Abstract
Background
MicroRNA regulates mammalian cell growth in terms of its proliferation and apoptosis by controlling the expression of target genes. MiRNA-323-5p plays an important role in regulating cell growth and death within various types of cells. The function of miRNA-323-5p and its possible molecular mechanism in human cerebral glioma U373 cells remains to be further confirmed. The aim of this study was to investigate the regulation function of miRNA-323-5p in human glioma U373 cell growth, proliferation, and apoptosis.
Material/Methods
We used human cerebral glioma U373 cells as the cell model; utilized liposome technology (transfected by Lipofectamine2000) in human cerebral glioma U373 cells to over-express miRNA-323-5p (microRNA used as control group); and selected MTT assay and flow cytometry to detect cell growth, proliferation, and apoptosis. We used RT-PCR and Western blotting techniques to study the expression levels of target insulin-like growth factor 1 (IGF-1) receptor protein in U373 cells transfected with miRNA-323-5p. We used liposome transfection techniques in human cerebral glioma U373 cells to over-express or processed knockdown of IGF-1R by siRNA, and then transferred with miRNA-323-5p, thereby investigating the treated human cerebral glioma U373 cells apoptosis situations.
Results
The over-expression of miRNA-323-5p inhibited the growth and proliferation of human cerebral glioma U373 cells and promoted its apoptosis. The over-expression of miRNA-323-5p also reduced the IGF-1R level. After processing the knockdown of IGF-1R and then transfection with miRNA-323-5p, U373 cells had enhanced apoptosis. The over-expression of IGF-1R inhibited the cells apoptosis induced by miRNA-323-5p.
Conclusions
MiRNA-323-5p inhibited human cerebral glioma U373 cell proliferation and promoted its apoptosis by reducing IGF-1R.
MeSH Keywords: BALB 3T3 Cells, MicroRNAs, Somatomedins, Technetium Tc 99m Aggregated Albumin
Background
Changes in lifestyle and rising living standards have contributed to increased incidence of cerebral glioma and its 2-year mortality rate, which is a serious threat to human quality of life and wellbeing [1]. At present, in China, treatments for related diseases mainly include surgery, radiation therapy, chemotherapy, and traditional Chinese medicine therapy. [2]. Compared with other tumor treatments, the molecular biology and gene therapy treatments for cerebral gliomas are very limited [3], mainly because the molecular mechanisms of the occurrence and the development of cerebral gliomas has yet to be discovered. Therefore, the study of pathogenesis of gliomas at the molecular and cellular levels has both theoretical and practical values. The purpose of this paper is to discuss the possibilities to treat cerebral gliomas with gene therapy in terms of microRNA-targeted therapy. MicroRNA is a small non-coding RNA, which regulates cell growth, proliferation, apoptosis, signal transduction, and autophagy [4]. Researchers are most likely to study miRNA126, miRNA397, miRNA-30e, miR-483-3p, MiR-221/222, miR148/152, miRNA-23a, miR-181a, and miR-150 [5,6]. At present, there are relatively few studies related to the functions of miRNA-323-5p in cell growth, proliferation, and apoptosis [7]. This article discusses whether miRNA-323-5p regulates cell proliferation and death by its regulation function of target gene expression, directly or indirectly.
Target insulin-like growth factor 1 (IGF-1) receptor protein is widely expressed at the cell surface [8]; it regulates cell growth, proliferation, and apoptosis through its combination with ligands in terms of target insulin-like growth factor (IGF) receptor proteins [9]. At present, there is relatively little research focused on whether target insulin-like growth factor (IGF) receptor proteins coordinate with microRNA to regulate cell growth and apoptosis.
The potential regulatory mechanisms and their possible molecular mechanisms of miRNA-323-5p in human cerebral glioma U373 cells are still unknown. Therefore, the purpose of this paper is to explore the role of miRNA-323-5p in regulating human cerebral glioma U373 cells growth, proliferation, and apoptosis.
