Abstract
Sporadic cases of gastroenteritis have been attributed to Arcobacter butzleri infection, but information about the underlying immunopathological mechanisms is scarce. We have recently shown that experimental A. butzleri infection induces intestinal, extraintestinal and systemic immune responses in gnotobiotic IL-10–/– mice. The aim of the present study was to investigate the immunopathological role of Toll-like Receptor-4, the receptor for lipopolysaccharide and lipooligosaccharide of Gram-negative bacteria, during murine A. butzleri infection. To address this, gnotobiotic IL-10–/– mice lacking TLR-4 were generated by broad-spectrum antibiotic treatment and perorally infected with two different A. butzleri strains isolated from a patient (CCUG 30485) or fresh chicken meat (C1), respectively. Bacteria of either strain stably colonized the ilea of mice irrespective of their genotype at days 6 and 16 postinfection. As compared to IL-10–/– control animals, TLR-4–/– IL-10–/– mice were protected from A. butzleri-induced ileal apoptosis, from ileal influx of adaptive immune cells including T lymphocytes, regulatory T-cells and B lymphocytes, and from increased ileal IFN-γ secretion. Given that TLR-4-signaling is essential for A. butzleri-induced intestinal inflammation, we conclude that bacterial lipooligosaccharide or lipopolysaccharide compounds aggravate intestinal inflammation and may thus represent major virulence factors of Arcobacter. Future studies need to further unravel the molecular mechanisms of TLR-4-mediated A. butzleri-host interactions.
Keywords: Arcobacter butzleri, Toll-like Receptor-4, lipopolysaccharide, lipooligosaccharide, pro-inflammatory immune responses, small intestine, spleen, apoptosis, innate immunity, host-pathogen interactions
Introduction
The motile, spiral-shaped Gram-negative Arcobacter species belong to the Campylobacteraceae family and can be isolated from a plethora of environmental habitats, including surface water. In animals, for instance, Arcobacter spp. form part of the commensal gastrointestinal microbiota [1]. Among the 19 so far described Arcobacter spp., A. butzleri and A. cryaerophilus are regarded as hazards for human health by the International Commission on Microbiological Specifications for Foods [2]. Notaby, detection of Arcobacter spp. may fail in applied microbacteriological routine procedures [3]. Hence, prevalence and incidence of Arcobacter associated human diseases are not known yet and the reported cases rather underestimating the epidemiological situation. Nevertheless, several single clinical cases and outbreaks of arcobacteriosis are documented in the literature to date, hence providing evidence for an important role of Arcobacter spp. in causing intestinal disease [4, 5]. Retrospective studies revealed that Arcobacter spp. are the fourth most common Campylobacterales that could be isolated from diarrheal patients [6–8]. Patients are mostly likely infected by contaminated food or water [5, 9] and present with symptoms of acute gastroenteritis such as abdominal pain, acute diarrhea, or prolonged watery diarrhea for up to 2 months [6, 7]. However, information about the underlying mechanisms of infection and induced host immune responses is scarce. Phenotypic assays revealed adhesive, invasive, and cytotoxic effects of A. butzleri in vitro [10–17]. A. butzleri has been further shown to compromize barrier function in monolayers of the human colon cell line HT-29/B6 pointing towards potential mechanisms by which diarrhea might be induced in humans [18].
We have recently shown that, upon peroral A. butzleri infection, gnotobiotic IL-10–/– mice with a depleted commensal microbiota following broad-spectrum antibiotic treatment [19] could be stably infected by the bacterium and displayed significant small and large intestinal pro-inflammatory immune responses [20, 21]. Remarkably, infection-induced sequelae were not restricted to the intestinal tract, given that even systemic inflammatory responses could be observed in infected mice.
Toll-like receptors (TLRs) are essentially involved in mediating innate immune responses of the host to commensals and pathogens [22]. Since bacterial lipopolysaccharides (LPS) and lipooligosaccharides (LOS) from Gram-negative bacteria are sensed by TLR-4 [22], we investigated the role of TLR-4 in mediating arcobacteriosis in vivo in the present study. To address this, IL-10 deficient mice lacking TLR-4 (TLR-4–/– IL-10–/–) were perorally infected either with A. butzleri reference strain CCUG 30485 (initially isolated from a diseased patient) [23] or C1 (derived from fresh chicken meat) [10], and the colonization and immunopathological properties of the respective bacterial strains were surveyed over time.
Materials and methods
Mice
IL-10–/– mice (in C57BL/10 background, B10) were bred and maintained in the facilities of the “Forschungseinrichtungen für Experimentelle Medizin” (FEM, Charité – Universitätsmedizin, Berlin, Germany) under specific pathogen-free (SPF) conditions. In order to generate TLR-4 IL-10 double deficient (TLR-4–/– IL-10–/–) mice, TLR-4–/– mice (in B10 background) were crossed to IL-10–/– mice and back crossed more than nine generations before use.
