Abstract
Background
Human immunodeficiency virus (HIV)–exposed, uninfected (HIV-EU) children are at increased risk of infectious illnesses and mortality compared with children of HIV-negative mothers (HIV-unexposed). However, treatment outcomes for lower respiratory tract infections among HIV-EU children remain poorly defined.
Methods
We conducted a hospital-based, prospective cohort study of N = 238 children aged 1–23 months with pneumonia, defined by the World Health Organization. Children were recruited within 6 hours of presentation to a tertiary hospital in Botswana. The primary outcome—treatment failure at 48 hours—was assessed by an investigator blinded to HIV exposure status.
Results
Median age was 6.0 months; 55% were male. One hundred fifty-three (64%) children were HIV-unexposed, 64 (27%) were HIV-EU, and 20 (8%) were HIV-infected; the HIV exposure status of 1 child could not be established. Treatment failure at 48 hours occurred in 79 (33%) children, including in 36 (24%) HIV-unexposed, 30 (47%) HIV-EU, and 12 (60%) HIV-infected children. In multivariable analyses, HIV-EU children were more likely to fail treatment at 48 hours (risk ratio [RR]: 1.83, 95% confidence interval [CI]: 1.27–2.64, P = .001) and had higher in-hospital mortality (RR: 4.31, 95% CI: 1.44–12.87, P = .01) than HIV-unexposed children. Differences in outcomes by HIV exposure status were observed only among children under 6 months of age. HIV-EU children more frequently received treatment with a third-generation cephalosporin, but this did not reduce the risk of treatment failure in this group.
Conclusions
HIV-EU children with pneumonia have higher rates of treatment failure and in-hospital mortality than HIV-unexposed children during the first 6 months of life. Treatment with a third-generation cephalosporins did not improve outcomes among HIV-EU children.
Keywords: HIV-exposed, pneumonia, treatment outcomes, uninfected
BACKGROUND
Improved access to antiretroviral prophylaxis and modifications of infant feeding practices have substantially reduced mother-to-child-transmission of human immunodeficiency virus (HIV) in sub-Saharan Africa (SSA) [1]. However, the prevalence of HIV among pregnant women remains high in many SSA countries, and the number of infants of HIV-infected mothers who do not themselves acquire the virus has risen rapidly. HIV-exposed, uninfected (HIV-EU) children have higher infectious morbidity and mortality during the first 2 years of life than the children of HIV-negative mothers (HIV-unexposed) [2–6]. Infant feeding practices may contribute to these poor health outcomes as HIV-infected mothers may be less able to breastfeed or may choose replacement feeding to reduce the risk of HIV transmission [7]. Other potential contributing factors include early maternal death, increased exposure to infections, and immune abnormalities resulting from in utero exposure to HIV and antiretroviral medications [7].
Pneumonia remains the leading killer of children worldwide [8]. Of the estimated 1.4 million child deaths from pneumonia in 2010, more than 99% occurred in developing countries, and 46% were in SSA [8]. In 2013, the World Health Organization (WHO) updated its recommendations for diagnosis and treatment of pneumonia in settings with limited diagnostic capabilities [9]. These guidelines classify children as having nonsevere or severe pneumonia, and offer specific treatment recommendations for children with known or suspected HIV infection. For HIV-uninfected children (including HIV-EU children), ampicillin or benzylpenicillin with gentamicin is recommended for severe pneumonia, while high-dose oral amoxicillin can be given for nonsevere disease. For HIV-infected children (or HIV-exposed children who have not had HIV testing), ampicillin or benzylpenicillin with gentamicin is recommended regardless of disease severity, and high-dose trimethoprim-sulfamethoxazole (TMP-SMX) should be considered for treatment of Pneumocystis jirovecii pneumonia (PCP) in infants. Cloxacillin and gentamicin are recommended regardless of HIV status if there is suspicion of staphylococcal pneumonia. Treatment with a third-generation cephalosporin is reserved for failure of first-line antimicrobial therapy.
The incidence of pneumonia among HIV-EU children is higher than HIV-unexposed children, and studies suggest that HIV exposure may also be associated with treatment failure among children treated as per WHO guidelines [2, 4, 10, 11]. McNally et al reported that HIV-EU infants with pneumonia who were given benzylpenicillin, gentamicin, and high-dose TMP-SMX had higher treatment failure rates than similarly treated HIV-unexposed infants [12]. Webb et al similarly found an increased treatment failure rate among children with a positive HIV antibody test, although this study did not distinguish infant HIV infection from in utero HIV exposure with placental acquisition of maternal antibody [13]. The authors of these studies concluded that WHO pneumonia treatment guidelines should be reexamined for settings with a high prevalence of HIV infection or exposure [12, 13].
We conducted a hospital-based, prospective cohort study to assess whether HIV-EU children have worse outcomes from pneumonia than HIV-unexposed children during the first 2 years of life. As a secondary objective, we investigated whether specific antimicrobial regimens might reduce the risk of treatment failure among HIV-EU children.
METHODS
Setting
The study was conducted from April 2012 to October 2013 at a tertiary hospital in Gaborone, Botswana. The country's prevalence of human immunodeficiency virus type 1 (HIV-1) among pregnant women aged 15–49 years was 30.4% in 2011 [14]. Haemophilus influenzae type B conjugate vaccine was included in the country's immunization schedule in 2010, while 13-valent pneumococcal conjugate vaccine was introduced in July 2012.
HIV-infected women in Botswana are counseled to exclusively breastfeed their children until at least 6 months of age unless replacement feeding is acceptable, feasible, affordable, sustainable, and safe (AFASS), and free infant formula is provided to HIV-infected women who meet these criteria [15, 16].
