Abstract
Background
M-M-RTMII (MMRII; Merck & Co) is currently the only measles-mumps-rubella (MMR) vaccine licensed in the United States. Another licensed vaccine would reinforce MMR supply. This study assessed the immunogenicity of a candidate vaccine (PriorixTM, GlaxoSmithKline Vaccines [MMR-RIT]) when used as a first dose among eligible children in the United States.
Methods
In this exploratory Phase-2, multicenter, observer-blind study, 1220 healthy subjects aged 12–15 months were randomized (3:3:3:3) and received 1 dose of 1 of 3 MMR-RIT lots with differing mumps virus titers (MMR-RIT-1 [4.8 log10]; MMR-RIT-2 [4.1 log10]; MMR-RIT-3 [3.7 log10] CCID50) or MMRII co-administered with hepatitis A vaccine (HAV), varicella vaccine (VAR) and 7-valent pneumococcal conjugate vaccine (PCV7). Immune response to measles, mumps, and rubella viruses was evaluated at Day 42 post-vaccination. Incidence of solicited injection site, general, and serious adverse events was assessed.
Results
Seroresponse rates for MMR vaccine viral components in MMR-RIT lots were 98.3–99.2% (measles), 89.7–90.7% (mumps), and 97.5–98.8% (rubella), and for MMRII were 99.6%, 91.1%, and 100%, respectively. Immune responses to HAV, VAR, and PCV7 were similar when co-administered with any of the 3 MMR-RIT lots or MMRII. There were no apparent differences in solicited or serious adverse events among the 4 groups.
Conclusions
Immune responses were above threshold levels for projected protection against the 3 viruses from MMR-RIT lots with differing mumps virus titers. MMR-RIT had an acceptable safety profile when co-administered with HAV, VAR, and PCV7.
Clinical Trials Registration
NCT00861744; etrack; 111870
Keywords: co-administration, immunogenicity, measles, mumps, rubella
INTRODUCTION
Despite the successful introduction of routine immunization with combined live attenuated measles-mumps-rubella (MMR) vaccines in the 1970s [1], outbreaks and generally increased prevalence of mumps and measles are still noted among the vaccinated and unvaccinated populations, respectively, across the United States [2–4]. Maintenance of high vaccine coverage rates remains an essential component of efforts to control these diseases. A 2-dose MMR vaccination schedule is recommended in the United States. Children receive dose-1 at 12–15 months concomitantly with other recommended vaccines, including hepatitis A vaccine (HAV), varicella vaccine (VAR), and pneumococcal conjugate vaccine (PCV) [5–8]. MMR dose-2 is administered at age 4–6 years to induce immune responses in those who fail to respond to the initial dose. Two-dose catch-up schedules, with a minimum 4-week interval between MMR doses, are recommended for children and adolescents who miss the first dose [9]. As Merck's M-M-RTMII (MMRII), a human serum albumin-free vaccine, is currently the only MMR vaccine licensed in the United States, any interruption in its availability would pose a critical public health risk. Therefore, GlaxoSmithKline Vaccines is currently evaluating its trivalent MMR vaccine PriorixTM (MMR-RIT) for use in the United States. MMR-RIT is routinely given in over 100 countries from the second year of life onwards [10]. The formulation of MMR-RIT used in this study does not contain human serum albumin, thereby minimizing any theoretical risk of microbial contamination as compared to previous formulations [11]. This formulation is also consistent with the recommendation from the European Medicines Agency to eliminate the use of blood-derived products of human origin [12, 13].
This Phase-2 exploratory study assessed immunologic responses to 3 lots of MMR-RIT (containing a range of mumps virus titers) and to MMRII used as a first dose in 12–15-month-old children in the United States. The study was used as a preliminary evaluation of the minimum effective mumps virus titer for the candidate vaccine to allow planning of a Phase-3 study, and was used also to generate preliminary data on the safety and immunogenicity of co-administration of MMR-RIT with routine childhood vaccines: VAR, HAV, and 7-valent PCV (PCV7).
METHODS
This randomized, observer-blind, Phase-2 study was conducted at 51 centers in the Unites States in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by a national, regional, or investigational center institutional review board or independent ethics committee. Written informed consent was obtained from parents/guardians before enrollment. The study consisted of an active phase (immediate post-vaccination interval; Days 0–42), an extended safety follow-up phase (Days 43–180), and an antibody persistence phase ending approximately 2 years post-vaccination. Only the results of a planned analysis conducted for immunogenicity and safety data from the active phase are reported here.
Eligible healthy 12- to 15-month-old male and female subjects had not been previously immunized against (and had no previous history of) measles, mumps, rubella, varicella, and hepatitis A, and had received 3 doses of PCV7 within the first year of life (third dose administered ≥30 days before enrollment). Other key exclusion criteria included: exposure to measles, mumps, rubella, or varicella ≤30 days before study start; previous (≤30 days before study start) or planned administration of investigational products during the study; administration of other vaccines (except influenza and Haemophilus influenzae type b) ≤30 days before study vaccination until Day 42; chronic immunosuppressants/immune-modifying drugs, polyclonal immunoglobulins, or blood products received ≤6 months before study vaccination; immunosuppressive or immunodeficient conditions; contraindication to vaccination; a history of neurologic disorders or seizures; acute disease at enrollment; and severe chronic illness or major congenital defects.
Subjects visited the study site at Days 0, 42, 180, 365, and 730. At Day 0 (Visit 1), subjects were randomized using a blocking scheme (3:3:3:[1:1:1] ratio) to 1 of 4 parallel treatment groups: 3 groups received a single dose of 1 of 3 MMR-RIT lots (containing either high [MMR-RIT-1], medium [MMR-RIT-2], or low [MMR-RIT-3] RIT 4385 mumps strain titers); the fourth group received a single dose of 1 of 3 commercial lots of MMRII (Merck & Co Inc. [14]) (Table 1). The randomization list was generated at GlaxoSmithKline Biologicals using SAS® software. Treatment allocation was performed at the investigator site via a central internet-based randomization system. Subjects concomitantly received a single dose each of HAV (HavrixTM, GlaxoSmithKline Vaccines [15]), VAR (VarivaxTM, Merck & Co. Inc. [16]), and PCV7 (PrevnarTM, Wyeth [17]) at Day 0. Immune response against measles, mumps, and rubella viruses was assessed at Day 42 (Visit 2). MMR-RIT or MMRII were administered subcutaneously into the upper right arm, VAR subcutaneously into the upper left arm, and HAV and PCV7 intramuscularly into the left and right thighs, respectively. A second HAV dose was administered at Day 180 (Visit 3). Vaccine recipients, parents/guardians, and those responsible for evaluation of study endpoints were blinded to study treatment. Vaccine reception, storage, preparation, reconstitution, and administration were performed by study personnel who did not participate in outcome evaluation.