To overcome physiological colonization resistance and assure stable pathogenic colonization, gnotobiotic TLR-4–/– IL-10–/– and IL-10–/– mice (with a virtually depleted gastrointestinal microbiota) were generated following broad-spectrum antibiotic treatment as described earlier [19, 24]. In brief, mice were transferred to sterile cages and treated by adding ampicillin–sulbactam (1 g/l; Pfizer, Berlin, Germany), vancomycin (500 mg/l; Hexal, Holzkirchen, Germany), ciprofloxacin (200 mg/l; Hexal), imipenem (250 mg/l; Fresenius Kabi, Graz, Austria), and metronidazole (1 g/l; Braun, Melsungen, Germany) to the drinking water ad libitum starting at 3 weeks of age immediately after weaning and continued for approximately 3 months before the infection experiment [25]. Three days prior infection, the antibiotic cocktail was replaced by sterile tap water (ad libitum). Germfree status of gnotobiotic mice was confirmed as described previously [24].
Arcobacter butzleri infection of mice
Age-matched female gnotobiotic TLR-4–/– IL-10–/– and IL-10–/– mice were perorally infected with approximately 109 viable colony forming units (CFU) of two different A. butzleri strains either (CCUG 30485 or C1 strain, respectively) by gavage in a total volume of 0.3 ml phosphate buffered saline (PBS) on two consecutive days (day 0 and day 1). Naive age- and sex-matched gnotobiotic TLR-4–/– IL-10–/– and IL-10–/– mice served as uninfected controls.
The A. butzleri reference strain CCUG 30485 was initially isolated from a fecal sample derived from a diarrheal patient [23], whereas the C1 strain was isolated from fresh chicken meat [10]. Both A. butzleri strains were grown on Karmali-Agar (Oxoid, Wesel, Germany) for 2 days at 37 °C under microaerobic conditions using CampyGen gas packs (Oxoid) as described earlier [20, 21, 26].
Clinical score
To assess clinical signs of A. butzleri-induced infection on a daily basis, a standardized cumulative clinical score (maximum 12 points), addressing the occurrence of blood in feces (0: no blood; 2: microscopic detection of blood by the Guajac method using Haemoccult, Beckman Coulter/PCD, Krefeld, Germany; 4: overt blood visible), diarrhea (0: formed feces; 2: pasty feces; 4: liquid feces), and the clinical aspect (0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromized locomotion, prefinal aspect), was used [19].
Sampling procedures
Mice were sacrificed by isofluran treatment (Abbott, Greifs wald, Germany) on day six or day 16 postinfection (p.i.). Tissue samples from mesenteric lymphnodes (MLNs), spleen, and ileum were removed under sterile conditions. Absolute small intestinal lengths were determined by measuring the distances from the transition of the stomach to the duodenum to the very distal terminal ileum by a ruler. Ileal ex vivo biopsies from each mouse were collected in parallel for immunohistochemical, microbiological, and immunological analyses. Immunohistopathological changes were determined in ileal samples immediately fixed in 5% formalin and embedded in paraffin. Sections (5 µm) were stained with hematoxylin and eosin (H&E) or respective antibodies for in situ immunohistochemistry as described earlier [25].
Histopathological grading of small intestinal lesions
To evaluate the severity of small intestinal histopathological lesions, an established scoring scheme with minor modifications was applied [27]. In detail, the character of immune cell infiltration (0: none; 1: mononuclear cells; 2: mononuclear cell dominated, fewer neutrophils; 3: neutrophil dominated, fewer mononuclear cells), quantity of immune cell infiltration (0: none; 1: mild; 2: moderate; 3: severe), vertical extent of inflammation (0: none; 1: mucosa; 2: mucosa and muscularis; 3: transmural), and horizontal extent of inflammation (0: no; 1: focal; 2: multifocal; 3: multifocal-coalescent; 4: diffuse) was assessed. The cumulative histologic scoring ranged from 0 to 13 for ileal samples.
Immunohistochemistry
In situ immunohistochemical analysis of ileal paraffin sections was performed as described previously [19, 26, 28–30]. Primary antibodies against cleaved caspase-3 (Asp175, Cell Signaling, Beverly, MA, USA, 1:200), F4/80 (no. 14-4801, clone BM8, eBioscience, 1:50), CD3 (no. N1580, Dako, 1:10), FOXP3 (FJK-16s, eBioscience, San Diego, CA, USA, 1:100), and B220 (eBioscience, 1:200) were used. The average number of positively stained cells within at least six high power fields (HPF, 0.287 mm2, 400× magnification) was determined for each mouse microscopically by a double-blinded investigator.
Quantitative analysis of A. butzleri
Viable A. butzleri was detected in luminal samples derived from the proximal (duodenum) and distal (ileum) part of the small intestines at days 6 or 16 p.i., dissolved in sterile PBS and cultured in serial dilutions on Karmali- and Columbia-Agar supplemented with 5% sheep blood (Oxoid) in parallel for 2 days at 37 °C under microaerobic conditions using CampyGen gas packs (Oxoid). To quantify bacterial translocation, MLNs and spleen (≈1 cm2) were homogenized in 1 ml sterile PBS, streaked onto Karmali-Agar and cultivated accordingly. The respective weights of fecal or tissue samples were determined by the difference of the sample weights before and after asservation. The detection limit of viable pathogens was ≈100 colony forming units (CFU) per g.
Cytokine detection in culture supernatants of ex vivo biopsies taken from ileum, mesenteric lymphnodes, and spleen
Ileal ex vivo biopsies were cut longitudinally and washed in PBS. Spleen, MLNs, or strips of approximately 1 cm2 ileum were placed in 24-flat-bottom well culture plates (Nunc, Wiesbaden, Germany) containing 500 µl serum-free RPMI 1640 medium (Gibco, Life Technologies, Paisley, UK) supplemented with penicillin (100 U/ml) and streptomycin (100 µg/ml; PAA Laboratories). After 18 h at 37 °C, culture supernatants were tested for IFN-γ by the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences, San Jose, CA, USA) on a BD FACSCanto II flow cytometer (BD Biosciences).