Study Population
Children 1–23 months of age with pneumonia, defined by the WHO as cough or difficulty in breathing with lower chest wall indrawing, were eligible for inclusion, provided that a legal guardian was available to provide written informed consent [9]. The presence of 1 or more danger signs (central cyanosis, convulsions, inability to drink, or abnormal sleepiness) further classified children as having severe pneumonia [9]. We excluded children with a chronic medical condition predisposing to pneumonia (other than HIV), hospitalization in the prior 14 days, asthma, wheezing with resolution of lower chest wall indrawing after 2 or fewer bronchodilator treatments, or previous enrollment in the study. All children were recruited within 6 hours of the triage time in the Emergency Department (ED).
Clinical care was provided by medical officers and pediatric residents on a ward supervised by pediatric specialists. Supplemental oxygen and continuous positive airway pressure (CPAP) were routinely available, while there was limited access to mechanical ventilation in an intensive care unit. Intravenous antibiotics that were routinely available included ampicillin, gentamicin, cloxacillin, ceftriaxone, cefotaxime, TMP-SMX, and vancomycin, while amoxicillin, amoxicillin-clavulanate, TMP-SMX, and erythromycin were available orally. Departmental guidelines for treatment of pneumonia were based on WHO recommendations, but antibiotic decisions were ultimately at the discretion of the treating pediatrician. Chest radiographs were routinely obtained for children with symptoms of lower respiratory tract infection, and interpretation was by the treating pediatrician. The hospital's microbiology laboratory uses a manual blood culture system but does not perform testing for respiratory viruses. Acid-fast stain and mycobacterial culture were intermittently available to clinicians during the study period.
Data Collection
Sociodemographic and clinical data were collected at enrollment from a physical examination, review of infant and maternal medical records, and a detailed face-to-face interview with the child's caregiver(s). Research staff assessed children and reviewed hospital charts daily until discharge (or death), recording additional clinical information including the dates and times of antibiotic doses.
Severe acute malnutrition was defined as weight-for-length <-3 standard deviations on WHO growth curves, mid–upper arm circumference <115 mm (for children ≥6 months), or bilateral edema of nutritional origin [17]. Children were considered to have a tuberculosis contact if their caregiver reported a household member was diagnosed with or treated for tuberculosis in the prior 12 months. Proximity to health care services was categorized as travel of <1 or ≥1 hour prior to first contact with the health system (at a clinic or hospital) on the enrollment date. Children were considered to have received a WHO antibiotic regimen if the prescribed regimen included at least the recommended antibiotic(s), even if they received additional antibiotics, and the first dose was given within 6 hours of ED triage time. Microbiological confirmation of PCP was not available, but clinicians frequently used lactate dehydrogenase (LDH) as a screening test in children with compatible illnesses. Children were considered to have probable PCP if they had an oxygen saturation <85% on room air, abnormal chest radiograph, and LDH >750 IU/L.
Study data were collected and managed using REDCap electronic data capture tools hosted at The Children's Hospital of Philadelphia (Philadelphia, PA).
Classification of HIV Exposure Status
Children of mothers with documented negative testing for HIV-1 during pregnancy, at delivery, or at enrollment were classified as HIV-unexposed. Children whose mothers tested positive for HIV-1 before or at delivery were considered HIV-exposed. Testing of infants <18 months of age for HIV-1 was performed using the Roche Amplicor 1.5 HIV DNA PCR (Roche, Alameda, CA). Infants ≥18 months and mothers were tested for HIV using dual parallel rapid testing with the Determine HIV 1/2 (Abbott Laboratories, North Chicago, IL) and Uni-Gold Recombigen HIV (Trinity Biotech, Inc., Wicklow, Ireland) tests. HIV-exposed children were classified as HIV-EU if they tested negative for HIV-1 after 6 weeks of age if exclusively formula fed, at least 6 weeks after breastfeeding cessation, or at enrollment.
Outcomes Assessment
The primary outcome, treatment failure, was assessed at 48 hours by a study physician or nurse blinded to HIV exposure status. This investigator was provided only with the name and location of the child on the ward, and did not have access to enrollment data or the child's medical record. Treatment failure was defined as persistent lower chest wall indrawing, the development of new WHO danger signs, oxygen saturation <80% (on room air), a continued requirement for CPAP or mechanical ventilation, or death. This definition was adapted for our setting from criteria used in a previous WHO-funded study of childhood pneumonia [18], and training sessions were held every 3 months during the study period to standardize the assessment process. Children discharged before 48 hours were considered treatment responders, but caregivers were contacted by telephone to confirm treatment response.
Secondary outcomes included days of supplemental oxygen, days of CPAP or mechanical ventilation, length of stay, and in-hospital mortality. For a given day, the highest level of respiratory support required by a child was recorded. Length of stay was calculated from the ED triage time to the time of discharge from the ward.
Statistical Analysis
Baseline characteristics of the exposure groups were described using frequencies and percentages for categorical variables and median and interquartile ranges (IQR) for continuous variables. We used Cox proportional hazards to directly estimate risk ratios (RR) for treatment failure at 48 hours and in-hospital mortality [19]. This approach, as opposed to logistic regression, was necessary given the commonality of both outcomes of interest. More specifically, when the outcome is not rare (ie, >10%), the odds ratio estimated by logistic regression will be an upwardly biased estimate of the underlying RR [20]. We used linear regression to estimate mean differences in days of supplemental oxygen, days of CPAP or mechanical ventilation, and length of stay according to HIV exposure status. Adjusted analyses included age and proximity to health care services, which were potential confounding variables identified based on subject matter knowledge of the proposed causal pathway between HIV exposure and pneumonia outcomes (Appendix 1). Measures of pneumonia disease severity and infant feeding practices were not included in adjusted analyses, as these variables are potential downstream consequences of in utero HIV exposure [21].
In analyses limited to HIV-EU children, we used Cox proportional hazards to directly estimate crude and adjusted RR for treatment failure at 48 hours according to receipt of a third-generation cephalosporin (versus ampicillin and gentamicin), adjusting for the following potential confounders: age, low birth weight (<2500 g), severe malnutrition, WHO disease severity, respiratory rate, and hypoxia (oxygen saturation <90%, room air).