Table 1.
Formulation of 3 Lots of Candidate MMR-RIT (Measles, Mumps, Rubella) Vaccine and Commercially Available Comparator Vaccine (MMRII, Merck & Co., Inc.)
| Vaccine | Lot Number(s) | Log10 CCID50 |
||
|---|---|---|---|---|
| Measlesa | Mumpsb | Rubellac | ||
| MMRII | 1291X | 4.0 | 4.8 | 4.2 |
| 1255X | 3.9 | 4.8 | 4.0 | |
| 1362X | 3.8 | 4.8 | 4.1 | |
| MMR-RIT-1 | AMJRB721A | 3.8 | 4.8 (high) | 3.9 |
| MMR-RIT-2 | DMJRA002A | 4.1 | 4.1 (medium) | 3.9 |
| MMR-RIT-3 | DMJRA003A | 4.0 | 3.7 (low) | 4.1 |
Abbreviation: CCID50, Median cell culture infective dose.
Note: All CCID50 values for vaccine components of MMRII and MMR-RIT were determined by GlaxoSmithKline.
aMeasles was Schwarz strain for GlaxoSmithKline vaccines and Moraten Edmonston-Enders strain for MMRII.
bMumps was RIT 4385 strain for GlaxoSmithKline vaccines and Jeryl Lynn for MMRII.
cRubella strain was the same for MMRII and each MMR-RIT (ie, Wistar RA 27/3).
Immunogenicity
Blood for antibody determination was obtained from subjects on Days 0 (pre-immunization), 42, 365, and 730. Analysis of blood obtained at Days 365 and 730 for evaluation of antibody persistence is ongoing and will be reported separately. Sera were stored at -20°C until assayed in a blinded manner at a central laboratory (GlaxoSmithKline Biologicals, Rixensart, Belgium). Mumps virus antibody response was determined using an in-house plaque-reduction assay (GlaxoSmithKline Biologicals [18, 19]) via neutralization of wild-type virus (Mu90LO1) in the presence of complement and anti-human globulin. Immunoglobulin (Ig) G antibodies to measles, rubella, and varicella-zoster virus (VZV) were measured with commercial enzyme-linked immunosorbent assay (ELISA) (Enzygnost, Dade Behring, Marburg GmbH, Germany); antibodies to hepatitis A virus were determined in a randomized subset of 50% of subjects. Antibodies to PCV7 pneumococcal serotypes were measured in the remaining 50% with an in-house ELISA (GlaxoSmithKline Biologicals [20]). Assay seronegativity cut-off values for antibodies to vaccine viral antigens were: measles <150 mIU/mL, mumps <24 ED50, rubella <4 IU/mL, VZV <25 mIU/mL, hepatitis A <15 mIU/mL, and Streptococcus pneumoniae <0.05 µg/mL. The seronegativity cut-offs evaluated in this study were determined empirically as part of the assay validation protocol and were accepted by the US Food and Drug Administration (FDA). Post-vaccination seroresponses for MMR vaccine viral antigens in initially seronegative subjects were defined as antibody concentrations/titers of: measles ≥200 mIU/mL [21]; mumps ≥51 ED50 (no known correlate of protection threshold) and rubella ≥10 IU/mL [22]. The seroresponse thresholds were accepted by the FDA as those defining active immunization offering clinical benefit.
VAR response was defined as a post-vaccination antibody concentration ≥75 mIU/mL in initially seronegative subjects. HAV response was defined as a post-vaccination antibody concentration ≥15 mIU/mL in initially seronegative subjects, or a ≥2-fold increase in the pre-vaccination antibody concentration in initially seropositive subjects.
Reactogenicity and Safety
Reactogenicity and safety were assessed at each visit and via subject diary cards completed by parents/guardians. Solicited injection site symptoms (pain, redness, swelling for study vaccines only) were recorded from Days 0–3. Solicited general symptoms (fever, rash, parotid/salivary gland swelling, febrile convulsions, irritability/fussiness, drowsiness, and loss of appetite), and unsolicited symptoms were recorded from Days 0–42. Serious adverse events (SAEs) were recorded throughout the study. Fever was assessed daily with a tympanic thermometer or rectally if the tympanic reading indicated fever (≥38.0°C). For each reported symptom, parents/guardians were asked what medical attention (if any) the subject had received.
Statistics
This was a “hypothesis-generating” exploratory study conducted to provide estimations of response rates, which will be used to develop statistical criteria for a formal Phase-3 trial to support licensure of the candidate vaccine on the basis of non-inferior immunogenicity compared to the licensed comparator. All analyses in this study were descriptive, and no formal statistical comparison was prespecified.
Enrollment of 1200 subjects (300/group) was planned to ensure ≥240 evaluable subjects/group. Subjects in the MMRII group were randomized across 3 commercial MMRII lots; no lot-by-lot analysis was done and results were pooled. The primary analysis of immunogenicity was conducted on the according-to-protocol (ATP) cohort for immunogenicity, which included eligible subjects who had received the study vaccine via the correct administration route and complied with study procedures, and who were below the assay cut-off for at least 1 MMR vaccine antigen at baseline, with pre-vaccination and post-vaccination serology results available. Safety analysis was performed on the total vaccinated cohort (TVC), which included all vaccinated subjects.