Statistical analysis
Medians and levels of significance were determined using Mann-Whitney U test (GraphPad Prism v5, La Jolla, CA, USA). Two-sided probability (P) values ≤ 0.05 were considered significant. Experiments were reproduced twice.
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration number G0184/12). Animal welfare was monitored twice daily by assessment of clinical conditions.
Results
Colonization properties of A. butzleri strains in small intestines of gnotobiotic IL-10–/– mice lacking TLR-4
Gnotobiotic IL-10–/– mice lacking TLR-4 (TLR-4–/– IL-10–/–) and gnotobiotic IL-10–/– control mice with a virtually depleted intestinal microbiota were generated by broad-spectrum antibiotic treatment and perorally infected with A. butzleri strains CCUG 30485 or C1. Six and 16 days following A. butzleri infection bacterial concentrations of either strain did not differ in the proximal (i.e., duodenum) and distal (i.e., ileum) part of the small intestines of TLR-4–/– IL-10–/– and IL-10–/– mice (Fig. 1). Irrespective of the bacterial strain and time point p.i., mice of either genotype harbored approximately 103 to 105 CFU per gram A. butzleri in their ileal lumen (Fig. 1B). Notably, neither in MLN nor in spleen, viable A. butzleri could be detected in infected mice of either genotype at days 6 or 16 p.i. by direct plating (not shown).
Fig. 1.
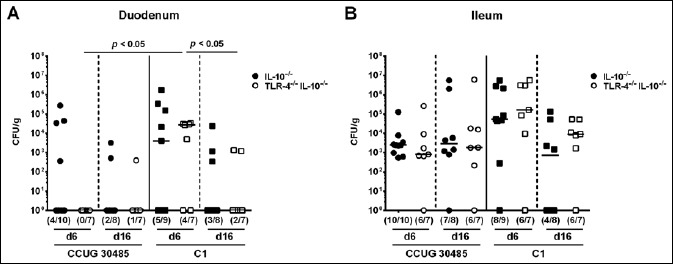
Colonization of Arcobacter butzleri in the small intestines of gnotobiotic TLR-4–/– IL-10–/– mice following peroral infection. Gnotobiotic TLR-4–/– IL-10–/– (open symbols) and IL-10–/– control mice (closed symbols) were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles) or strain C1 (squares). A. butzleri loads were determined in luminal samples derived from the (A) duodenum and (B) ileum at days 6 or 16 p.i. and expressed as colony forming units (CFU) per gram (g) sample. Medians (black bars), numbers of mice harboring the pathogen out of the total number of analyzed animals (in parentheses), and levels of significance (p-value) determined by Mann–Whitney U test are indicated. Data shown were pooled from three independent experiments
Clinical aspects and intestinal apoptosis induced by A. butzleri in gnotobiotic IL-10–/– mice lacking TLR-4
Despite intestinal colonization, however, infected mice of either genotype did not exhibit any clinical signs of enteric disease such as wasting, diarrhea, or occurence of blood in stool, neither early (i.e., at day 6 p.i.) nor later (i.e., day 16 p.i.) in the course of A. butzleri infection with either strain (not shown).
Since intestinal inflammation leads to significant shortening of the intestinal tract [19, 24], we assessed small intestinal lengths at days of necropsy. Neither at day 6 nor at day 16 following A. butzleri infection with either strain, shortening of the small intestines in infected gnotobiotic TLR-4–/– IL-10–/– or IL-10–/– mice could be observed when compared to uninfected control animals (not shown), thus, further supporting absence of macroscopic disease.
We next surveyed potential microscopic intestinal sequelae of infection in H&E stained small intestinal paraffin sections applying a standardized histopathological scoring system. Histopathological scores did not differ between naive and infected TLR-4–/– IL-10–/– and IL-10–/– mice, irrespective of the applied strain and time point p.i. (not shown).
Given that apoptosis is a commonly used diagnostic marker in the histopathological evaluation and grading of intestinal inflammation and a hallmark of C. jejuni-induced enterocolitis in gnotobiotic IL-10–/– mice [19], we next assessed numbers of caspase-3+ cells within the small intestinal mucosa of infected mice. Numbers of apoptotic ileal epithelial cells increased in IL-10–/– mice until day 16 following infection with either strain (p < 0.005; Fig. 2A and B). However, TLR-4–/– IL-10–/– mice exhibited lower apoptotic cell numbers than IL-10–/– control animals at day 16 following A. butzleri strain CCUG 30485 infection (p < 0.005; Fig. 2A; Fig. S1A). Furthermore, apoptotic cell numbers were lower in the ileal mucosa of TLR-4–/– IL-10–/– as compared to IL-10–/– mice at days 6 and 16 following C1 strain infection (p < 0.05; Fig. 2B), and relative infection-induced increases were less pronounced in mice of the former genotype (Fig. S1B). Taken together, despite lack of clinical and histopathological changes, TLR-4 gene deficiency was associated with lower abundances of ileal apoptotic cells during A. butzleri infection with either strain.
Fig. 2.