All statistical analyses were conducted using SAS software version 9.3 (SAS Institute, Cary, NC). This study was approved by the Health Research and Development Committee (Ministry of Health, Botswana) and institutional review boards at Princess Marina Hospital, The University of Pennsylvania, and The Children's Hospital of Philadelphia.
RESULTS
Patient Characteristics
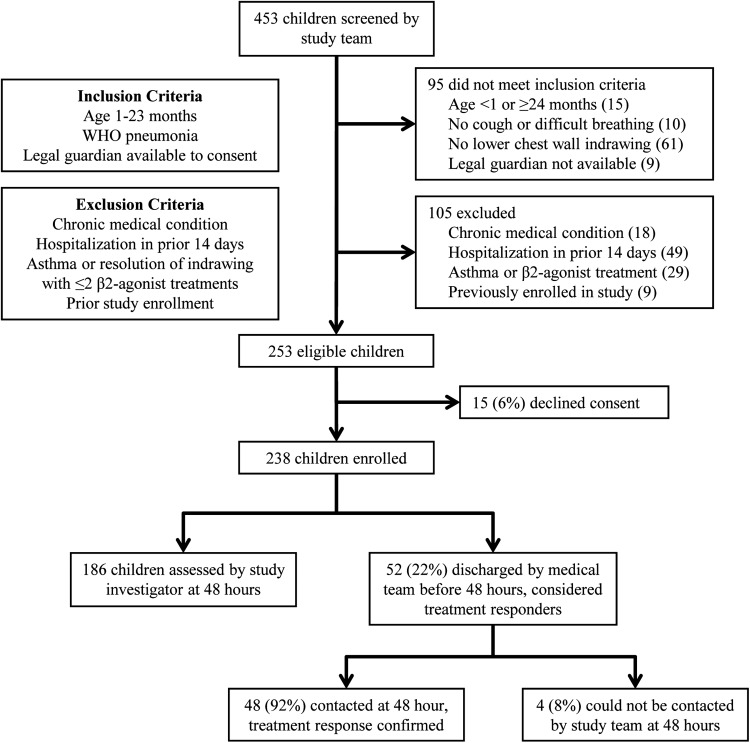
Four hundred fifty-three children were screened by the study team for enrollment, and 253 children were eligible (Figure 1). The legal guardians of 15 children (6%) declined consent, but the age and gender of these children did not differ from enrolled children.
Figure 1.
Screening and enrollment of study participants presenting with pneumonia to a tertiary hospital in Gaborone, Botswana, April 2012 to October 2013. Abbreviation: WHO, World Health Organization.
Table 1 presents baseline characteristics of the study population. The median age was 6.0 months and 55% were male. Eighty-five (36%) children presented with severe pneumonia. One hundred fifty-three children (64%) were HIV-unexposed, 64 (27%) were HIV-EU (27%), 20 were HIV-infected (8%), and the HIV exposure status of 1 child could not be established. Of children classified as HIV-EU, 48 (75%) were tested for HIV at enrollment, while the remaining 16 HIV-EU children were exclusively formula-fed and had previously tested negative for HIV by DNA PCR at ≥6 weeks of age. All the mothers of children classified as HIV-unexposed tested negative for HIV at least once during the pregnancy, and in 91 (59%) cases either the mother or infant also tested negative for HIV at enrollment. Fifteen (75%) HIV-infected children were diagnosed with HIV during the enrollment hospitalization, and only 3 HIV-infected children were on antiretroviral therapy at the time of enrollment. Four (20%) HIV-infected and 15 (23%) HIV-EU children were on TMP-SMX prophylaxis at enrollment; an additional seven (11%) HIV-EU children were enrolled in an ongoing double-blind trial comparing TMP-SMX prophylaxis with placebo.
Table 1.
Baseline Characteristics of N = 238 Children Aged 1–23 Months Presenting With Pneumonia to a Tertiary Hospital in Gaborone, Botswana, April 2012–October 2013
| N (column %) |
|||||
|---|---|---|---|---|---|
| Characteristic (n with data) | Total (N = 238a) | HIV-Unexposed (N = 153) | HIV-EU (N = 64) | HIV-Infected (N = 20) | Pb |
| Demographics | |||||
| Age, mo (n = 238) | 0.21 | ||||
| 1 to <6 | 120 (50.4) | 70 (45.8) | 36 (56.3) | 14 (70.0) | — |
| 6 to <12 | 53 (22.3) | 36 (23.5) | 14 (21.9) | 2 (10.0) | — |
| 12 to <24 | 65 (27.3) | 47 (30.7) | 14 (21.9) | 4 (20.0) | — |
| Median (IQR) | 6.0 (2.8, 13.3) | 7.5 (3.3, 14.0) | 5.4 (2.5,12.1) | 3.9 (2.4, 8.6) | 0.08 |
| Male gender (n = 238) | 130 (54.6) | 83 (54.3) | 36 (56.3) | 10 (50.0) | 0.88 |
| Birth weight <2500 g (n = 238) | 51 (21.4) | 27 (17.7) | 17 (26.6) | 6 (30.0) | 0.20 |
| Nutrition and infant feeding practices | |||||
| Severe malnutritionc (n = 238) | 20 (8.8) | 11 (7.3) | 3 (5.3) | 6 (30.0) | 0.002 |
| Current breastfeeding (n = 238) | 100 (42.0) | 89 (58.2) | 4 (6.3) | 7 (35.0) | <0.0001 |
| Socioeconomic factors | |||||
| Maternal education level (n = 238) | <0.0001 | ||||
| None or primary | 27 (11.3) | 13 (8.5) | 8 (12.5) | 6 (30.0) | — |
| Secondary | 164 (68.9) | 99 (64.7) | 51 (79.7) | 14 (70.0) | — |
| Tertiary | 47 (19.8) | 41 (26.8) | 5 (7.8) | 0 (0.0) | — |
| Electricity in household (n = 238) | 154 (64.7) | 102 (66.7) | 38 (59.4) | 13 (65.0) | 0.59 |
| Municipal water (n = 238) | 206 (86.6) | 136 (88.9) | 54 (84.4) | 15 (75.0) | 0.20 |
| Refrigerator in household (n = 238) | 143 (60.1) | 97 (63.4) | 35 (54.7) | 10 (50.0) | 0.31 |
| Wood as primary cooking fuel (n = 238) | 72 (30.3) | 44 (28.8) | 22 (34.4) | 6 (30.0) | 0.71 |