The primary endpoint was seroresponse rates for antibodies to measles, mumps, and rubella viruses at Day 42; the proportions of subjects with antibody concentration/titer at or above specified assay cut-offs were calculated with exact 95% confidence intervals (CIs) both pre- and post-vaccination. Secondary endpoints included pre- and post-vaccination (Day 42) antibody concentration/titers, summarized by geometric mean concentrations/titers (GMC/Ts) with 95% CI. Exploratory analyses included standardized asymptotic 2-sided 95% CIs calculated for group differences (MMR-RIT group minus MMRII) in Day-42 seroresponse rates for antibodies to MMR viruses. In addition, 95% CIs for GMC ratios (MMR-RIT:MMRII) for antibodies to hepatitis A virus and PCV7 pneumococcal serotypes were obtained using an analysis of covariance model on the logarithm10-transformed Day-42 concentrations. For the safety analysis, the number and percentage of subjects reporting a symptom were calculated with exact 95% CIs. Symptoms were categorized according to intensity and relationship to study vaccine. All data processing and analyses were performed using SAS® version 9.2 (SAS Institute Inc., Cary, NC). Proc StatXact 8.1 derived exact 95% CIs for a proportion within a group as well as standardized asymptotic 95% CI for the group difference in proportions.
RESULTS
Subject Disposition and Baseline Demography
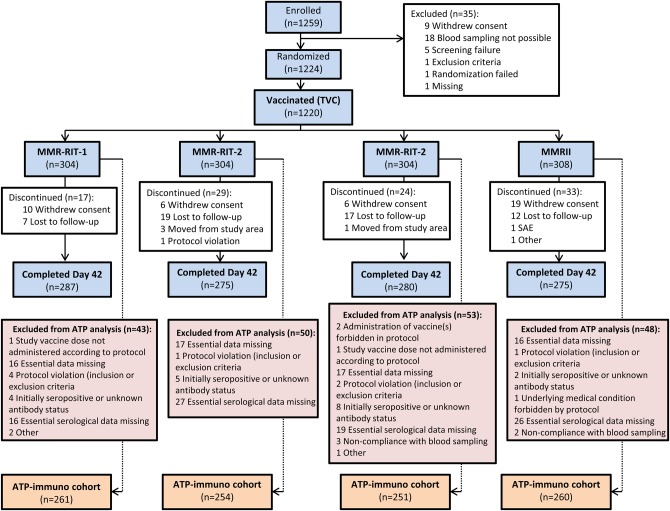
The first subject was enrolled on June 3, 2009, and the last visit of the active (43-day) phase was completed on July 21, 2010. Of 1259 enrolled subjects, 1224 were randomized and 4 did not receive study vaccine. The TVC consisted of 1220 subjects: MMR-RIT-1 (n = 304), MMR-RIT-2 (n = 304), MMR-RIT-3 (n = 304), and MMRII (n = 308). Of these, 1117 completed Day 42 and 103 were withdrawn (Figure 1).
Figure 1.
Disposition of subjects in the total vaccinated cohort (TVC) (enrolled = 1259 subjects, randomized = 1224 subjects, vaccinated = 1220 subjects). Abbreviations: MMR, measles-mumps-rubella; SAE, serious adverse event; ATP, according-to-protocol.
Overall, of 1220 subjects in the TVC, 1026 subjects were included in the ATP-immunogenicity cohort (194 subjects were excluded): MMR-RIT-1 (n = 261); MMR-RIT-2 (n = 254); MMR-RIT-3 (n = 251), and MMRII (n = 260). Demographic characteristics of the 4 treatment groups were comparable between the TVC and ATP-immunogenicity cohorts. Mean (standard deviation) age in the TVC was 12.3 (0.71) months, 75.8% of subjects were white, and 51.1% were male. In the ATP-immunogenicity cohort, 100%, 86.2%, and 99.8%, of subjects were seronegative for measles, mumps and rubella antibody, respectively, before study vaccination; overall baseline seronegativity rates were comparable across the 4 groups (Table 2).
Table 2.
Demographic and Baseline Data for the Total Vaccinated Cohort (TVC) and According-to-Protocol (ATP) Cohort for Immunogenicity
| MMR-RIT-1 |
MMR-RIT-2 |
MMR-RIT-3 |
MMRII |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TVC N = 304 | ATP N = 261 | TVC N = 304 | ATP N = 254 | TVC N = 304 | ATP N = 251 | TVC N = 308 | ATP N = 260 | TVC N = 1220 | ATP N = 1026 | ||
| Age (mo), mean (SD) | 12.4 (0.75) | 12.4 (0.69) | 12.4 (0.73) | 12.4 (0.73) | 12.2 (0.56) | 12.2 (0.57) | 12.4 (0.75) | 12.4 (0.73) | 12.3 (0.71) | 12.3 (0.69) | |
| Gender | Female, n (%) | 156 (51.3) | 134 (51.3) | 144 (47.4) | 120 (47.2) | 157 (51.6) | 128 (51.0) | 139 (45.1) | 118 (45.4) | 596 (48.9) | 500 (48.7) |
| Male, n (%) | 148 (48.7) | 127 (48.7) | 160 (52.6) | 134 (52.8) | 147 (48.4) | 123 (49.0) | 169 (54.9) | 142 (54.6) | 624 (51.1) | 526 (51.3) | |
| Pre-vaccination status of ATP cohort for immunogenicity, no. of seronegative subjects (%)a | |||||||||||
| Antibody (cut-off point) | MMR-RIT-1 N = 261b | MMR-RIT-2 N = 254b | MMR-RIT-3 N = 251b | MMRII N = 260b | Total N = 1026b | ||||||
| Measles (<150 mIU/mL) | 259/259 (100) | 253/253 (100) | 249/249 (100) | 258/258 (100) | 1019/1019 (100) | ||||||
| Mumps (ED50 <24) | 216/252 (85.7) | 219/248 (88.3) | 213/246 (86.6) | 213/243 (86.6) | 861/999 (86.2) | ||||||
| Rubella (<4 IU/mL) | 259/259 (100) | 251/252 (99.6) | 248/249 (99.6) | 258/258 (100) | 1016/1018 (99.8) | ||||||
Abbreviations: MMR, measles-mumps-rubella; N, total number of subjects; SD, standard deviation.
aCalculated for subjects in TVC for which pre-vaccination status was known (ie, % = n/[n + seropositive] × 100, where n = no. of seronegative subjects).
bNumber of subjects regardless of the status of baseline detection of antibody (ie, sum of seronegative, seropositive, and unknown).