Material and Methods
Reagents and cells
MTT cell survival and growth detection reagent [3-(4,5-dimethyl-2-thiazolyl) -2,5-diphenyl-2-H-tetrazolium bromide] was purchased from Sigma (United States). Both monoclonal antibody of anti-IGF-1R protein in mice and goat anti-mouse secondary antibody with horseradish peroxidase-labeled IgG were purchased from Santa Cruz (United States). The monoclonal mouse anti-human actin antibodies were purchased from Sigma (United States). Cell culture media DMEM and FBS were both purchased from Santa Cruz (United States). Human cerebral glioma cell line U373 was purchased from the American Type Culture Collection (ATCC). RT-PCR kits were purchased from Biyuntian Institute of Biotechnology. Reagents FITC-Annexin-V for the detection of phosphatidylserine valgus and reagents kits for the detection of Caspase-3 activity were purchased from Beijing Dingguo Biotechnology (Beijing, China). MiRNA-323-5p and its control group (miRNA) were designed and synthesized by Jima Biotechnology (Shanghai, China). We obtained ethics approval from the Ethics Committee of Shandong University.
Cell culture
Cell culture used a previously reported method [10], in which human cerebral glioma cell line U373 was cultured at 37°C in the incubator with 5% CO2.
MTT test
MTT testing was performed as per the literature to determine cell growth and activity [11,12].
IGF-1R siRNA transfection
siRNA was designed and synthesized to transfect IGF-1R (nucleotide sequence is: (5′-3′)R TTGTTACGACTTGGTTACC\F CCTTTGATTGGCTCAAGAG), transfected with siRNA to U373 cells in according with the reference [13].
Flow cytometry
Flow cytometry techniques were applied to detect the phosphatidylserine valgus situation of U373 cells in each group [14].
Colony formation test
We utilized means to detect the colony-forming ability of U37 cells in each group [15].
RT-PCR
We used means to process RNA extraction and RT-PCR detection testing [4]. We collected cells, used RT-PCR instructions to extract RNA and then reverse transcript into cDNA, used the cDNA as template. We used IGF-1R and internal actin primers to run RT-PCR and agarose gel electrophoresis tests.
Western blot
We used means process Western blot testing [16]. We resuspended the cells once in cell lysates (contains protease inhibitors) to prepare cell lysate samples for Western blot testing. We treated with primary antibodies in anti-IGF-1R protein antibody and anti-Actin antibody, incubated secondary antibody in goat anti-mouse, developed and fixed, and analyzed IGF-1R protein levels in U373 cells in each group.
Caspase-3 activity assay
We used a caspase-3 activity assay kit from Biyuntian Institute of Biotechnology to detect and analyze caspase-3 activity for U373 cells in each group [17]. We collected U373 cells in each group, resuspended the cells in cell lysates in the kit, then added Ac-DEVD-pNA (2 mM) and mixed evenly, incubating at 37°C for 60 min. Reactants were placed in microplates to detect its absorption of 490-nm light. We set absorption values of cells in the control group as standard 1, then detected and calculated relative value of other cells samples to indentify caspase-3 activity in each group.
Statistical methods
We used SPSS 15.0 to count all statistical data, and measured data in mean ± standard deviation (χ̄±s) format. Comparisons between U373 from each group were tested by one-way analysis of variance [13]. P<0.05 indicated a statistically significant difference and P<0.01 indicated an extremely statistically significant difference.
Results
MiRNA-323-5p transfection inhibited the growth and proliferation of U373 cells
MTT test results indicated that, compared with U373 cells transfected with 1 μg miRNA in the control group, the growth and activity of U373 cells transfected with 1 μg miRNA-323-5p were significantly reduced (P=0.0027), as shown in Figure 1.
Figure 1.
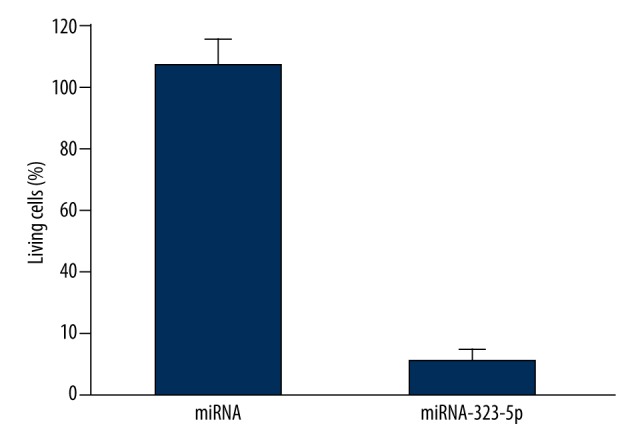
miRNA-323-5p transfection inhibited the growth of U373 cells.