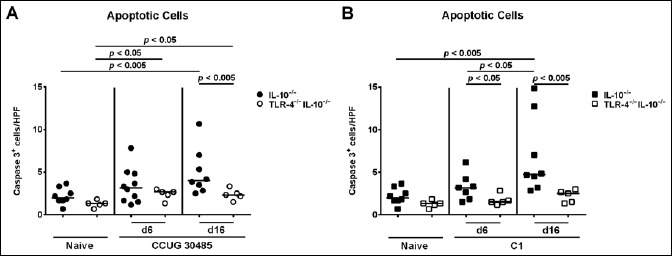
Kinetics of apoptotic cells in ileal tissue of gnotobiotic TLR-4–/– IL-10–/– mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open symbols) and IL-10–/– mice (closed symbols) were generated by antibiotic treatment and perorally infected either with A. butzleri (A) strain CCUG 30485 (circles) or (B) strain C1 (squares). Uninfected mice served as negative controls (Naive). The average numbers of apoptotic cells (positive for caspase-3, Casp3) from at least six high power fields (HPF, 400× magnification) per mouse were determined microscopically in immunohistochemically stained ileal sections at days 6 or 16 p.i. Medians (black bars) and levels of significance (p-value) determined by Mann–Whitney U test are indicated. Data shown were pooled from three independent experiments
Fig. S1.

Relative increases in adaptive and innate immune cell populations and apoptotic cells in ileal tissue of gnotobiotic TLR-4–/– IL-10–/– mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open bars) and IL-10–/– mice (closed bars) were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 or strain C1 as indicated. Uninfected mice served as controls. The average number of (A) apoptotic cells (positive for caspase-3, Casp3+), (B) macrophages and monocytes (F4/80+), (C) T lymphocytes (CD3+), (D) regulatory T cells (Tregs, FOXP3+), and (E) B lymphocytes (B220+) per animal was determined microscopically in immunohistochemically stained ileal section at days 6 or 16 p.i. Respective bars indicate fold increases of respective cell abundance upon infection. Data shown were pooled from three independent experiments
Role of TLR-4 in small intestinal inflammation induced by A. butzleri in gnotobiotic IL-10–/– mice
Given that recruitment of innate and adaptive immune cells as well as of effector cells to sites of inflammation is a hallmark of enteric infection including campylobacteriosis, we next quantitatively determined distinct immune cell populations by in situ immunohistochemical staining of small intestinal paraffin sections. Interestingly, already in the naive state, TLR-4–/– IL-10–/– mice exhibited lower numbers of F4/80+ macrophages and monocytes as compared to IL-10–/– mice (p < 0.005; Fig. 3), which also held true at day 6 following infection with either strain (p < 0.0005 and p < 0.005, respectively; Fig. 3). Six and 16 days following A. butzleri strain C1 infection, F4/80+ cell numbers increased in the ileal mucosa of TLR-4–/– IL-10–/– mice (p < 0.05 and p < 0.005, respectively; Fig. 3B), whereas ileal macrophages and monocytes increased as early as 6 days following A. butzleri CCUG 30485 strain infection in TLR-4–/– IL-10–/– mice only (p < 0.05; Fig. 3A). As compared to uninfected mice, numbers of CD3+ T lymphocytes were higher in small intestines of IL-10–/– than TLR-4–/– IL-10–/– mice at days 6 and 16 p.i. with either strain (p < 0.05–0.0005; Fig. 4A). Furthermore, A. butzleri induced relative increases in T lymphocytes were less pronounced in TLR-4–/– IL-10–/– as compared to IL-10–/– mice (Fig. S1C). Ileal Treg numbers increased in IL-10–/– but not TLR-4–/– IL-10–/– mice until days 6 and 16 following A. butzleri CCUG 30485 strain infection (p < 0.05; Fig. 4B, left panel; Fig. S1D). At day 16, but not day 6 following A. butzleri C1 strain infection, only IL-10–/– mice displayed higher Tregs in their small intestines as compared to uninfected control animals (p < 0.05; Fig. 4B, right panel; Fig. S1D). Furthermore, Treg numbers were higher in small intestines of IL-10–/– mice at day 6 following A. butzleri CCUG 30485 strain as compared to C1 strain infection (p < 0.05; Fig. 4B). As for the innate F4/80+ cells, naive as well as infected TLR-4–/– IL-10–/– mice exhibited lower ileal B220+ B lymphocytes as compared to respective IL-10–/– control mice (p < 0.05– 0.0001; Fig. 4C). Only 16 days following A. butzleri strain C1 infection, elevated B220+ cells could be determined in the ilea of IL-10–/– mice (p < 0.005; Fig. 4C, right panel; Fig. S1E) that were significantly higher as compared to those observed in A. butzleri CCUG 30485 strain infected mice at day 6 (p < 0.01; Fig. 4C). Taken together, TLR-4–/– IL-10–/– mice exhibited lower numbers of ileal innate and adaptive immune cell populations following A. butzleri infection with either strain.
Fig. 3.