Immunogenicity
Seroresponse to MMR Vaccines
Measles virus seroresponse rates were 98.3–99.2% for MMR-RIT groups and 99.6% for the MMRII group; GMCs of measles virus antibodies were >2500 mIU/mL in all 4 groups. Day-42 seroresponse rates for mumps virus antibodies were 89.7% for MMR-RIT-3 (low mumps titer), 90.6% for MMR-RIT-2 (medium mumps titer), 90.7% for MMR-RIT-1 (high mumps titer), and 91.1% for MMRII. Mumps virus antibody GMTs were at least 10-fold greater than the assay cut-off for seronegativity in all 4 groups. Day-42 rubella virus seroresponse rates were 97.5–98.8% for MMR-RIT groups and 100% for the MMRII group. Observed rubella virus antibody GMCs for MMR-RIT groups (68.2–77.7 IU/mL) and MMRII (89.4 IU/mL) were above the assay seronegativity cut-off of 10 IU/mL (Table 3).
Table 3.
Seroresponse Rates and Geometric Mean Concentrations/Titers (GMC/Ts) for Antibodies to Measles, Mumps, and Rubella Viruses at Day 42 in Initially Seronegative Subjects (According-to-Protocol Cohort for Immunogenicity)
| Measles (≥200 mIU/mL) |
Mumps (≥51 ED50) |
Rubella (≥10 IU/mL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Seroresponse |
GMC (95% CI) | N | Seroresponse |
GMC (95% CI) | N | Seroresponse |
GMC (95% CI) | |||
| n (%) (95% CI) | % Diff. vs. MMRII (95% CI) | n (%) (95% CI) | % Diff. vs. MMRII (95% CI) | n (%) (95% CI) | % Diff. vs. MMRII (95% CI) | ||||||
| MMR-RIT-1 | |||||||||||
| 247 | 245 (99.2) (97.1; 99.9) | −0.41 (−2.55; 1.50) | 2799 (2545; 3078) | 193 | 175 (90.7) (85.7; 94.4) | −0.47 (−6.42; 5.46) | 242 (205; 287) | 247 | 244 (98.8) (96.5; 99.7) | −1.21 (−3.51; 0.32) | 72.2 (65.6; 79.6) |
| MMR-RIT-2 | |||||||||||
| 240 | 236 (98.3) (95.8; 99.5) | −1.27 (−3.85; 0.74) | 2878 (2607; 3178) | 202 | 183 (90.6) (85.7; 94.2) | −0.55 (−6.41; 5.35) | 265 (222; 317) | 238 | 235 (98.7) (96.4; 99.7) | −1.26 (−3.64; 0.27) | 77.7 (70.4; 85.7) |
| MMR-RIT-3 | |||||||||||
| 240 | 236 (98.3) (95.8; 99.5) | −1.27 (−3.85; 0.74) | 2593 (2350; 2861) | 195 | 175 (89.7) (84.6; 93.6) | −1.40 (−7.47; 4.62) | 253 (213; 301) | 239 | 233 (97.5) (94.6; 99.1) | −2.51 (−5.37; −0.97) | 68.2 (61.8; 75.3) |
| MMRII | |||||||||||
| 249 | 248 (99.6) (97.8; 100) | Reference | 2950 (2698; 3224) | 192 | 175 (91.1) (86.2; 94.8) | Reference | 268 (224; 320) | 249 | 249 (100) (98.5; 100) | Reference | 89.4 (81.4; 98.2) |
Abbreviations: MMR, measles-mumps-rubella; N, number of subjects with available results; n (%), number/percentage of subjects who seroconverted; 95% CI, 95% confidence interval; GMC/T, geometric mean concentrations/titers for measles, mumps, and rubella virus antibodies.
Co-administration of VAR, HAV, and PCV7
Day-42 seroresponse rates for antibodies to VZV among initially seronegative subjects when VAR was co-administered with MMR-RIT or MMRII were 95.8–98.0%. GMCs for VZV antibodies were >200 mIU/mL in all 4 groups. Day-42 response rates for antibodies to hepatitis A virus were 83.0–89.3% among initially seronegative vaccinees across the 4 groups. Day-42 GMCs adjusted for baseline serostatus for antibodies to hepatitis A virus or pneumococcal serotypes appeared to be similar for all 4 groups, as shown by calculated GMC ratios in Table 4.
Table 4.