| Household contact with tuberculosis, past 12 mod (n = 237) | 17 (7.2) | 10 (6.6) | 6 (9.4) | 1 (5.0) | 0.71 |
| Current illness factors | |||||
| WHO severe diseasee (n = 238) | 85 (35.7) | 52 (34.0) | 24 (37.5) | 9 (45.0) | 0.60 |
| Respiratory rate (breaths/min), median (IQR) (n = 238) | 62 (54, 72) | 62 (54, 72) | 60 (53, 76) | 58 (54, 71) | 0.97 |
| Oxygen saturation <90%, room air (n = 237) | 92 (39.0) | 56 (36.8) | 25 (39.1) | 11 (55.0) | 0.29 |
| Days of cough, median (IQR) (n = 237) | 3 (2, 5) | 3 (2, 5) | 2 (2, 5) | 5 (3, 8) | 0.04 |
| Days of fever, median (IQR) (n = 236) | 2 (1, 3) | 2 (1, 3) | 1 (0, 2) | 3 (1, 7) | 0.02 |
| Nasal congestion or discharge (n = 238) | 192 (80.7) | 130 (85.0) | 48 (75.0) | 14 (70.0) | 0.10 |
| History of convulsions (n = 238) | 9 (3.8) | 5 (3.3) | 4 (6.3) | 0 (0.0) | 0.38 |
| White blood cell count (cells/μL), median (IQR) (n = 206) | 12.4 (9.4–16.9) | 11.9 (9.1–16.9) | 13.5 (10.6–16.3) | 14.2 (9.6–19.0) | 0.27 |
| Hemoglobin (g/dL), median (IQR) (n = 206) | 10.6 (9.4–11.5) | 10.8 (9.8–11.6) | 10.7 (9.5–11.5) | 8.3 (6.7–9.8) | <0.0001 |
| Travel of more than 1 h to clinic or hospital (n = 237) | 24 (10.1) | 13 (8.6) | 8 (12.5) | 3 (15.0) | 0.52 |
| Received antibiotics prior to hospital presentation (n = 237) | 152 (64.1) | 95 (62.5) | 43 (67.2) | 13 (65.0) | 0.80 |
| Traditional medicine usef (n = 238) | 29 (12.2) | 16 (10.5) | 8 (12.5) | 5 (25.0) | 0.17 |
Abbreviations: HIV-EU, HIV-exposed uninfected; IQR, interquartile range; WHO, World Health Organization.
aHIV exposure status could not be established for N = 1 child.
bWald χ2 P-values.
cDefined as weight-for-length <-3 SD on standard WHO growth curves, mid–upper arm circumference <115 mm (for children ≥6 months of age), or the presence of bilateral edema of nutritional origin.
dContact within household who was diagnosed with or treated for tuberculosis in the prior 12 months.
ePneumonia accompanied by WHO danger signs (central cyanosis, convulsions, inability to drink, or abnormal sleepiness).
fCaregivers were asked if the child had been seen or had treatment recommended outside of a local health post, clinic, or hospital for the current illness.
Severe malnutrition, current breastfeeding, maternal education levels, reported days of cough and fever, and hemoglobin levels differed significantly by HIV exposure status (Table 1). However, in comparisons limited to HIV-uninfected children, only current breastfeeding (P < .0001) and maternal education levels (P = .01) differed between HIV-EU and HIV-unexposed children. There were no significant differences in WHO disease severity, respiratory rate, or hypoxia by HIV exposure status.
LDH was sent on the date of enrollment for 10 HIV-infected, 16 HIV-EU, and 8 HIV-unexposed children. Eight (40%) HIV-infected, 4 (6%) HIV-EU children, and 1 (<1%) HIV-unexposed child met clinical criteria for probable PCP.
Treatment Outcomes
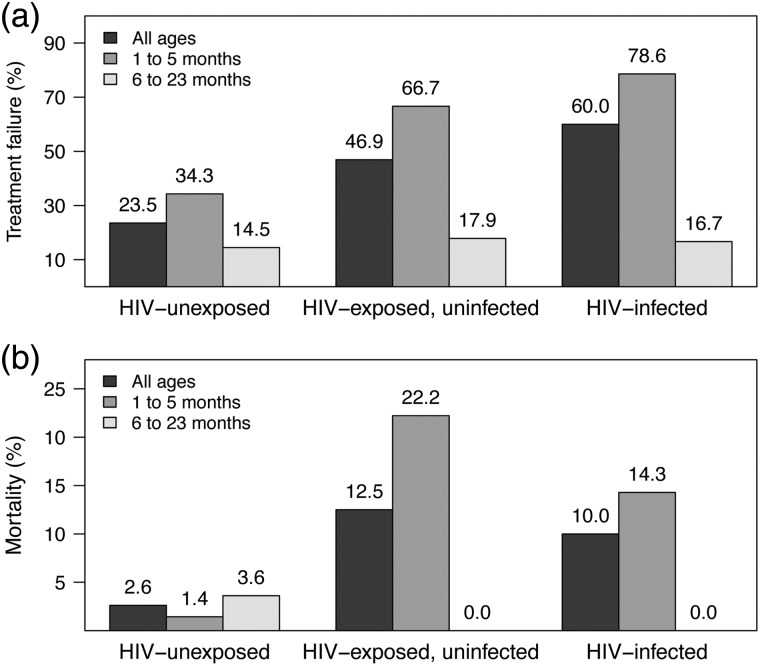
Seventy-eight (33%) participants failed treatment at 48 hours: 36 (24%) HIV-unexposed, 30 (47%) HIV-EU, and 12 (60%) HIV-infected children (Figure 2). One hundred forty-five (61%) children received supplemental oxygen, 26 (11%) required CPAP, and 5 (2%) children were mechanically ventilated during the hospitalization. Fourteen (5.9%) children died, including 4 (2.6%) HIV-unexposed, 8 (12.5%) HIV-EU, and 2 (10.0%) HIV-infected children (Figure 2). Median length of stay for the 224 children surviving to hospital discharge was 3.6 days (IQR: 1.9, 8.0 days). Fifty-two (22%) children were discharged before the 48-hour treatment failure assessment; the caregivers of 48 (92%) of these children were contacted by phone, and no child was reported to have required further medical care.