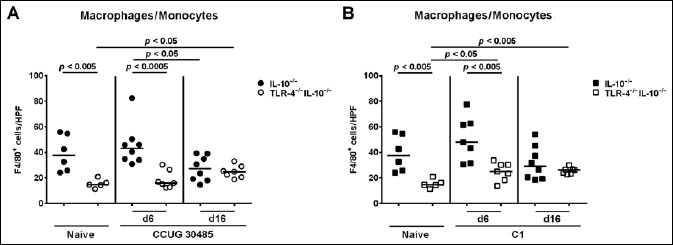
Kinetics of innate immune cell influx into ileal tissue of gnotobiotic TLR-4–/– IL-10–/– mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open symbols) and IL-10–/– mice (closed symbols) were generated by antibiotic treatment and perorally infected either with A. butzleri (A) strain CCUG 30485 (circles) or (B) strain C1 (squares). Uninfected mice served as negative controls (naive). The average numbers of macrophages and monocytes (positive for F4/80) from at least six high power fields (HPF, 400× magnification) per mouse were determined microscopically in immunohistochemically stained ileal sections at days 6 or 16 p.i. Medians (black bars) and levels of significance (p-value) determined by Mann–Whitney U test are indicated. Data shown were pooled from three independent experiments
Fig. 4.
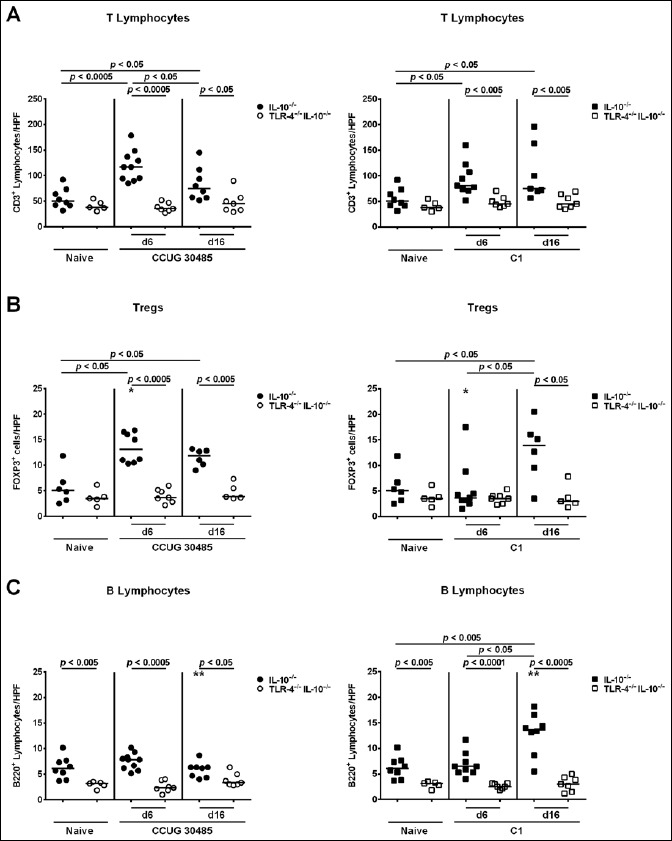
Kinetics of adaptive immune cell influx into ileal tissue of gnotobiotic TLR-4–/– IL-10–/– mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open symbols) and IL-10–/– mice (closed symbols) were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 (circles; left panels) or strain C1 (squares; right panels). Uninfected mice served as negative controls (Naive). The average number of cells positive for (A) CD3 (T lymphocytes), (B) FOXP3 (regulatory T cells, Tregs), and (C) B220 (B lymphocytes) per mouse was determined microscopically in immunohistochemically stained ileal sections at days 6 or 16 p.i. Medians (black bars) and levels of significance (p-value) determined by Mann–Whitney U test are indicated. Significant differences between A. butzleri strains are indicated by stars within the graph (*p < 0.05, **p < 0.01). Data shown were pooled from three independent experiments
Small intestinal and systemic pro-inflammatory cytokine levels in A. butzleri-infected gnotobiotic IL-10–/– mice lacking TLR-4
We next measured concentrations of the pro-inflammatory cytokine IFN-γ in supernatants of ex vivo biopsies derived from ileum, MLN, and spleen. Irrespective of the applied strain, ileal IFN-γ concentration significantly increased as early as 6 days following A. butzleri infection of IL-10–/–, but not TLR-4–/– IL-10–/– mice (p < 0.005 and p < 0.05, respectively; Fig. 5, Fig. S2A). At day 6 p.i., IFN-γ concentrations were higher in small intestines of A. butzleri CCUG 30485 strain as compared to C1 strain infected IL-10–/– mice (p < 0.05; Fig. 5). Six days following A. butzleri CCUG 30485 strain infection, IFN-γ levels increased in MLNs of mice of either genotype, but less distinctly in IL-10–/– mice lacking TLR-4 (p < 0.005 and p < 0.05; Fig. 6A, Fig. S2B). There was, however, a trend towards lower IFN-γ concentrations in MLN of IL-10–/– mice lacking TLR-4 as compared to IL-10–/– control animals (n.s.; Fig. 6A). Interestingly, no significant increases of the pro- inflammatory cytokine could be observed in MLN at either time point following A. butzleri C1 strain infection, ir respective of the genotype of mice (Fig. 6B). Remarkably, pro-inflammatory cytokine responses were not restricted to the intestinal tract, given that IFN-γ concentrations significantly increased in spleens of IL-10–/– versus TLR-4–/– IL-10–/– mice as early as 6 days following CCUG 30485 strain infection (p < 0.005; Fig. 7A, Fig. S2C), but declined back to naive levels until day 16 p.i. (Fig. 7A). At day 6 p.i., splenic IFN-γ concentrations were lower in C1 strain as compared to CCUG 30485 strain infected IL-10–/– mice (p < 0.05; Fig. 7). Interestingly, IFN-γ concentrations were higher in spleens of TLR-4–/– IL-10–/– as compared to IL-10–/– mice at day 16 following A. butzleri infection with either strain (p < 0.005; Fig. 7, Fig. S2C). In TLR-4–/– IL-10–/– mice, however, splenic IFN-γ levels measured at day 16 p.i. did not differ from uninfected, naive mice (Fig. 7). Taken together, A. butzleri-induced IFN-γ production was strain dependent, but less pronounced in the small intestinal compartment of IL-10–/– mice lacking TLR-4–/–.
