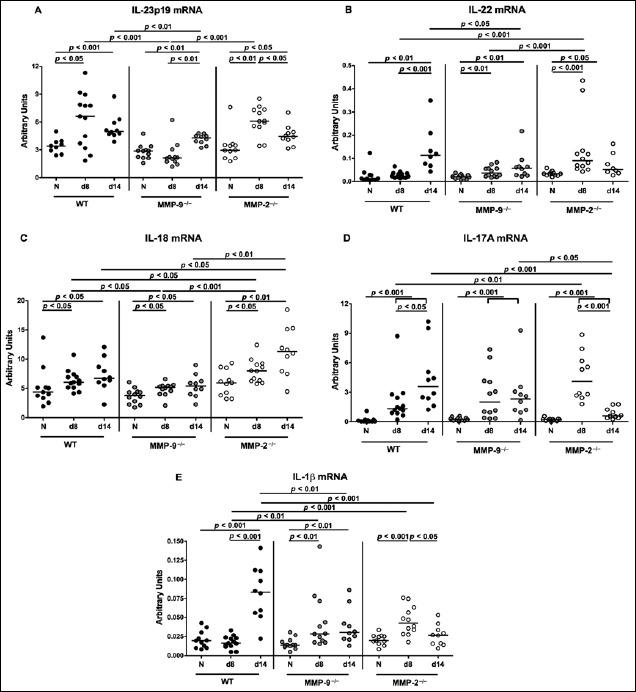
Expression of pro- and anti-inflammatory mediators in the colon of C. jejuni-infected gnotobiotic mice lacking MMP-2 or MMP-9. Gnotobiotic wildtype (WT; black circles), MMP-9–/– (grey circles), and MMP-2–/– (white circles) mice were perorally infected with C. jejuni strain 81-176 at days 0 and 1. (A) IL-23p19, (B) IL-22, (C) IL-18, (D) IL-17A, and (E) IL-1β mRNA expression levels were determined in colonic ex vivo biopsies derived at day (d) 8 and day 14 postinfection (p.i.) by real-time PCR and expressed in arbitrary units (fold expression). Naive (N) mice served as uninfected controls. Medians (black bars) and significance levels as determined by the Mann–Whitney U test are indicated. Data were pooled from three independent experiments
Discussion
The gelatinases MMP-2 and MMP-9 are pivotally involved in modulating intestinal inflammation of mice and men [18–23, 29, 40]. To date, however, only limited data regarding the role of gelatinases in host interactions with enteric pathogens including C. jejuni exist. Results from our recent experiments revealed that selective gelatinase blockage by the synthetic compound RO28-2653 ameliorated colonic pro-inflammatory immune responses and apoptosis in C. jejuni 81-176 strain infected gnotobiotic IL-10–/– mice [29]. This provided strong evidence that gelatinases are involved in the pathogenesis of campylobacteriosis. To address the question which gelatinase in particular modulates the course of murine C. jejuni infection, we investigated campylobacteriosis in gnotobiotic MMP-2- or MMP-9-deficient mice at distinct time points p.i. Results revealed that C. jejuni stably colonized the intestinal tract of gnotobiotic MMP-2–/–, MMP-9–/–, and WT mice at high loads. Viable bacteria were further isolated from MLNs, but did not translocate to extra-intestinal compartments such as liver, kidney, spleen, or blood. This is well supported by our previous campylobacteriosis studies in different murine models including gnotobiotic, with human microbiota reconstituted, conventional WT infant and gnotobiotic IL-10–/– mice [11, 31–33, 37, 38, 41, 42]. Upon C. jejuni infection, gnotobiotic MMP-2–/– mice developed less colonic histopathology, whereas both infected MMP-2–/– and MMP-9–/– mice displayed less apoptotic, but more proliferating cells in the colonic epithelium as compared to WT mice. These data are partly supported by our very recent study obtained from different C. jejuni infection models. Following peroral infection with the C. jejuni B2 strain, conventionally colonized infant MMP-2–/–, but not MMP-9–/– mice exhibited less pronounced colonic histopathological and apoptotic changes as compared to WT controls [43]. Interestingly, in both studies, less influx of effector as well as of innate and adaptive immune cells including neutrophils, T lymphocytes, and Tregs into the colonic mucosa and lamina propria of either gelatinase deficient mice could be observed. In contrast to infected infant MMP-2–/– and MMP-9–/– mice, which expelled the pathogen rather early in the course of infection [43], gnotobiotic mice of either genotype harbored the pathogen at high loads throughout the experiment.