Figure 2.
Summary of (a) treatment failure at 48 hours and (b) in-hospital mortality among N = 238 children aged 1–23 months with pneumonia at a tertiary hospital in Gaborone, Botswana, according to human immunodeficiency virus (HIV) status and age.
Table 2 presents the results of univariate and multivariable analyses by HIV exposure status. Both HIV-EU and HIV-infected children were significantly more likely to fail treatment at 48 hours than HIV-unexposed children (HIV-EU versus HIV-unexposed, RR: 1.83, 95% confidence interval [CI]: 1.27–2.64, P = .001; HIV-infected versus HIV-unexposed, RR: 2.08, 95% CI: 1.39–3.12, P = .0004). HIV-EU and HIV-infected children also required significantly more days of CPAP or mechanical ventilation and tended to have longer lengths of stay than HIV-unexposed infants. Among HIV-uninfected children, in-hospital mortality was strongly associated with in utero HIV exposure (HIV-EU versus HIV-unexposed, RR: 4.31, 95% CI: 1.44–12.87, P = .01). Mortality did not differ between HIV-EU and HIV-infected children (HIV-EU versus HIV-infected, RR: 1.58, 95% CI: 0.39–6.49, P = .40), although power for this comparison was limited by the small number of HIV-infected children in the cohort. When analyses were stratified by age (1–5 months versus 6–23 months), differences in the risk of treatment failure and in-hospital mortality by HIV exposure status were observed only among children <6 months of age (Figure 2). The effects of HIV exposure on treatment failure at 48 hours (RR: 2.10, 95% CI: 1.42–3.08, P = .001) and in-hospital mortality (RR: 6.05, 95% CI: 2.08–17.58, P = .0009) were not significantly changed when we excluded HIV-EU children who were not tested for HIV at enrollment (n = 20). Similarly, we conducted additional analyses in which we excluded the following subpopulations: (1) children who had been breastfed in the prior 6 weeks or were <6 weeks of age (n = 115), (2) children on TMP-SMX prophylaxis or enrolled in the placebo-controlled trial of TMP-SMX prophylaxis (n = 26), or (3) children discharged before the 48-hour treatment failure assessment (n = 52). The effect of HIV exposure status on treatment failure at 48 hours was unchanged in these sensitivity analyses (data not shown).
Table 2.
Crude and Adjusted Risk Ratios (RR) or Mean Differences (MD) and 95% Confidence Intervals (CI) for Treatment Outcomes According to Human Immunodeficiency Virus (HIV) Exposure Status Among N = 238 Children Aged 1–23 Mo With Pneumonia in Gaborone, Botswana, April 2012–October 2013
| Crude |
Adjustedb |
|||||
|---|---|---|---|---|---|---|
| RR or MD | (95% CI) | Pa | RR or MD | (95% CI) | Pa | |
| HIV-EU vs. HIV-unexposed | ||||||
| Primary outcome | ||||||
| Treatment failure at 48 h | 1.99 | (1.35, 2.93) | .0005 | 1.83 | (1.27, 2.64) | 0.001 |
| Secondary outcomes | ||||||
| Days of supplemental oxygen | +0.88 days | (−0.54, +2.31 days) | .22 | +0.68 days | (−0.73, +2.09 days) | 0.35 |
| Days of CPAP or mechanical ventilation | +0.55 days | (+0.15, +0.95 days) | .008 | +0.49 days | (+0.10, +0.89 days) | 0.02 |
| Length of stay, days | +1.89 days | (0.00, +3.78 days) | .05 | +1.74 days | (−0.12, +3.61 days) | 0.07 |
| In-hospital mortality | 4.78 | (1.49, 15.32) | .009 | 4.31 | (1.44, 12.87) | 0.01 |
| HIV-infected vs. HIV-unexposed | ||||||
| Primary outcome | ||||||
| Treatment failure at 48 h | 2.55 | (1.61, 4.03) | <.0001 | 2.08 | (1.39, 3.12) | 0.0004 |
| Secondary outcomes | ||||||
| Days of supplemental oxygen | +7.27 days | (+5.00, +9.54 days) | <.0001 | +6.84 days | (+4.58, +9.10 days) | <0.0001 |
| Days of CPAP or mechanical ventilation | +1.08 days | (+0.44, +1.73 days) | .001 | +0.95 days | (+0.31, +1.59 days) | 0.004 |
| Length of stay, days | +12.29 days | (+9.28, +15.29 days) | <.0001 | +11.82 days | (+8.83, +14.80 days) | <0.0001 |
| In-hospital mortalityc | 3.83 | (0.75, 19.56) | .11 | 3.05 | (0.66, 14.06) | 0.15 |
Abbreviations: HIV-EU, HIV-exposed uninfected; CPAP, continuous positive airway pressure.
aWald χ2 P values.
bRisk ratios (or differences in means) estimated from Cox proportional hazards models (or linear regression models) adjusted for age and proximity to health care services.
cEstimates are based on only N = 2 deaths among HIV-infected children.