Fig. 5.
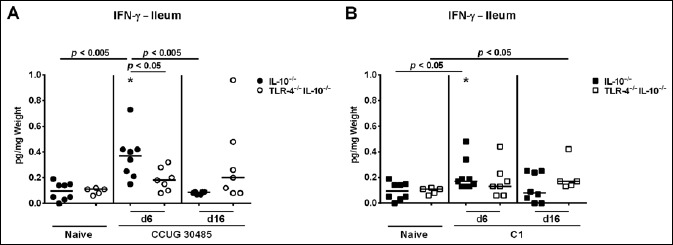
Kinetics of pro-inflammatory cytokine responses in the ileum of gnotobiotic TLR-4–/– IL-10–/– mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open symbols) and IL-10–/– mice (closed symbols) were generated by antibiotic treatment and perorally infected either with A. butzleri (A) strain CCUG 30485 (circles) or (B) strain C1 (squares). Uninfected mice served as negative controls (Naive). IFN-γ concentrations (pg per mg ileal tissue) were determined in supernatants of ex vivo ileal biopsies taken at days 6 or 16 p.i. Medians (black bars) and significance levels as determined by the Mann–Whitney U test are indicated. Significant differences between A. butzleri strains are indicated by stars within the graph (*p < 0.05). Data shown were pooled from three independent experiments
Fig. S2.

Relative increases in intestinal and systemic IFN-γ concentrations in gnotobiotic TLR-4–/– IL-10–/–- mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open bars) and IL-10–/– mice (closed bars) were generated by antibiotic treatment and perorally infected either with A. butzleri strain CCUG 30485 or strain C1 as indicated. Uninfected mice served as controls. IFN-γ concentrations were determined in supernatants of ex vivo biopsies taken from (A) ilea, (B) mesenterial lymphnodes (MLN), and (C) spleen at days 6 or 16 p.i. Bars indicate fold increases of IFN-γ levels in respective compartments upon infection. Data shown were pooled from three independent experiments
Fig. 6.

Kinetics of pro-inflammatory cytokine responses in mesenetric lymphnodes of gnotobiotic TLR-4–/– IL-10–/– mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open symbols) and IL-10–/– mice (closed symbols) were generated by antibiotic treatment and perorally infected either with A. butzleri (A) strain CCUG 30485 (circles) or (B) strain C1 (squares). Uninfected mice served as negative controls (Naive). IFN-γ concentrations (pg per mg tissue) were determined in supernatants of ex vivo biopsies taken from mesenterial lymphnodes (MLN) at days 6 or 16 p.i. Medians (black bars) and significance levels as determined by the Mann– Whitney U test are indicated. Significant differences between A. butzleri strains are indicated by stars within the graph (**p < 0.01). Data shown were pooled from three independent experiments
Fig. 7.
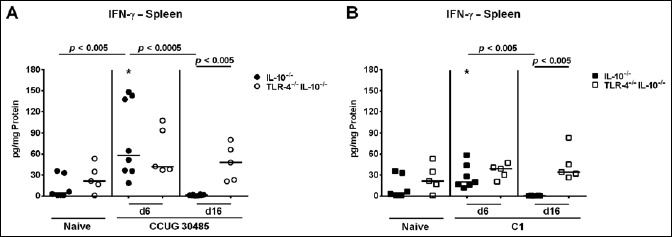
Kinetics of pro-inflammatory cytokine responses in spleens of gnotobiotic TLR-4–/– IL-10–/– mice following A. butzleri infection. Gnotobiotic TLR-4–/– IL-10–/– (open symbols) and IL-10–/– mice (closed symbols) were generated by antibiotic treatment and perorally infected either with A. butzleri (A) strain CCUG 30485 (circles) or (B) strain C1 (squares). Uninfected mice served as negative controls (Naive). IFN-γ concentrations (pg per mg total protein) were determined in supernatants of ex vivo splenic biopsies taken at days 6 or 16 p.i. Medians (black bars) and significance levels as determined by the Mann–Whitney U test are indicated. Significant differences between A. butzleri strains are indicated by stars within the graph (*p < 0.05). Data shown were pooled from three independent experiments
Discussion
The immunopathological impact of A. butzleri infection in vivo is under current debate. Data regarding the molecular mechanisms underlying potential host interactions with this emerging pathogen are lacking due to the scarcity of appropriate in vivo models [20]. In the present study, we have applied our gnotobiotic (i.e., secondary abiotic) IL-10–/– mouse infection model in order to unravel the role of TLR-4, the major innate immune receptor for sensing of LPS and LOS derived from the cell wall of Gram-negative bacteria, in murine Arcobacter infection. Both gnotobiotic IL-10–/– mice lacking TLR-4 and gnotobiotic IL-10–/– control animals harbored comparable A. butzleri loads in their proximal (i.e., duodenum) and distal (i.e., ileum) small intestines following peroral infection. The small intestinal colonization potential of A. butzleri was strain-independent, given that either strain colonized the proximal as well as distal small intestines of gnotobiotic mice at comparably high concentrations. Interestingly, A. butzleri strain-dependent differences in infection-induced immune cell responses could be observed in IL-10–/– control, but not IL-10–/– mice lacking TLR-4. For instance, small intestinal Treg numbers were lower at day 6 following C1 versus CCUG 30485 strain infection, whereas at day 16 p.i., B cell counts were higher in ilea of C1 as compared to CCUG 30485 strain-infected IL-10–/– mice. Remarkably, TLR-4 was essential for A. butzleri-induced ileal apoptosis and inflammatory immune