Geometric Mean Concentrations (GMCs) for Varicella-Zoster Virus (VZV) Antibodies, and Baseline-Adjusted GMCs and GMC Ratios for Antibodies to Hepatitis A Virus and PCV7 Pneumococcal Serotypes at Day 42 (According-to-Protocol Cohort for Immunogenicity)
| Antibody | MMRII |
MMR-RIT-1 |
MMR-RIT-2 |
MMR-RIT-3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | GMC (95% CI) | N | GMC (95% CI) | N | GMC (95% CI) | N | GMC (95% CI) | ||||
| VZV | 246 | 256 (240; 272) | 245 | 246 (229; 263) | 238 | 235 (217; 254) | 240 | 256 (240; 272) | |||
| MMRII |
MMR-RIT-1 |
MMR-RIT-2 |
MMR-RIT-3 |
||||||||
| N | Adjusted GMC | N | Adjusted GMC | Adjusted GMC ratioa (95% CI) | N | Adjusted GMC | Adjusted GMC ratioa (95% CI) | N | Adjusted GMC | Adjusted GMC ratioa (95% CI) | |
| Hepatitis A virus | 124 | 42.0 | 117 | 33.9 | 0.81 (0.64; 1.02) | 112 | 39.2 | 0.93 (0.74; 1.18) | 111 | 39.5 | 0.94 (0.74; 1.19) |
| S.PNEU-4 | 116 | 3.68 | 122 | 3.69 | 1.00 (0.82; 1.24) | 125 | 3.78 | 1.03 (0.84; 1.26) | 124 | 3.26 | 0.89 (0.72; 1.09) |
| S.PNEU-6B | 111 | 6.50 | 117 | 5.86 | 0.90 (0.75; 1.09) | 122 | 5.87 | 0.90 (0.75; 1.09) | 123 | 5.81 | 0.89 (0.74; 1.07) |
| S.PNEU-9V | 120 | 7.32 | 121 | 6.65 | 0.91 (0.76; 1.08) | 125 | 7.23 | 0.99 (0.83; 1.17) | 127 | 5.80 | 0.79 (0.64; 0.94) |
| S.PNEU-14 | 118 | 7.87 | 127 | 8.91 | 1.13 (0.95; 1.35) | 127 | 8.29 | 1.05 (0.88; 1.26) | 126 | 7.89 | 1.00 (0.84; 1.20) |
| S.PNEU-18C | 119 | 6.58 | 123 | 6.29 | 0.96 (0.79; 1.15) | 126 | 6.62 | 1.01 (0.84; 1.21) | 126 | 5.98 | 0.91 (0.76; 1.09) |
| S.PNEU-19F | 115 | 2.39 | 122 | 2.41 | 1.01 (0.83; 1.22) | 126 | 2.52 | 1.05 (0.87; 1.27) | 126 | 2.33 | 0.97 (0.80; 1.18) |
| S.PNEU-23F | 113 | 10.21 | 121 | 9.56 | 0.94 (0.76; 1.16) | 127 | 9.69 | 0.95 (0.77; 1.17) | 126 | 8.37 | 0.82 (0.67; 1.01) |
Abbreviations: Adjusted GMC, geometric mean antibody concentration adjusted for baseline antibody concentration; MMR, measles-mumps-rubella; N, Number of subjects with both pre- and post-vaccination results available; 95% CI, 95% confidence interval for the adjusted GMC ratio (ANCOVA model: adjustment for baseline concentration - pooled variance with more than 2 groups); S.PNEU, Streptococcus pneumoniae.
aRatio of MMR-RIT lot: MMRII.
Safety and Reactogenicity
Overall incidence of solicited and unsolicited symptoms in the TVC (Days-0–42) was 80.9% (95% CI: 76.0; 85.2) for MMR-RIT-1, 75.7% (70.4; 80.4) for MMR-RIT-2, 74.0% (68.7; 78.9) for MMR-RIT-3, and 75.3% (70.1; 80.0) for MMRII. The most frequently observed solicited symptom (Days 0–3) at MMR-RIT and MMRII injection sites was pain, reported in ≥24.5% of subjects in each group (Table 5), although Grade-3 pain (subject cried when the limb was moved/spontaneously painful) was reported in ≤1.5%. Two subjects (1 each in the MMR-RIT-2 and MMRII groups) had Grade-3 injection site swelling (diameter >20 mm).
Table 5.
Incidence of Solicited Injection Site (Days 0; 3) and General Symptoms During the 43-Day Post-vaccination Period (Total Vaccinated Cohort)
| Symptom | MMR-RIT-1 (N = 283) |
MMR-RIT-2 (N = 275) |
MMR-RIT-3 (N = 283) |
MMRII (N = 277) |
||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Days 0; 3 | ||||||||
| Pain | 70 | 24.8 (19.9; 30.3) | 70 | 25.5 (20.5; 31.1) | 79 | 28.0 (22.9; 33.6) | 67 | 24.5 (19.5; 30.0) |
| Redness | 45 | 16.0 (11.9; 20.8) | 47 | 17.2 (12.9; 22.1) | 41 | 14.5 (10.6; 19.2) | 47 | 17.2 (12.9; 22.1) |
| Swelling | 20 | 7.1 (4.4; 10.7) | 26 | 9.5 (6.3; 13.6) | 19 | 6.7 (4.1; 10.3) | 15 | 5.5 (3.1; 8.9) |
| Days 0; 14 | ||||||||
| Irritability/fussiness | 180 | 63.6 (57.7; 69.2) | 141 | 51.3 (45.2; 57.3) | 150 | 53.0 (47.0; 58.9) | 153 | 55.2 (49.2; 61.2) |
| Drowsiness | 133 | 47.0 (41.1; 53.0) | 106 | 38.5 (32.8; 44.6) | 113 | 39.9 (34.2; 45.9) | 109 | 39.4 (33.6; 45.4) |
| Loss of appetite | 111 | 39.2 (33.5; 45.2) | 77 | 28.0 (22.8; 33.7) | 110 | 38.9 (33.2; 44.8) | 94 | 33.9 (28.4; 39.8) |
| Fever (rectal temp. ≥38.0°C) | 65 | 23.0 (18.2; 28.3) | 79 | 28.7 (23.5; 34.5) | 64 | 22.6 (17.9; 27.9) | 56 | 20.2 (15.6; 25.4) |
| Fever (rectal temp. >39.5°C) | 10 | 3.5 (1.7; 6.4) | 7 | 2.5 (1.0; 5.2) | 9 | 3.2 (1.5; 6.0) | 8 | 2.9 (1.3; 5.6) |
| Days 0; 42 | ||||||||
| Fever (rectal temp. ≥38.0°C) | 103 | 36.4 (30.8; 42.3) | 104 | 37.8 (32.1; 43.8) | 104 | 36.7 (31.1; 42.7) | 85 | 30.7 (25.3; 36.5) |
| Fever (rectal temp. >39.5°C) | 20 | 7.1 (4.4; 10.7) | 14 | 5.1 (2.8; 8.4) | 18 | 6.4 (3.8; 9.9) | 13 | 4.7 (2.5; 7.9) |
| Localized/generalized rash | 72 | 25.4 (20.5; 30.9) | 74 | 26.9 (21.8; 32.6) | 60 | 21.2 (16.6; 26.4) | 69 | 24.9 (19.9; 30.4) |
| Parotid gland swelling | 3 | 1.1 (0.2; 3.1) | 3 | 1.1 (0.2; 3.2) | 5 | 1.8 (0.6; 4.1) | 2 | 0.7 (0.1; 2.6) |
Abbreviations: MMR, measles-mumps-rubella; N, number of subjects having received the documented dose; n/%, number/percentage of subjects reporting a specified symptom; 95% CI, exact 95% confidence interval.