As shown in Figure 2, the result of colony formation test showed that, compared with U373 cells transfected with 1 μg miRNA in the control group, the colony-forming ability of U373 cells transfected with 1 μg miRNA-323-5p were significantly reduced (P=0.012).
Figure 2.

miRNA-323-5p transfection inhibited the proliferation of U373 cells.
MiRNA-323-5p Transfection induced the apoptosis of U373 cells
As shown in Figure 3, the result of flow cytometry showed that, compared with U373 cells transfected with 1 μg miRNA in the control group, the phosphatidylserine valgus situation of U373 cells transfected with 1 μg miRNA-323-5p were significantly increased (P=0.0073).
Figure 3.
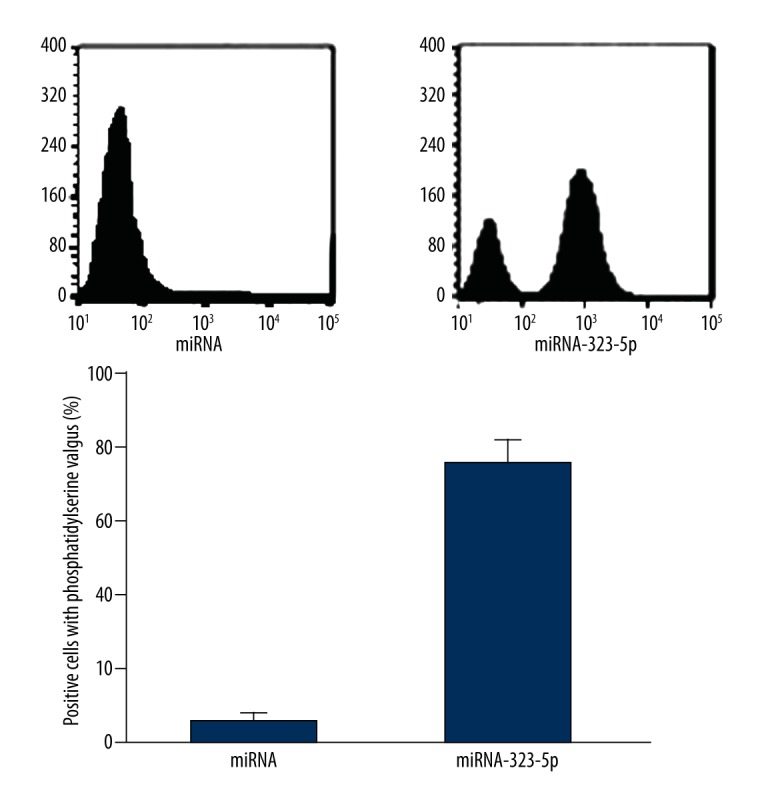
miRNA-323-5p transfection induced the apoptosis of U373 cells.
As shown in Figure 4, the result of caspase-3 activity assay showed that, compared with U373 cells transfected with 1 μg miRNA in the control group, the activation of caspase-3 in U373 cells transfected with 1 μg miRNA-323-5p were significantly enhanced (P=0.018).
Figure 4.
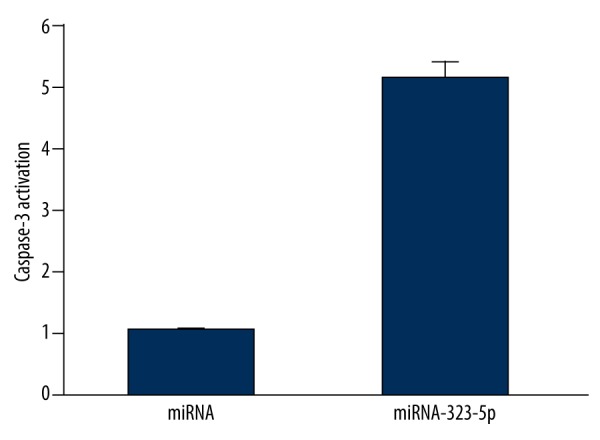
miRNA-323-5p transfection induced the caspase-3 activation in U373 cells.