cell responses as indicated by lower numbers of ileal T lymphocytes, Tregs and B cells as well as less distinct small intestinal pro-inflammatory IFN-γ secretion in infected IL-10–/– mice lacking TLR-4. Surprisingly, splenic IFN-γ concentrations were even higher in TLR-4–/– IL-10–/– as compared to IL-10–/– control mice 16 but not 6 days following A. butzleri infection with either strain. Notably, viable bacteria could not be detected in extra-intestinal and systemic compartments including the spleen by direct plating in infected mice of either genotype. It is tempting to speculate that, in the course of the systemic immune response upon infection, more immune cells such as dendritic cells and lymphocytes might have been imprinted and activated in the spleen of IL-10–/– mice lacking TLR-4 as compared to control mice. The TLR-4 dependence of local (i.e., small intestinal) pro-inflammatory immune responses in A. butzleri infected mice is well in line with results from our C. jejuni in vivo studies demonstrating that C. jejuni-induced colonic and extra-intestinal disease is mediated by TLR-4 signalling of pathogenic LOS [19, 26, 31]. The structure of the carbohydrate backbone of LOS has been successfully characterized in the halophilic bacterium A. halophilus [32]. To date, however, molecular structures of neither LPS nor LOS have been identified in A. butz leri. It is highly likely, however, that, besides TLR-4, other so far unknown pattern recognition factors including TLR and NOD-like receptors are involved in mediating Arcobacter infection. For murine C. jejuni infection, for instance, we were able to demonstrate that, in addition to TLR-4- dependent signalling of C. jejuni LOS, sensing of pathogenic lipoproteins and CpG-DNA by TLR-2 and -9, respectively, contributed to infection-induced pro-inflammatory sequelae [19, 26].
Taken together, we here show that TLR-4 is essential for pro-inflammatory immune responses induced by A. butzleri in the small intestinal tract of infected mice.
In conclusion, further in vivo studies should further elucidate the molecular mechanisms underlying pathogen–host interactions in arcobacteriosis.
Acknowledgements
We thank Michaela Wattrodt, Ursula Rüschendorf, Silvia Schulze, Alexandra Bittroff-Leben, Ines Puschendorf, Ulrike Hagen, Uwe Lohmann, Gernot Reifenberger, and the staff of the animal research facility of the Charité – University Medicine Berlin for excellent technical assistance and animal breeding.
References
- 1.Ho HT, Lipman LJ, Gaastra W: Arcobacter, what is known and unknown about a potential foodborne zoonotic agent! Vet Microbiol 115(1–3), 1-13 (2006) [DOI] [PubMed] [Google Scholar]
- 2.ICMSF ICoMSfF (2002). In: Microbiological Testing in Food Safety Management, 7, ed. Tompkin RB, Kluwer Academic/Plenum Publishers, New York, NY, p. 171. [Google Scholar]
- 3.Ferreira S, Queiroz JA, Oleastro M, Domingues FC: Insights in the pathogenesis and resistance of Arcobacter: a review. Crit. Rev. Microbiol. 1-20 (2015) [DOI] [PubMed] [Google Scholar]
- 4.Collado L, Figueras MJ: Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev 24(1), 174–192 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lappi V, Archer JR, Cebelinski E, Leano F, Besser JM, Klos RF, et al. : An outbreak of foodborne illness among attendees of a wedding reception in Wisconsin likely caused by Arcobacter butzleri. Foodborne Pathog Dis 10(3), 250–255 (2013) [DOI] [PubMed] [Google Scholar]
- 6.Van den Abeele AM, Vogelaers D, Van Hende J, Houf K: Prevalence of Arcobacter species among humans, Belgium, 2008–2013. Emerg Infect Dis 20(10), 1746–1749 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenberg O, Dediste A, Houf K, Ibekwem S, Souayah H, Cadranel S, et al. : Arcobacter species in humans. Emerg Infect Dis 10(10), 1863–1867 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prouzet-Mauleon V, Labadi L, Bouges N, Menard A, Megraud F: Arcobacter butzleri: underestimated enteropathogen. Emerg Infect Dis 12(2), 307–309 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong TT, Mansfield LS, Wilson DL, Schwab DJ, Molloy SL, Rose JB: Massive microbiological groundwater contamination associated with a waterborne outbreak in Lake Erie, South Bass Island, Ohio. Environ Health Perspect 115(6), 856–864 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karadas G, Sharbati S, Hanel I, Messelhausser U, Glocker E, Alter T, et al. : Presence of virulence genes, adhesion and invasion of Arcobacter butzleri. J Appl Microbiol 115(2), 583–590 (2013) [DOI] [PubMed] [Google Scholar]