Results presented here support the important role of MMP-2 in intestinal inflammation and are well in line with results from our earlier studies showing that MMP-2, but not MMP-9, is essential for the pathogenesis of acute ileitis [19] and colitis [20] in mice.
Less severe intestinal inflammation observed in C. jejuni-infected gnotobiotic MMP-2–/– mice was accompanied by increased IL-22 mRNA levels in the colon. IL-22 belongs to the IL-10 cytokine family and is well known for its tissue-protective and wound-healing responses from epithelial cells following infection and inflammation [44, 45]. In the intestinal tract, IL-22 exerts its dichotomous actions in a tissue-depending manner. Whereas IL-22 has been shown to act as an anti-inflammatory mediator in the large intestine, we have previously reported its pro-inflammatory properties in the small intestine. In acute Toxoplasma gondii-induced ileitis, IL-23 caused small intestinal immunopathology via the upregulation of MMP-2 and, surprisingly, via the induction of IL-22 [19, 46]. Besides IL-22, higher IL-17A, IL-1β, and IL-18 mRNA expression levels were detected in large intestines of MMP-2–/–, but not MMP-9–/– mice at day 8 p.i. as compared to infected WT mice in the present study. These results are supported by a study demonstrating that IL-22, IL-17A, and IL-1β secretion was increased in MLNs and colons of IL-10–/– mice as early as 4 days following oral C. jejuni infection, whereas both T cells and innate lymphoid cells (ILCs) induced upregulation of IL-17A and IL-22 in an organ- and time-specific manner [47]. Moreover, C. jejuni infection resulted in an upregulation of IL-22, IL-17A, and IL-1β in ex vivo colonic biopsies derived from humans [48]. Results further revealed that C. jejuni induced an innate IL-22/IL-17 as well as an adaptive Th17/Th1 host response [48]. Taken together, these data indicate that cytokines of the IL-22 and IL-17 families are essential mediators of host immunity in the acute and effector phase of C. jejuni infection in vivo. Upregulated IL-22 mRNA expression levels in the large intestines of C. jejuni-infected MMP-2–/– mice were accompanied by increased IL-18 concentrations. Data on the role of IL-18 in C. jejuni–host interaction are lacking so far, but we could recently show that IL-22 induced IL-18 expression in gut epithelial cells after oral Toxoplasma gondii or Citrobacter rodentium infection [49]. Furthermore, IL-18 amplified Th1-mediated intestinal inflammation and IL-22 production from ILCs during pathogenic infection [49]. It is therefore tempting to speculate that IL-22 and IL-18 might also exert a mutual regulation during intestinal C. jejuni infection.
Conclusion
In conclusion, both MMP-2 and MMP-9 are differentially involved in mediating C. jejuni-induced intestinal immunopathology with MMP-2 holding a more prominent and MMP-9 rather a minor role. In future studies, the regulatory pathways of MMP-2, IL-22, and IL-18 in murine C. jejuni infection need to be further elucidated in order to better understand molecular mechanisms underlying human campylobacteriosis.
Acknowledgements
We thank Michaela Wattrodt, Ursula Rüschendorf, Silvia Schulze, Alexandra Bittroff-Leben, Ines Puschendorf, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility at Charité – University Medicine Berlin for excellent technical assistance and animal breeding. We are grateful to Simone Spieckermann for immunohistochemical staining of paraffin sections.