MiRNA-323-5p transfection decreased the mRNA and protein levels of IGF-1R
As shown in Figure 5, the result of RT-PCR and Western blot showed that, compared with U373 cells transfected with 1 μg miRNA in the control group, the mRNA and protein levels of IGF-1R in U373 cells transfected with 1 μg miRNA-323-5p were significantly decreased.
Figure 5.
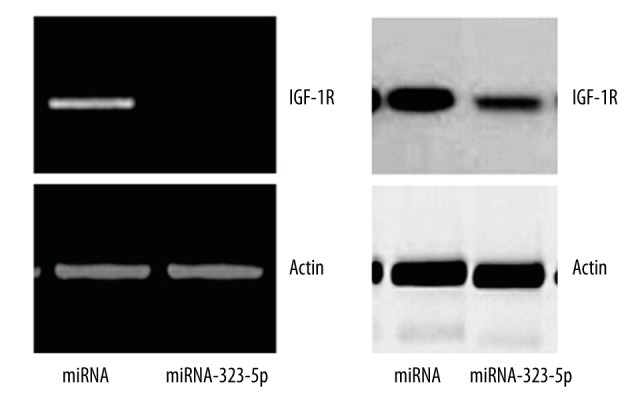
miRNA-323-5p transfection decreased the mRNA and protein levels of IGF-1R.
Knockdown of IGF-IR enhanced the apoptosis of U373 cells induced nu miRNA-323-5p
As shown in Figure 6, the result of caspase-3 activity assay (after knockdown of IGF-IR by siRNA) showed that, compared with U373 cells transfected with 1 μg miRNA in control group, the activation of caspase-3 in U373 cells transfected with 1 μg miRNA-323-5p were significantly enhanced (P=0.0049).
Figure 6.
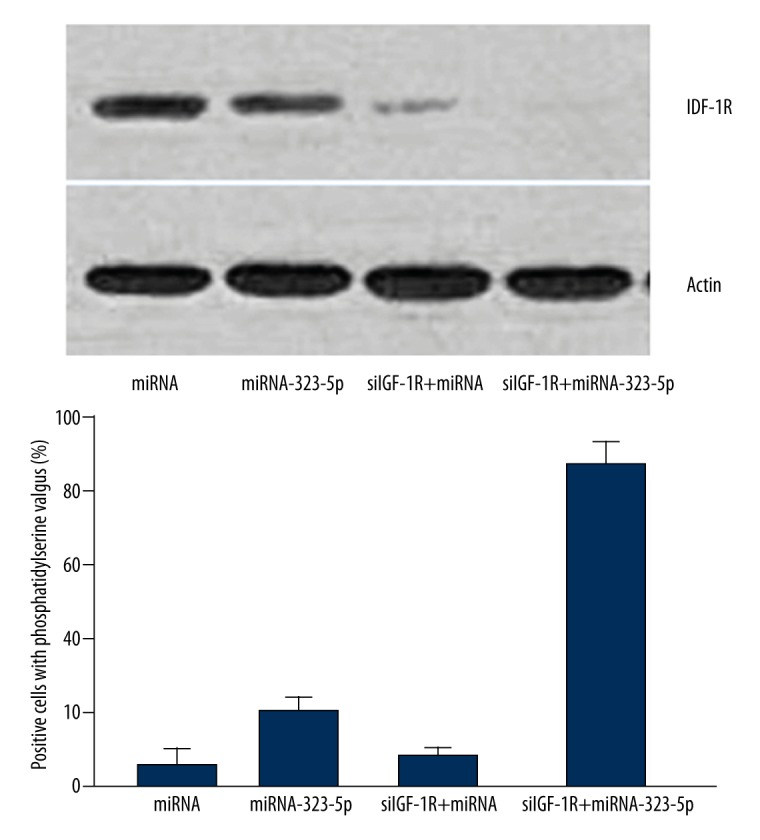
Knockdown of IGF-IR enhanced the apoptosis of U373 cells induced nu miRNA-323-5p.