As infant-feeding practices may contribute to the observed differences in treatment outcomes by HIV exposure status, we sought to examine whether current breastfeeding was associated with treatment failure within categories of HIV exposure status. Only 4 HIV-EU and 7 HIV-infected children were breastfeeding at enrollment, and we did not have sufficient power to assess whether reduced breastfeeding contributed to the poor outcomes of these children. However, current breastfeeding did not alter the risk of treatment failure among HIV-unexposed children (RR: 0.85, 95% CI: 0.46–1.56, P = .59) in analyses adjusting for age and low birth weight.
Antimicrobial Treatment
One hundred fifty (63%) children received a WHO-recommended antibiotic regimen during the first 48 hours, with the most common regimen being ampicillin and gentamicin (Table 3). Among children who did not receive antibiotics or for whom antibiotics were discontinued before 48 hours (n = 71), only 16% failed treatment and none died; 66% had a discharge diagnosis of bronchiolitis. Six of 17 (35%) children who started antibiotics >6 hours after presentation failed treatment, and one HIV-unexposed child died.
Table 3.
Antimicrobial Treatment Regimens Received by N = 238 Children Aged 1–23 Months from 0 to 48 H After Presentation to a Tertiary Hospital in Gaborone, Botswana, April 2012–October 2013
| N (column %) |
||||
|---|---|---|---|---|
| Total (N = 238)a | HIV-unexposed (N = 153) | HIV-EU (N = 64) | HIV-infected (N = 20) | |
| Received WHO antibiotic treatmentb | 150 (63.0) | 84 (54.9) | 46 (71.9) | 19 (95.0) |
| Amoxicillin (oral, >80 mg/kg per day)c,d | 12 (5.0) | 7 (4.6) | 5 (7.8) | 0 (0.0) |
| Ampicillin and gentamicin | 84 (35.3) | 53 (34.6) | 23 (35.9) | 7 (35.0) |
| Ampicillin and gentamicin only | 60 (25.2) | 41 (26.8) | 13 (20.3) | 5 (25.0) |
| Ampicillin, gentamicin and high-dose TMP-SMXe | 4 (1.7) | 0 (0.0) | 3 (4.7) | 1 (5.0) |
| Switched to amoxicillin (oral) before 48 hc | 7 (2.9) | 5 (3.3) | 2 (3.1) | 0 (0.0) |
| Switched to 3rd-gen cephalosporin ± vancomycin before 48 h | 13 (5.5) | 7 (2.9) | 5 (7.8) | 1 (5.0) |
| 3rd-generation cephalosporin (ceftriaxone or ceftriaxone) | 54 (22.7) | 24 (15.7) | 18 (28.1) | 12 (60.0) |
| 3rd-gen cephalosporin only | 23 (9.7) | 16 (10.5) | 4 (6.3) | 3 (15.0) |
| 3rd-gen cephalosporin with vancomycin | 12 (5.0) | 7 (4.6) | 3 (4.7) | 2 (10.0) |
| 3rd-gen cephalosporin and high-dose TMP-SMXe | 5 (2.1) | 0 (0.0) | 2 (3.1) | 3 (15.0) |
| 3rd-gen cephalosporin, vancomycin, and high-dose TMP-SMXe | 14 (5.9) | 1 (0.7) | 9 (14.1) | 4 (20.0) |
| Did not receive WHO antibiotic treatment | 88 (37.0) | 69 (45.1) | 18 (28.1) | 1 (5.0) |
| Did not receive antibiotics | 51 (21.4) | 41 (26.8) | 10 (15.6) | 0 (0) |
| WHO antibiotic regimen started >6 h after presentation | 17 (7.1) | 10 (6.5) | 7 (10.9) | 1 (5.0) |
| WHO antibiotics discontinued before 48 h after presentation | 20 (8.4) | 18 (11.8) | 2 (3.1) | 0 (0) |
Abbreviations: HIV-EU, human immunodeficiency virus–exposed uninfected; WHO, World Health Organization; TMP-SMX, trimethoprim-sulfamethoxazole.
aHIV exposure status could not be established for N = 1 child.
bChildren were considered to have received a WHO antibiotic regimen if they were on a regimen that included at least the recommended antibiotic(s), even if the child received additional antimicrobial agents.
cAmoxicillin is recommended only for treatment of nonsevere pneumonia in HIV-negative children; all N = 19 children who received this treatment were HIV-negative and had nonsevere disease.
dN = 7 children received treatment with amoxicillin-clavulanate.
eN = 15 (65%) children who received high-dose trimethoprim-sulfamethoxazole also received corticosteroids.
Compared with HIV-unexposed children, HIV-EU children were more likely to receive an initial regimen containing a third-generation cephalosporin (P = .03), and were more often treated for PCP with high-dose TMP-SMX (P < .0001). Thirty HIV-EU children failed treatment at 48 hours, including 26 (87%) who received a WHO antibiotic regimen and 15 (50%) who received a third-generation cephalosporin. In multivariable analyses limited to HIV-EU children, treatment with a third-generation cephalosporin did not reduce the risk of treatment failure compared with ampicillin and gentamicin (RR: 1.01, 95% CI: 0.56–1.82, P = .98).
CONCLUSIONS
Among children with pneumonia in Botswana, HIV-EU children were significantly more likely to fail treatment at 48 hours, required more days of CPAP or mechanical ventilation, and had higher in-hospital mortality than HIV-unexposed children. These differences in outcomes by HIV exposure status were observed only among children <6 months of age and were not attenuated by receipt of second-line antimicrobial therapy.