References
- 1.Young KT, Davis LM, Dirita VJ: Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 5, 665–679 (2007) [DOI] [PubMed] [Google Scholar]
- 2.Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U: Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol 300, 205–211 (2010) [DOI] [PubMed] [Google Scholar]
- 3.Lane JA, Mehra RK, Carrington SD, Hickey RM: The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol 142, 1–13 (2010) [DOI] [PubMed] [Google Scholar]
- 4.Guerry P, Szymanski CM: Campylobacter sugars sticking out. Trends Microbiol 16, 428–435 (2008) [DOI] [PubMed] [Google Scholar]
- 5.Alter T Bereswill S Glunder G Haag LM Hanel I et al.: [Campylobacteriosis of man: livestock as reservoir for Campylobacter species]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 54, 728–734 (2011) [DOI] [PubMed] [Google Scholar]
- 6.Kist M, Bereswill S: Campylobacter jejuni. Contrib Microbiol 8, 150–165 (2001) [DOI] [PubMed] [Google Scholar]
- 7.Wakerley BR, Uncini A, Yuki N, Group GBSC, Group GBSC: Guillain-Barre and Miller Fisher syndromes – new diagnostic classification. Nat Rev Neurol 10, 537–544 (2014) [DOI] [PubMed] [Google Scholar]
- 8.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, et al. : Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut 26, 945–951 (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker RI Caldwell MB Lee EC Guerry P Trust TJ et al.: Pathophysiology of Campylobacter enteritis. Microbiol Rev 50, 81–94 (1986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masanta WO Heimesaat MM Bereswill S Tareen AM Lugert R et al.: Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol 2013, 526860 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, et al. : Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One 6, e20953 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkedal-Hansen H Moore WG Bodden MK Windsor LJ Birkedal-Hansen B et al.: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4, 197–250 (1993) [DOI] [PubMed] [Google Scholar]
- 13.Goetzl EJ, Banda MJ, Leppert D: Matrix metalloproteinases in immunity. J Immunol 156, 1–4 (1996) [PubMed] [Google Scholar]
- 14.Brinckerhoff CE, Matrisian LM: Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 3, 207–214 (2002) [DOI] [PubMed] [Google Scholar]
- 15.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM: Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 18, 1135–1149 (2000) [DOI] [PubMed] [Google Scholar]
- 16.Crawford HC, Matrisian LM: Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein 49, 20–37 (1996) [DOI] [PubMed] [Google Scholar]
- 17.Saren P, Welgus HG, Kovanen PT: TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol 157, 4159–4165 (1996) [PubMed] [Google Scholar]
- 18.Salmela MT MacDonald TT Black D Irvine B Zhuma T et al.: Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut 51, 540–547 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz M Heimesaat MM Danker K Struck D Lohmann U et al.: Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetallo-proteinase-2 and IL-22 but independent of IL-17. J Exp Med 206, 3047–3059 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimesaat MM Dunay IR Fuchs D Trautmann D Fischer A et al.: The distinct roles of MMP-2 and MMP-9 in acute DSS colitis. Eur J Microbiol Immunol (Bp) 1, 302–310 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, et al. : Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. J Clin Pathol 47, 113–116 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baugh MD Perry MJ Hollander AP Davies DR Cross SS et al.: Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 117, 814–822 (1999) [DOI] [PubMed] [Google Scholar]
- 23.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S: Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47, 63–73 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanlaere I, Libert C: Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev 22, 224-239 (2009), Table of Contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handley SA, Miller VL: General and specific host responses to bacterial infection in Peyer’s patches: a role for stromelysin-1 (matrix metalloproteinase-3) during Salmonella enterica infection. Mol Microbiol 64, 94–110 (2007) [DOI] [PubMed] [Google Scholar]
- 26.Kundu P Mukhopadhyay AK Patra R Banerjee A Berg DE et al.: Cag pathogenicity island-independent up-regulation of matrix metalloproteinases-9 and -2 secretion and expression in mice by Helicobacter pylori infection. J Biol Chem 281, 34651–34662 (2006) [DOI] [PubMed] [Google Scholar]