The over-expression of IGF-1R inhibited the cells apoptosis situations induced by miRNA-323-5p
As shown in Figure 7, the result of caspase-3 activity assay (after increasing IGF-1R levels by over-expression techniques) showed that, compared with U373 cells transfected with 1 μg miRNA in the control group, the activation of caspase-3 in U373 cells transfected with 1 μg miRNA-323-5p were significantly deceased (P=0.003).
Figure 7.
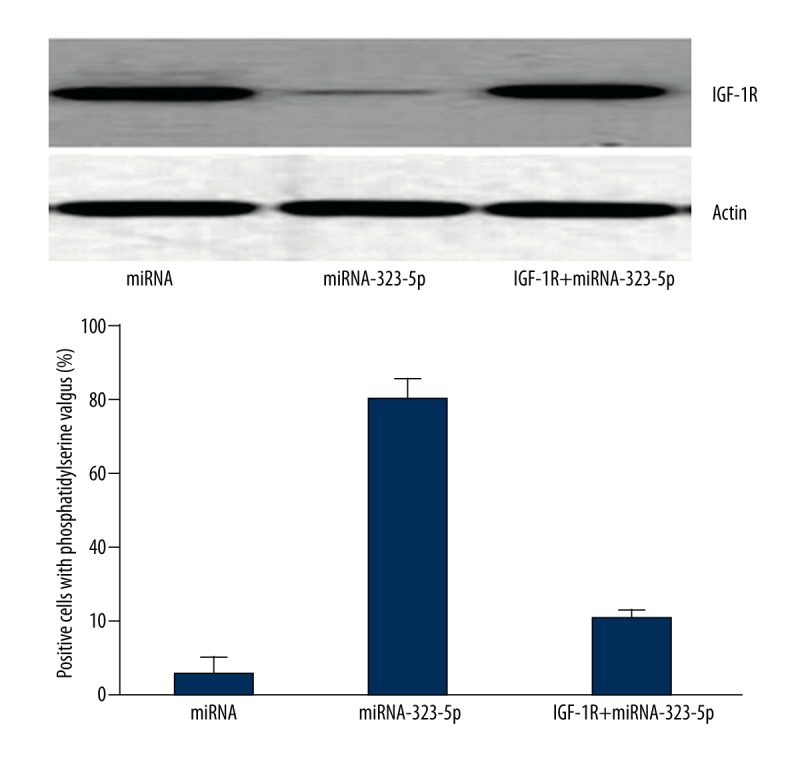
The over-expression of IGF-1R inhibited the cells apoptosis situations induced by miRNA-323-5p.
Discussion
Cerebral glioma is a common clinical intracranial tumor [1]. As quality of life improves and lifestyles change, the incidence of cerebral glioma is growing [1]. Consequently, the study of pathogenesis of the growth and apoptosis of cerebral glioma U363 cells at the molecular level has both theoretical and practical values.
At present, treatments for cerebral glioma mainly include surgery, chemotherapy, radiation therapy, and targeted therapy [2]. All of those therapeutic methods play important roles in the treatment of cerebral glioma. Nevertheless, there still remain many problems to be solved [18]. Radiation therapy and chemotherapy often cause a variety of adverse effects [19]. Target points of targeted molecular therapy are subjects that could be further discussion. The present study investigated targeted molecular therapy in terms of basic theories and cellular levels.
MiRNA-323-5p is an important small RNA molecule, which has been studied frequently. This article discusses the miRNA-323-5p regulation function in the growth, proliferation, and apoptosis of cerebral glioma cells. Results showed that over-expression of miRNA-323-5p inhibited the growth and proliferation of human cerebral glioma U373 cells and promoted apoptosis, which is consistent with past research [3].