There are several potential explanations for the poor outcomes observed among HIV-EU children. In utero exposure to HIV and antiretroviral medications results in a broad spectrum of abnormalities of the adaptive and innate immune systems. These immune abnormalities are present at birth and resolve with age, although differences in several parameters remain detectable until at least 24 months of age [22–24]. Abnormalities of lymphocyte number are perhaps the most extensively studied, with HIV-EU infants having fewer naïve and total CD4+ lymphocytes and CD8+ lymphocytes than HIV-unexposed infants [22, 25, 26]. These abnormalities appear to be more pronounced among HIV-EU infants exposed in utero to antiretroviral therapy. However, small but significant reductions in lymphocyte number are still observed among HIV-EU infants whose mothers did not receive antiretroviral therapy, suggesting that exposure to an altered maternal immune system or HIV itself might also contribute [23–25, 27]. HIV-EU infants also have lower neutrophil counts, altered cytokine production, and acquire lower levels of maternal antibodies specific to many common pathogens including Streptococcus pneumoniae [22–25, 28, 29]. Finally, breastfeeding has established immunologic benefits and reduces pneumonia incidence and all-cause mortality among HIV-EU infants in developing countries [30–33].
Although reserved for second-line therapy in current WHO guidelines, third-generation cephalosporins are widely available alternatives that provide improved coverage of several bacterial pathogens, including penicillin-resistant S pneumoniae and some Gram-negative bacteria. Few studies have compared the effectiveness of third-generation cephalosporins to other antimicrobial regimens used to treat childhood pneumonia. A recent study that examined data from 43 children's hospitals in the United States reported similar outcomes among hospitalized children treated with a third-generation cephalosporin or ampicillin (or penicillin) [34]. In our cohort, receipt of a third-generation cephalosporin did not reduce the risk of treatment failure among HIV-EU children compared with ampicillin and gentamicin. This may relate to the relatively small differences in spectra between these antimicrobial regimens, or to the importance of pathogens not treated by either regimen, including respiratory viruses and tuberculosis. Several case series also describe PCP among HIV-EU infants, and McNally et al detected P jirovecii from bronchoalveolar lavage in one-third of HIV-EU children failing treatment at 48 hours [12, 35, 36]. Although microbiological confirmation was not possible in our setting, 4 HIV-EU children met clinical criteria for probable PCP and 3 of these children died, suggesting that PCP may have contributed to the increased mortality observed among HIV-EU children.
Our study has several limitations. First, this research was conducted at a tertiary hospital staffed by pediatricians with access to CPAP and mechanical ventilation, and the findings may not be generalizable to facilities with more limited capabilities. Second, HIV testing of children at enrollment was at the discretion of the clinical team, and it is possible that a child classified as HIV-EU might have been infected through recent breastfeeding. However, the effect of HIV exposure on the risk of treatment failure was unchanged in sensitivity analyses excluding HIV-EU children who were not tested for HIV at enrollment or HIV-EU and HIV-unexposed children who had breastfed in the prior 6 weeks or were not yet 6 weeks of age. False-negative HIV testing is also unlikely as the HIV tests we used have sensitivity >98–99% for the diagnosis of HIV-1 infection [37–39]. Given the observational study design, antibiotic regimens were not systematically assigned to study participants, and the potential for unmeasured confounding by indication exists. Our case definition for PCP may also not reliably estimate the contribution of P jirovecii to the poor outcomes of HIV-EU and HIV-infected children. Finally, few HIV-EU infants in our cohort were breastfed, and we did not have statistical power to determine whether reduced breastfeeding contributed to the poor outcomes of these children. Current breastfeeding did not prevent treatment failure among HIV-unexposed children, but a benefit of breastfeeding on clinical outcomes may differ by HIV exposure status.
Our results indicate that HIV exposure is associated with treatment failure and mortality among HIV-uninfected children with WHO-defined pneumonia during the first 6 months of life. Treatment with a third-generation cephalosporin did not prevent treatment failure among HIV-EU children, suggesting that altering treatment recommendations to expand coverage of typical bacterial pathogens may not improve outcomes among these children. Further research is needed to confirm these findings and to investigate the roles of infant feeding practices and PCP in mediating the poor outcomes of HIV-EU children meeting clinical criteria for pneumonia.
SUPPLEMENTARY DATA
Supplementary materials are available at The Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We would like to thank Ms. Boitshepo Seme, Ms. Pearl Monate, and other staff at the Botswana-University of Pennsylvania Partnership who assisted in data collection. We offer sincere thanks to the children and families who participated in this study.
Financial support. This work was supported by an Early Career Award from the Thrasher Research Fund (to M. S. K.), by the Children's Hospital of Philadelphia (to A. P. S. and K. A. F.) and Pincus Family Foundation, and through core services and support from the Penn Center for AIDS Research, a National Institutes of Health (NIH)–funded program (P30-AI045008). Funding for this project was also made possible in part by a CIPHER grant from the International AIDS Society, supported by ViiV Healthcare. The views expressed in this publication do not necessarily reflect the official policies of the International AIDS Society or ViiV Healthcare. C. K. C. received financial support from the National Institutes of Health through the Duke Center for AIDS Research (P30-AI064518).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.The United Nations Joint Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic. Available at: http://www.unaids.org Accessed January 12, 2014.
- 2.Koyanagi A, Humphrey JH, Ntozini R, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J 2011; 30:45–51. [DOI] [PubMed] [Google Scholar]
- 3.Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 2007; 26:519–26. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro RL, Lockman S, Kim S, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis 2007; 196:562–9. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis 2005; 41:1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell ML, Coovadia H, Cotrina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–43. [DOI] [PubMed] [Google Scholar]
- 7.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health 2009; 14:276–87. [DOI] [PubMed] [Google Scholar]
- 8.United Nations Children's Fund (UNICEF). Pneumonia and diarrhea: tackling the deadliest diseases for the world's poorest children. Available at: http://www.unicef.org Accessed December 10, 2013.