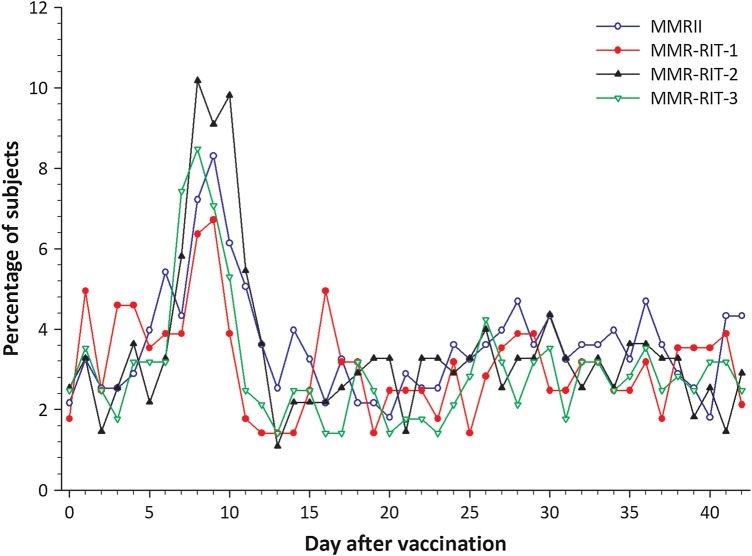
During Days 0–14, irritability or fussiness (overall incidence, 51.3–63.6%) and drowsiness (38.5–47.0%) were the most frequently reported solicited general symptoms (Table 5). Incidence of fever (rectal temperature ≥38.0°C) during Days 0–14 was 20.2–28.7% across the 4 groups; fever >39.5°C occurred in ≤3.5% subjects in any group. Incidence of fever requiring medical attention reported during Days 0–14 in each group was as follows: 6.0% (95% CI: 3.5; 9.4) for MMR-RIT-1, 8.0% (5.1; 11.9) for MMR-RIT-2, 8.5% (5.5; 12.4) for MMR-RIT-3, and 6.9% (4.2; 10.5) for MMRII. Overall incidence of fever during Days 0–42 was 36.4–37.8% for MMR-RIT groups and 30.7% for MMRII recipients (Table 5). For all groups, prevalence of fever peaked 5–12 days after study vaccination (Figure 2); in an exploratory post hoc analysis, the observed incidence of fever between Days 5 and 12 was 14.8% (95% CI: 10.9; 19.5) for MMR-RIT-1, 23.3% (18.4; 28.7) for MMR-RIT-2, 17.3% (13.1; 22.2) for MMR-RIT-3, and 14.8% (10.8; 19.5) for MMRII.
Figure 2.
Prevalence of any fever from Day 0 to Day 42 after vaccination (total vaccinated cohort). Abbreviation: MMR, measles-mumps-rubella.
For Days 0–42, incidence of rash of any type varied between 21.2% and 26.9% across the 4 groups. Measles or rubella-like rashes were reported in MMR-RIT-1 (n = 6), MMR-RIT-2 (n = 7), MMR-RIT-3 (n = 5), and MMRII (n = 9) recipients; varicella-like rash was reported for MMR-RIT-2 (n = 4) and MMRII (n = 2) subjects. Overall incidence of parotid-gland swelling was low (Table 5). There were 2 cases of febrile convulsion during Days 0–42. One MMR-RIT-2 recipient experienced a simple febrile convulsion at Day 29. This was not considered vaccine-related by the investigator since the peak prevalence of vaccine-related fever, and hence febrile convulsions, occurs in the second week following vaccination with measles-containing vaccines [23].
One MMRII recipient had a complex febrile seizure on Day 0, which led to hospitalization and the mother's withdrawal of the subject from the study; this event, classified as an SAE, was considered to be related to the study vaccine.
Of 15 SAEs reported in 11 subjects (Days 0–42), 2 SAEs were considered to be related to study treatment: febrile convulsion in a 12-month-old female MMRII recipient (described above), and idiopathic thrombocytopenic purpura (onset at Day 20) in a 13-month-old female MMR-RIT-2 recipient who was hospitalized for treatment and discharged after 3 days. Both subjects with vaccine-related SAEs were withdrawn from the study. All SAEs resolved without sequelae.
Concomitant Medication
Rates of concomitant medication use (Days 0–42) were the following: MMR-RIT-1 (76.6%), MMR-RIT-2 (72.0%), MMR-RIT-3 (74.0%), and MMRII (70.1%). Rates of antipyretic medication use were the following: MMR-RIT-1 (65.1%), MMR-RIT-2 (59.2%), MMR-RIT-3 (64.1%), and MMRII (57.5%).
DISCUSSION
This Phase-2 multicenter exploratory study assessed immune responses to the first dose of MMR-RIT with 3 differing mumps virus titers and to commercially available MMRII when concomitantly administered with HAV, VAR, and PCV7 in healthy children in the United States, aged 12–15 months. The results indicate that a single dose of any 1 of the 3 MMR-RIT lots elicited an acceptable immune response with respect to seroresponse rates to MMR viruses, 42 days post-vaccination. Previous randomized comparative studies have shown that MMR-RIT administered as a primary vaccination to children in the second year of life produced similar seroconversion rates for antibodies to MMR vaccine viruses compared to those seen with MMRII [24–28].
The current formulation of MMR-RIT used in this study, when co-administered to young children with other recommended vaccines (HAV, VAR, and PCV7), elicited measles and rubella virus antibody concentrations meeting the predefined threshold for seroresponse in ≥97.5% MMR-RIT recipients, and in ≥99.6% MMRII recipients when co-administered with the same vaccines.