IGF-1R is an important receptor widely expressed at cell surfaces; it regulates the growth, proliferation, and apoptosis of cells through its combination with its ligands in target insulin-like growth factor (IGF) receptor proteins [20]. At present, relatively few researchers are focused on whether target insulin-like growth factor (IGF) receptor proteins coordinate with microRNA to regulate cell growth and apoptosis [21,22]. This article indicated that the over-expression of miRNA-323-5p reduced IGF-1R level. After processing the knockdown of IGF-1R and then the transfection with miRNA-323-5p, U373 cells showed enhanced apoptosis. The over-expression of IGF-1R inhibited the cells apoptosis situations induced by miRNA-323-5p.
In this study, there are 3 aspects of evidence proving that U373 cells apoptosis situations induced by miRNA-323-5p had a close relationship with the expression levels of IGF-1R protein. First, the result of Western blotting showed that, under the impact of miRNA-323-5p, the expression levels of IGF-1R protein in U373 cells were significantly decreased. Second, after interference in IGF-1R protein levels by siRNA, and then utilizing miRNA-323-5p to induce U373 cells, human cerebral glioma U373 cells showed more obvious apoptosis situations. Third, after using the over-expression system to transfect with IGF-1R DNA, IGF-1R protein levels were increased, then adding miRNA-323-5p to induce U373 cells, the apoptosis situation was remarkably inhibited. Those results thoroughly indicated that IGF-1R protein played key roles in the inducting process, in which miRNA-323-5p induced U373 cells apoptosis. More importantly, the results also suggested that IGF-1R protein therapy might become a new clinical method for the treatment of cerebral glioma.
Future research can be done in 3 ways. First, explore the different development stages of cerebral glioma specimens at clinical levels, use RT-PCR and Western blot to detect the IGF-1R protein expression levels in cerebral glioma tissues and normal tissues, discussing the relationship between IGF-1R levels and the occurrence and development of cerebral glioma, in order to oppose or support the conclusion of this paper [21]. Second, collect various clinical specimens from cerebral glioma patients treated with different kind of chemotherapeutic agents, using RT-PCR and Western blot to detect the IGF-1R protein expression levels in cerebral glioma tissues and normal tissues, and discuss the relationship between IGF-1R levels and the occurrence and development of cerebral glioma, which might provide evidence needed to determine if IGF-1R protein can be cerebral glioma marker. Third, establish a cerebral glioma rat model, while establishing a gene knockdown or knockout IGF-1R cerebral glioma model; inject the later model into the former model, use miRNA-323-5p for treatment, and discuss the effects at animal levels to provide meaningful information for clinical practice.
Conclusions
Results in this paper showed that miRNA-323-5p inhibited the proliferation of human cerebral glioma U373 cells and promoted its apoptosis by reducing IGF-1R.
Footnotes
Source of support: Departmental sources
References
- 1.Akil H, Abbaci A, Lalloué F, et al. IL22/IL-22R Pathway Induces Cell Survival in Human Glioblastoma Cells. PLoS One. 2015;20:3. doi: 10.1371/journal.pone.0119872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Tellingen O, Yetkin-Arik B, De Gooijer MC, et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015 doi: 10.1016/j.drup.2015.02.002. pii: S1368-7646: 00012. [DOI] [PubMed] [Google Scholar]
- 3.Cominelli M, Grisanti S, Mazzoleni S, et al. EGFR amplified and overexpressing glioblastomas and association with better response to adjuvant metronomic temozolomide. J Natl Cancer Inst. 2015;107(5) doi: 10.1093/jnci/djv041. pii: djv041. [DOI] [PubMed] [Google Scholar]