- 9.World Health Organization (WHO), Department of Child and Adolescent Health and Development. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. 2nd ed Geneva, Switzerland: WHO Press; 2013. [PubMed] [Google Scholar]
- 10.Kourtis AP, Wiener J, Kayira D, et al. Health outcomes of HIV-exposed uninfected African infants. AIDS 2013; 27:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mussi-Pinhata MM, Freimanis L, Yamamoto AY, et al. Infectious disease morbidity among young HIV-1-exposed but uninfected infants in Latin American and Caribbean countries: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Pediatrics 2007; 119:e694–704. [DOI] [PubMed] [Google Scholar]
- 12.McNally LM, Jeena PM, Gajee K, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet 2007; 369:1440–51. [DOI] [PubMed] [Google Scholar]
- 13.Webb C, Ngama M, Ngatia A, et al. Treatment failure among Kenyan children with severe pneumonia—a cohort study. Pediatr Infect Dis J 2012; 31:e152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The United Nations Joint Programme on HIV/AIDS (UNAIDS). Botswana Global AIDS Response Report, 2012. Available at: http://www.unaids.org Accessed February 16, 2014.
- 15.World Health Organization (WHO). Guidelines on HIV and Infant Feeding: Principles and Recommendations for Infant Feeding in the Context of HIV and a Summary of Evidence. Geneva, Switzerland: WHO Press; 2010. [PubMed] [Google Scholar]
- 16.Botswana Ministry of Health. Botswana National HIV & AIDS Treatment Guidelines. Gaborone, Botswana: Botswana Ministry of Health, 2012. [Google Scholar]
- 17.World Health Organization (WHO), United Nations Children's Fund (UNICEF). WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. Geneva, Switzerland: WHO Press, 2009. [PubMed] [Google Scholar]
- 18.Addo-Yobo E, Chisaka N, Hassan M, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomised multicentre equivalency study. Lancet 2004; 364:1141–8. [DOI] [PubMed] [Google Scholar]
- 19.Tchetgen Tchetgen EJ. Estimation of risk ratios in cohort studies with a common outcome: a simple and efficient two-stage approach. Int J Biostats 2013; 16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott, Williams & Wilkins, 2008. [Google Scholar]
- 21.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009; 20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 2000; 96:3866–71. [PubMed] [Google Scholar]
- 23.Pacheco SE, McIntosh K, Lu M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: an analysis of the Women and Infants Transmission Study. J Infect Dis 2006; 194:1089–97. [DOI] [PubMed] [Google Scholar]
- 24.Bunders M, Thorne C, Newell ML. Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. AIDS 2005; 19:1071–9. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen SD, Jeppesen DL, Kolte L, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood 2001; 98:398–404. [DOI] [PubMed] [Google Scholar]
- 26.Embree J, Bwayo J, Nagelkerke N, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr Infect Dis J 2001; 20:397–403. [DOI] [PubMed] [Google Scholar]
- 27.Le Chenedac J, Mayaux MJ, Guihenneuc-Jouyaux C, et al. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS 2003; 17:2051–61. [DOI] [PubMed] [Google Scholar]
- 28.Chougnet C, Kovacs A, Baker R, et al. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis 2000; 181:1590–7. [DOI] [PubMed] [Google Scholar]
- 29.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011; 305:576–84. [DOI] [PubMed] [Google Scholar]
- 30.Cournil A, De Vincenzi I, Gaillard P, et al. Relationship between mortality and feeding modality among children born to HIV-infected mothers in a research setting: the Kesho Bora Study. AIDS 2013; 27:1621–30. [DOI] [PubMed] [Google Scholar]
- 31.Taha TE, Hoover DR, Chen S, et al. Effects of cessation of breastfeeding in HIV-1-exposed, uninfected children in Malawi. Clin Infect Dis 2011; 53:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Uria G, Midde M, Pakam R, Bachu L, Kumar Naik P. Effect of formula feeding and breastfeeding on child growth, infant mortality, and HIV transmission in children born to HIV-infected pregnant women who received triple antiretroviral therapy in a resource-limited setting: data from an HIV cohort study in India. ISRN Pediatr 2012; 2012:763591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asbjornsdottir KH, Slyker JA, Weiss NS, et al. Breastfeeding is associated with decreased pneumonia incidence among HIV-exposed, uninfected Kenyan infants. AIDS 2013; 27:2809–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams DJ, Hall M, Shah SS, et al. Narrow vs. broad-spectrum antimicrobial therapy for children hospitalized with pneumonia. Pediatrics 2013; 132:e1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heresi GP, Caceres E, Atkins JT, Reuben J, Doyle M. Pneumocystis carinii pneumonia in infants who were exposed to human immunodeficiency virus but were not infected: an exception to the AIDS surveillance case definition. Clin Infect Dis 1997; 25:739–40. [DOI] [PubMed] [Google Scholar]
- 36.Slogrove AL, Cotton MF, Esser MM. Severe infections in HIV-exposed uninfected infants: clinical evidence of immunodeficiency. J Trop Pediatr 2010; 56:75–81. [DOI] [PubMed] [Google Scholar]
- 37.Sherman GG, Cooper PA, Coovadia AH, et al. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J 2005; 24:993–7. [DOI] [PubMed] [Google Scholar]
- 38.Creek T, Tanuri A, Smith M, et al. Early diagnosis of human immunodeficiency virus in infants using polymerase chain reaction on dried blood spots in Botswana's national program for prevention of mother-to-child transmission. Pediatr Infect Dis J 2008; 27:22–6. [DOI] [PubMed] [Google Scholar]
- 39.Piwowar-Manning EM, Tustin NB, Sikateyo P, et al. Validation of rapid HIV antibody tests in 5 African countries. J Int Assoc Physicians AIDS Care 2010; 9:170–2. [DOI] [PMC free article] [PubMed] [Google Scholar]