- 11.Levican A, Alkeskas A, Günter C, Forsyth SJ, Figueras MJ: The adherence and invasion of human intestinal cells by Arcobacter species and their virulence genotype. Appl Environ Microbiol 79(16), 4951–4957 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karadas G, Bucker R, Sharbati S, Schulzke JD, Alter T, Golz G: Arcobacter butzleri isolates exhibit pathogenic potential in intestinal epithelial cell models. J Appl Microbiol. Oct 19 doi: 10.1111/jam.12979 [Epub ahead of print] (2015) [DOI] [PubMed] [Google Scholar]
- 13.Golla SC, Murano EA, Johnson LG, Tipton NC, Cureington EA, Savell JW: Determination of the occurrence of Arcobacter butzleri in beef and dairy cattle from Texas by various isolation methods. J Food Prot 65(12), 1849–1853 (2002) [DOI] [PubMed] [Google Scholar]
- 14.Musmanno RA, Russi M, Lior H, Figura N: In vitro virulence factors of Arcobacter butzleri strains isolated from superficial water samples. New Microbiol 20(1), 63–68 (1997). [PubMed] [Google Scholar]
- 15.Carbone M, Maugeri TL, Giannone M, Gugliandolo C, Midiri A, Fera MT: Adherence of environmental Arcobacter butzleri and Vibrio spp. isolates to epithelial cells in vitro. Food Microbiol 20(5), 611–616 (2003) [Google Scholar]
- 16.Villarruel-Lopez A, Marquez-Gonzalez M, Garay-Martinez LE, Zepeda H, Castillo A, de la Garza Mota L, et al. : Isolation of Arcobacter spp. from retail meats and cytotoxic effects of isolates against vero cells. J Food Prot 66(8), 1374–1378 (2003) [DOI] [PubMed] [Google Scholar]
- 17.Gugliandolo C, Irrera GP, Lentini V, Maugeri TL: Pathogenic Vibrio, Aeromonas and Arcobacter spp. associated with copepods in the Straits of Messina (Italy). Mar Pollut Bull 56(3), 600–666 (2008) [DOI] [PubMed] [Google Scholar]
- 18.Bucker R, Troeger H, Kleer J, Fromm M, Schulzke JD: Arcobacter butzleri induces barrier dysfunction in intestinal HT-29/B6 cells. J Infect Dis 200(5), 756–764 (2009) [DOI] [PubMed] [Google Scholar]
- 19.Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, Gobel UB, et al. : Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10−/− mice via Toll-like-receptor -2 and -4 signaling. PLoS One 7(7), e40761(2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golz G, Karadas G, Alutis ME, Fischer A, Kuhl AA, Breithaupt A, et al. : Arcobacter butzleri Induce colonic, extra-intestinal and systemic inflammatory responses in gnotobiotic IL-10 deficient mice in a strain-dependent manner. PLoS One 10(9), e0139402 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimesaat MM, Karadas G, Alutis M, Fischer A, Kuhl AA, Breithaupt A, et al. : Survey of small intestinal and systemic immune responses following murine Arcobacter butzleri infection. Gut Pathog 7, 28 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janeway CA, Jr., Medzhitov R: Innate immune recognition. Annu Rev Immunol 20, 197-216 (2002) [DOI] [PubMed] [Google Scholar]
- 23.Vandamme P, Pugina P, Benzi G, Van Etterijck R, Vlaes L, Kersters K, et al. : Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. J Clin Microbiol 30(9), 2335–2337 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, et al. : Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol 177(12), 8785–8795 (2006) [DOI] [PubMed] [Google Scholar]
- 25.Heimesaat MM, Lugert R, Fischer A, Alutis M, Kuhl AA, Zautner AE, et al. : Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PLoS One 9(2), e90148 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, et al. : Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One 6(6), e20953 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN: Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterol 116(5), 1107–1114 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, et al. : MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59(8), 1079–1087 (2010) [DOI] [PubMed] [Google Scholar]
- 29.Haag LM, Fischer A, Otto B, Grundmann U, Kuhl AA, Gobel UB, et al. : Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur J Microbiol Immunol (Bp) 2(1), 2–11 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimesaat MM, Haag LM, Fischer A, Otto B, Kuhl AA, Gobel UB, et al. : Survey of extra-intestinal immune responses in asymptomatic long-term Campylobacter jejuni-infected mice. Eur J Microbiol Immunol (Bp) 3(3), 174–182 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto B, Haag LM, Fischer A, Plickert R, Kuhl AA, Gobel UB, et al. : Campylobacter jejuni induces extra-intestinal immune responses via Toll-like-receptor-4 signaling in conventional IL-10 deficient mice with chronic colitis. Eur J Microbiol Immunol (Bp) 2(3), 210–219 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silipo A, Sturiale L, Perino V, Garozzo D, Lanzetta R, Parrilli M, et al. : The structure of the carbohydrate backbone of the lipooligosaccharide from the halophilic bacterium Arcobacter halophilus. Carbohydr Res 345(6), 850–853 (2010) [DOI] [PubMed] [Google Scholar]