- 4.Buitrago DH, Patnaik SK, Kadota K, et al. Small RNA sequencing for profiling microRNAs in long-term preserved formalin-fixed and paraffin-embedded non-small cell lung cancer tumor specimens. PLoS One. 2015;10:e0121521. doi: 10.1371/journal.pone.0121521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun C, Alkhoury K, Wang YI, et al. IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal. Circ Res. 2012;111:1054. doi: 10.1161/CIRCRESAHA.112.270314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weili M. The expression and significance of five types of miRNAs in breast cancer. Med Sci Monit Basic Res. 2014;20:97–104. doi: 10.12659/MSMBR.891246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Momen-Heravi F, Trachtenberg AJ, Kuo WP, et al. Genomewide study of salivary microRNAs for detection of oral cancer. J Dent Res. 2014;93:86S. doi: 10.1177/0022034514531018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holgersson G, Bergström S, Harmenberg J, et al. A phase I pilot study of the insulin-like growth factor 1 receptor pathway modulator AXL1717 in combination with gemcitabine HCl and carboplatin in previously untreated, locally advanced, or metastatic non-small cell lung cancer. Med Oncol. 2015;32(4):129. doi: 10.1007/s12032-015-0578-y. [DOI] [PubMed] [Google Scholar]
- 9.Streckel E, Braun-Reichhart C, Herbach N, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide in juvenile transgenic pigs modeling a pre-diabetic condition. J Transl Med. 2015;13:73. doi: 10.1186/s12967-015-0431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genady AR, Ioppolo JA, Azaam MM, et al. New functionalized ercaptoundecahydrododecaborate derivatives for potential application in boron neutron capture therapy: Synthesis, characterization and dynamic visualization in cells. Eur J Med Chem. 2015;93:574–83. doi: 10.1016/j.ejmech.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Fagali NS, Grillo CA, Puntarulo S. Cytotoxicity of corrosion products of degradable Fe-based stents: Relevance of pH and insoluble products. Colloids Surf B Biointerfaces. 2015;128:480–88. doi: 10.1016/j.colsurfb.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 12.Tosun M, Soysal Y, Mas NG, et al. Comparison of the Effects of 13-cis Retinoic Acid and Melatonin on the Viabilities of SH-SY5Y Neuroblastoma Cell Line. J Korean Neurosurg Soc. 2015;57:147–51. doi: 10.3340/jkns.2015.57.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzmitruk V, Szulc A, Shcharbin D, et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int J Pharm. 2015;485:288–94. doi: 10.1016/j.ijpharm.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Marczak A, Denel-Bobrowska M, Rogalska A, et al. Cytotoxicity and induction of apoptosis by formamidinodoxorubicins in comparison to doxorubicin in human ovarian adenocarcinoma cells. Environ Toxicol Pharmacol. 2015;39:369–83. doi: 10.1016/j.etap.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Zischka M, Künne CT, Blom J, et al. Comprehensive molecular, genomic and phenotypic analysis of a major clone of Enterococcus faecalis MLST ST40. BMC Genomics. 2015;16:175. doi: 10.1186/s12864-015-1367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leisching GR, Loos B, Botha MH, Engelbrecht AM. The role of mTOR during cisplatin treatment in an in vitro and ex vivo model of cervical cancer. Toxicology. 2015;335:72–78. doi: 10.1016/j.tox.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Jun-Jiang C, Huan-Jiu X. Vascular endothelial growth factor 165-transfected adipose-derived mesenchymal stem cells promote vascularization-assisted fat transplantation. Artif Cells Nanomed Biotechnol. 2015 doi: 10.3109/21691401.2015.1011808. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Zhao L, Liu J, et al. Cisplatin induces platelet apoptosis through the ERK signaling pathway. Thromb Res. 2012;130:81–91. doi: 10.1016/j.thromres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Xu T, Jiang Y, et al. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia. 2015;17:239–55. doi: 10.1016/j.neo.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen ZR, Ramishetti S, Peshes-Yaloz N, et al. Localized RNAi therapeutics of chemoresistant grade iv glioma using hyaluronan-grafted lipid-based nanoparticles. ACS Nano. 2015;9:1581–91. doi: 10.1021/nn506248s. [DOI] [PubMed] [Google Scholar]
- 21.Fassnacht M, Berruti A, Baudin E, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–35. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- 22.Patterson SD, Rossi JM, Paweletz KL, et al. Functional EpoR pathway utilization is not detected in primary tumor cells isolated from human breast, non-small cell lung, colorectal, and ovarian tumor tissues. PLoS One. 2015;10(3):e0122149. doi: 10.1371/journal.pone.0122149. [DOI] [PMC free article] [PubMed] [Google Scholar]