Day-42 mumps virus antibody titers after vaccination with all 3 MMR-RIT lots met the seroresponse threshold in 90.7%, 90.6%, and 89.7% recipients, respectively, and no obvious dose–response relationship was observed. Of note, 13.8% of subjects were seropositive for mumps at baseline. This high baseline seropositivity rate is likely attributed to complement enhancement of the mumps PRN assay rather than prior exposure to mumps or persistence of maternal antibodies. Complement-enhanced mumps PRN assays have been shown to have higher seroresponse rates than unenhanced assays [29], and this may also be reflected in the baseline seropositivity. Rubella seroresponse rates across all 4 groups were high (97.5–100%) and GMCs for antibodies to rubella virus following all 3 MMR-RIT lots were substantially higher than the cut-off level of ≥10 IU/mL and would provide effective immunization. Long-term follow-up in this study will allow the evaluation of antibody persistence until approximately 2 years post-vaccination.
The immune response to vaccines routinely co-administered with MMR dose-1 in the United States (HAV, VAR, and PCV7) was also assessed. Observed seroresponse rates for antibodies to VZV were consistently high (≥95.8%) across all 4 treatment groups, and hepatitis A virus antibody response rates to HAV dose-1 were ≥83.0% in each group. Furthermore, Day-42 baseline-adjusted GMCs for antibodies to hepatitis A virus and Streptococcus pneumoniae serotypes appeared comparable when these vaccines were administered with MMR-RIT or MMRII in this exploratory analysis of between-group GMC ratios.
MMR-RIT had an acceptable reactogenicity profile when co-administered with HAV, VAR, and PCV7. Injection site symptoms at the MMR injection site occurred in all 4 treatment groups within 4 days of vaccination, although the incidence of severe symptoms was low. Consistent with previous reports [25–28], vaccination with MMR-RIT or MMRII was associated with fever (rectal temperature ≥38.0°C) during the first 2 weeks, which peaked during days 5–12 after vaccine administration. MMR-RIT, which contains the Jeryl Lynn–derived RIT 4385 strain, has demonstrated a good reactogenicity profile in previous clinical trials [25–28, 30]. Accordingly, in this study, a low incidence of other solicited general symptoms, including measles/rubella- or varicella-like rash, parotid gland swelling, and febrile convulsions, was reported among both MMR-RIT and MMRII recipients during the follow-up period.
Strengths of this study include use of a computer-generated randomization list and blinding of study vaccines, thereby addressing potential biases in study conduct, and comparing the immune responses to the study vaccine with the US-licensed standard of care (MMRII). Furthermore, the MMR-RIT formulation used in this study was free of human serum albumin, thus eliminating the theoretical risk of microbial contamination associated with human serum albumin–containing vaccines [14]. Additionally, omitting a human blood–derived component from the MMR-RIT formulation might make it more socially acceptable. Lastly, concomitant administration of routine vaccines (HAV, VAR, and PCV7) did not affect the immunogenicity of MMR-RIT and vice versa.
Limitations of the study include the relatively small study population that is consistent with a Phase-2 planning study. Larger Phase-3 clinical trials are required in future to substantiate the immunogenicity and safety profile of MMR-RIT. Furthermore, extended follow-up studies are important to evaluate the long-term protection offered by MMR-RIT.
CONCLUSIONS
This Phase-2 study demonstrated an acceptable immune response to 3 candidate MMR-RIT lots containing differing mumps virus titers with respect to seroresponse rates to all 3 MMR virus components. There was no obvious dose–response relationship for the 3 mumps virus titers evaluated; based on the current results, all 3 lots would provide effective immunization, with an acceptable reactogenicity profile. MMR-RIT can be given concomitantly with HAV, VAR, and PCV7 without interfering with the immune response to these co-administered vaccines, as was shown for MMRII. Confirmatory Phase-3 studies to support licensure of MMR-RIT on the basis of immunogenic noninferiority to the licensed comparator are warranted.
Trademark Statement
MMRII is a registered trademark of Merck & Co. Inc., Whitehouse Station, NJ, United States.
Priorix, Havrix, and Varivax are registered trademarks of the GlaxoSmithKline group of companies.
Prevnar is a trademark of Wyeth LLC, New York, NY.
Enzygnost is a registered trademark of Dade Behring, Marburg GmbH, Germany.
SAS is a registered trademark of SAS Institute Inc., Cary, NC.
Author Contributions
C.D.C. and B.I. conceived the study, designed the trial, and obtained research funding. All authors substantially contributed to the conception, the design, the acquisition of data, and the analysis and interpretation of data. M.M., C.D., M.L., C.H., S.G., A.C., S.C.T., R.J., and A.Q. recruited patients. M.P. provided statistical advice on study design and analyzed the data. All authors contributed substantially to the drafting of the article. They revised it critically for important intellectual content and approved the version to be published.
Acknowledgments
The authors would like to thank the parents, children, and investigators who participated in this clinical trial. We also gratefully acknowledge the work of the nurses and other staff members involved. The authors also thank Dr. Sarah Hopwood (Scinopsis) for medical writing and Véronique Duquenne, Shruti MP, and Ashmita Ravishankar (GlaxoSmithKline group of companies) for editorial support and publication coordination.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA. As study sponsor, GlaxoSmithKline Biologicals SA was involved in all stages of study conduct, including data analysis and took charge of all costs associated with the study, including the development and publication of the manuscript.