- 27.Bergin PJ Raghavan S Svensson H Starckx S Van Aelst I et al.: Gastric gelatinase B/matrix metalloproteinase-9 is rapidly increased in Helicobacter felis-induced gastritis. FEMS Immunol Med Microbiol 52, 88–98 (2008) [DOI] [PubMed] [Google Scholar]
- 28.Takeda M, Imada K, Sato T, Ito A: Activation of human progelatinase A/promatrix metalloproteinase 2 by Escherichia coli-derived serine proteinase. Biochem Biophys Res Commun 268, 128–132 (2000) [DOI] [PubMed] [Google Scholar]
- 29.Alutis ME Grundmann U Fischer A Kuhl AA Bereswill S et al.: Selective gelatinase inhibition reduces apoptosis and pro-inflammatory immune cell responses in Campylobacter jejuni-infected gnotobiotic IL-10 deficient mice. Eur J Microbiol Immunol (Bp) 4, 213–222 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimesaat MM Bereswill S Fischer A Fuchs D Struck D et al.: Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol 177, 8785–8795 (2006) [DOI] [PubMed] [Google Scholar]
- 31.Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, et al. : Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10–/– mice via Toll-like-receptor-2 and -4 signaling. PLoS One 7, e40761 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimesaat MM, Lugert R, Fischer A, Alutis M, Kuhl AA, et al. : Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PLoS One 9, e90148 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heimesaat MM, Alutis M, Grundmann U, Fischer A, Tegtmeyer N, et al. : The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol 4. 77 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paclik D, Danese S, Berndt U, Wiedenmann B, Dignass A, et al. : Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS One 3, e2629 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heimesaat MM Nogai A Bereswill S Plickert R Fischer A et al.: MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59, 1079–1087 (2010) [DOI] [PubMed] [Google Scholar]
- 36.Bereswill S, Munoz M, Fischer A, Plickert R, Haag LM, et al. : Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One 5, e15099 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimesaat MM Fischer A Alutis M Grundmann U Boehm M et al.: The impact of serine protease HtrA in apoptosis, intestinal immune responses and extra-intestinal histopathology during Campylobacter jejuni infection of infant mice. Gut Pathog 6, 16 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haag LM Fischer A Otto B Grundmann U Kuhl AA et al.: Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur J Microbiol Immunol (Bp) 2, 2–11 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scholzen T, Gerdes J: The Ki-67 prote in: from the known and the unknown. J Cell Physiol 182, 311–322 (2000) [DOI] [PubMed] [Google Scholar]
- 40.Heimesaat MM Dunay IR Fuchs D Trautmann D Fischer A et al.: Selective gelatinase blockage ameliorates acute DSS colitis. Eur J Microbiol Immunol (Bp) 1, 228–236 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, et al. : Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One 7, e35988 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimesaat MM, Bereswill S: Murine infection models for the investigation of Campylobacter jejuni – host interactions and pathogenicity. Berl Munch Tierarztl Wochenschr 128, 98–103 (2015) [PubMed] [Google Scholar]
- 43.Alutis ME, Grundmann U, Hagen U, Fischer A, Kuhl AA, Gobel UB, et al. : Matrix metalloproteinase-2 mediates intestinal immunopathogenesis in Campylobacter jejuni-infected infant mice. Eur J Microbiol Immunol (Bp) (2015), doi: 10.1556/1886.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eidenschenk C, Rutz S, Liesenfeld O, Ouyang W: Role of IL-22 in microbial host defense. Curr Top Microbiol Immunol 380, 213–236 (2014) [DOI] [PubMed] [Google Scholar]
- 45.Ouyang WJ, Rutz S, Crellin NK, Valdez PA, Hymowitz SG: Regulation and Functions of the IL-10 Family of Cytokines in Inf lammation and Disease. Annual Review of Immunology, Vol 29 29, 71–109 (2011) [DOI] [PubMed] [Google Scholar]
- 46.Munoz M, Liesenfeld O, Heimesaat MM: Immunology of Toxoplasma gondii. Immunol Rev 240, 269–285 (2011) [DOI] [PubMed] [Google Scholar]
- 47.Malik A, Sharma D, St Charles J, Dybas LA, Mansfield LS: Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol 7, 802–817 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards LA, Nistala K, Mills DC, Stephenson HN, Zilbauer M, et al. : Delineation of the innate and adaptive T-cell immune outcome in the human host in response to Campylobacter jejuni infection. PLoS One 5, e15398 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munoz M Eidenschenk C Ota N Wong K Lohmann U et al.: Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity 42, 321–331 (2015) [DOI] [PubMed] [Google Scholar]