Potential conflicts of interest. C.D.C, B.L.I., and O.N., former and current employees of the sponsor, may own stock or stock options in the company. M.P. is an employee of GlaxoSmithKline group of companies. M.A.M. received travel support for a meeting for the study and payment for lectures, including service on speakers bureau (Merck). C.J.H. declares that his institution receives grants to conduct the trials for which he is the investigator; he has also received honoraria for lectures, including service on speakers bureau (Merck and Sanofi). S.G. received support for travel, consulting fees, payment for lectures, and fees for participation in review. M.L. has in the past, and is currently being paid by the sponsor as a principal investigator for vaccine research. C.D, A.C, S.C.T., R.JF, A.Q.D.R., and G.B have indicated they have no financial relationship or conflict of interest relevant to this article to disclose.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention (CDC). Summary of Notifiable Diseases, United States, 1998. MMWR Morb Mortal Wkly Rep 1999; 47:ii–92. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Notes from the field: Measles outbreak—Hennepin County, Minnesota, February-March 2011. MMWR Morb Mortal Wkly Rep 2011; 60:421. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). Measles: United States, January-May 20, 2011. MMWR Morb Mortal Wkly Rep 2011; 60:666–8. [PubMed] [Google Scholar]
- 4.Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580–9. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics 2007; 120:221–31. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 2000; 106:362–6. [DOI] [PubMed] [Google Scholar]
- 7.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–23. [PubMed] [Google Scholar]
- 8.Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1998; 47:1–57. [PubMed] [Google Scholar]
- 9.Committee on Infectious Diseases; American Academy of Pediatrics. Policy statement—recommended childhood and adolescent immunization schedules—United States, 2011. Pediatrics 2011; 127:387–8. [DOI] [PubMed] [Google Scholar]
- 10.GOV.UK. Immunisation against infectious disease: Mumps: the green book, chapter 23. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/147975/Green-Book-Chapter-23-v2_0.pdf Accessed February 12, 2014.
- 11.Offit PA, Jew RK. Addressing parents’ concerns: do vaccines contain harmful preservatives, adjuvants, additives, or residuals? Pediatrics 2003; 112:1394–7. [DOI] [PubMed] [Google Scholar]
- 12.EMEA–CHMP. EMEA–CHMP Position Statement on Creutzfeldt–Jakob Disease and Plasma-derived and Urine-derived Medicinal Products. 2004. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003741.pdf Accessed February 12, 2014.
- 13.EMEA–CHMP. EMEA–CHMP Guideline on the Investigation of Manufacturing Processes for Plasma-derived Medicinal Products With Regard to VCJD Risk. 2004. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003741.pdf Accessed February 12, 2014.
- 14.Merck & Co. I. M-M-R(R) II (Measles, Mumps, and Rubella Virus Vaccine Live). December 2010. Available at: http://merck.com/product/usa/pi_circulars/m/mmr_ii/mmr_ii_pi.pdf Accessed February 12, 2014.
- 15.GlaxoSmithKline. HAVRIX (Hepatitis A Vaccine). Highlights of Prescribing Information. HVX:35PI. July 2011. Available at: http://us.gsk.com/products/assets/us_havrix.pdf Accessed February 12, 2014.
- 16.Merck & Co. I. VARIVAX(R). Varicella Virus Vaccine Live. Highlights of Prescribing Information. August 2011. Available at: http://merck.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf Accessed February 12, 2014.
- 17.Wyeth Pharmaceuticals Inc. Pneumococcal 7-valent Conjugate Vaccine (Diphtheria CRM197 Protein). July 2009. Available at: http://labeling.pfizer.com/showlabeling.aspx?id=134 Accessed February 12, 2014.
- 18.Sato H, Albrecht P, Hicks JT, Meyer BC, Ennis FA. Sensitive neutralization test for virus antibody. 1. Mumps antibody. Arch Virol 1978; 58:301–11. [DOI] [PubMed] [Google Scholar]
- 19.Dodet M, Dessy F, Parent I, Gerard P. Use of a neutralization assay as an alternative for assessing mumps immunization status. In: Program and Abstracts of the 24th Annual Meeting of the European Society for Pediatric Infectious Diseases (Basel, Switzerland) 2006. Abstract 387.
- 20.Poolman JT, Frasch CE, Kayhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiepiela P, Coovadia HM, Loening WE, Coward P, Abdool Karim SS. Loss of maternal measles antibody in black South African infants in the first year of life—implications for age of vaccination. S Afr Med J 1991; 79:145–8. [PubMed] [Google Scholar]
- 22.Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol 1996; 106:170–4. [DOI] [PubMed] [Google Scholar]
- 23.Klein NP, Fireman B, Yih WK, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatr 2010; 126:e1–8. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein H, Marchant C, Rathfon H, Marchand P, Kimura A. Safety and immunogenicity of a new, live, attenuated combined measles, mumps and rubella vaccine in healthy U.S. children 12 to 18 months of age. Antimicrob Agents Chemother 1999; 39:719 (abstract no. 1086). [Google Scholar]
- 25.Usonis V, Bakasenas V, Chitour K, Clemens R. Comparative study of reactogenicity and immunogenicity of new and established measles, mumps and rubella vaccines in healthy children. Infection 1998; 26:222–6. [DOI] [PubMed] [Google Scholar]
- 26.Usonis V, Bakasenas V, Kaufhold A, Chitour K, Clemens R. Reactogenicity and immunogenicity of a new live attenuated combined measles, mumps and rubella vaccine in healthy children. Pediatr Infect Dis J 1999; 18:42–8. [DOI] [PubMed] [Google Scholar]
- 27.Gatchalian S, Cordero-Yap L, Lu-Fong M, et al. A randomized comparative trial in order to assess the reactogenicity and immunogenicity of a new measles mumps rubella (MMR) vaccine when given as a first dose at 12-24 months of age. Southeast Asian J Trop Med Public Health 1999; 30:511–7. [PubMed] [Google Scholar]
- 28.Lee CY, Tang RB, Huang FY, Tang H, Huang LM, Bock HL. A new measles mumps rubella (MMR) vaccine: a randomized comparative trial for assessing the reactogenicity and immunogenicity of three consecutive production lots and comparison with a widely used MMR vaccine in measles primed children. Int J Infect Dis 2002; 6:202–9. [DOI] [PubMed] [Google Scholar]
- 29.Hishiyama M, Tsurudome M, Ito Y, Yamada A, Sugiura A. Complement-mediated neutralization test for determination of mumps vaccine-induced antibody. Vaccine 1988; 6:423–7. [DOI] [PubMed] [Google Scholar]
- 30.Lim FS, Han HH, Bock HL. Safety, reactogenicity and immunogenicity of the live attenuated combined measles, mumps and rubella vaccine containing the RIT 4385 mumps strain in healthy Singaporean children. Ann Acad Med Singapore 2007; 36:969–73. [PubMed] [Google Scholar]