Abstract
Background
Limited studies incorporating population-based pharmacokinetic modeling have been conducted to determine pharmacodynamic indices associated with nephrotoxicity during vancomycin exposure in children.
Methods
A retrospective cohort analysis was conducted from September 2003 to December 2011 at 2 hospitals. Nephrotoxicity was defined as an increase in serum creatinine concentration (SCr) by ≥0.5 mg/dL, or ≥50% increase in baseline SCr, either persisting for ≥2 consecutive days. A 1-compartment model with first-order kinetics was used in NONMEM 7.2 to estimate trough concentrations (Cmin) and area under the curve over 24 hours (AUC). Univariate, classification and regression tree (CART), and multivariate analyses were conducted to identify factors contributing to nephrotoxicity.
Results
The analyses included 680 pediatric subjects with 1576 vancomycin serum concentrations. Based on univariate analysis, median Cmin (14.2 [interquartile range, IQR, 7.1–25.4] vs 8.4 [IQR, 5.5–12.4] mcg/mL; P = .001) and AUC (544 [IQR, 359–801] vs 378 [IQR, 304–494]; P < .001) were significantly higher in the nephrotoxic group compared with the non-nephrotoxic group. Using CART, we discovered that subjects with doses ≥60 mg/kg per day and AUC >1063 mg-h/L had a significantly higher occurrence of nephrotoxicity (P = .005). Adjusting for intensive care unit stay and concomitant nephrotoxic drugs, steady-state vancomycin Cmin ≥15 mcg/mL (adjusted odds ratio [aOR], 2.5; 95% confidence interval [CI], 1.1–5.8; P = .028) and AUC ≥800 mg-h/L (aOR, 3.7; 95% CI, 1.2–11.0; P = .018) were associated with increased risk of nephrotoxicity.
Conclusions
Our study describes the pediatric exposure-nephrotoxicity relationships for vancomycin. Vancomycin Cmin ≥15 mcg/mL and AUC ≥800 mg-h/L in children are independently associated with a > 2.5-fold increased risk of nephrotoxicity and may provide justification for use of alternative antibiotics in selected situations.
Keywords: nephrotoxicity, pediatrics, pharmacodynamic, pharmacokinetics, vancomycin
Vancomycin, a bactericidal glycopeptide antibiotic, is the treatment of choice for certain methicillin-resistant Staphylococcus aureus (MRSA) infections. Observations of increasing vancomycin minimum inhibitory concentrations (MICs) in MRSA that are still interpreted as “susceptible,” associated with apparent poor clinical responses, has led to the use of higher doses of vancomycin in adults [1–5]. The area under the curve over 24 hours (AUC) to MIC ratio (AUC/MIC) is the pharmacodynamic (PD) index that best predicts the efficacy of vancomycin [6]. A study involving adult patients with S aureus lower respiratory infections reported that an AUC/MIC ≥400 was associated with a statistically significant improved clinical response and microbiological eradication compared with an exposure below this level [7]. In addition, AUC/MIC ≥400 best correlates to serum trough concentrations (Cmin) of 15–20 mcg/mL, and doses up to 3–4 g/day may be necessary to achieve this AUC/MIC target in adults with normal renal function [1, 8]. In a recent large, 2-center study in children, doses of 60–70 mg/kg per day, calculated using a population-based pharmacokinetic (PK) model with Monte Carlo simulation, could be used to treat children infected by pathogens whose MIC's were ≤2 mg/L, to achieve an AUC/MIC ≥400 in 75% of subjects. At this dosage, the predicted Cmin was noted to be only 8–9 mcg/mL [9, 10].
Not unexpectedly, higher vancomycin dosages maybe associated with an increase in nephrotoxicity [11–16]. Studies in adults suggest increasing rates of nephrotoxicity with escalating doses of vancomycin to achieve the Cmin of 15–20 mcg/mL [12–14, 16]. The vancomycin exposure-nephrotoxicity response in adults is best predicted by initial vancomycin Cmin≥ 9.9 mcg/mL and doses >4 grams/day [11, 12].
Studies of vancomycin serum trough concentrations in children with respect to nephrotoxicity are limited, but an association between serum trough concentrations ≥15 mcg/mL and nephrotoxicity has been reported [15, 17]. Previous pediatric studies have not incorporated population-based modeling using post hoc Bayesian analysis to evaluate the exposure-response relationship between vancomycin exposure and nephrotoxicity. In particular, the vancomycin AUC has not been examined in the context of achieving a specific AUC/MIC target in exposure-toxicity studies in children, although an AUC/MIC of 400 (compared with a Cmin of 15–20 mcg/mL) has been demonstrated to be a more achievable target in children [9, 10, 18]. The primary objective of this study was to determine the PK indexes (including Cmin and AUC) that best predict nephrotoxicity in children receiving vancomycin.
METHODS
Study Design
This retrospective cohort analysis was conducted in children who received vancomycin from September 2003 to December 2011 at 2 pediatric hospitals. Miller Children's Hospital (MCH) is a community-based, tertiary care, teaching hospital with 249 beds (34 pediatric intensive care, 69 neonatal intensive care, 94 general pediatrics, and 52 hematology/oncology beds). Rady Children's Hospital of San Diego (RCHSD) is a tertiary care, teaching hospital with 308 beds (44 pediatric intensive care, 49 neonatal intensive care, 177 general medical/surgical, and 38 hematology/oncology beds). This study was approved by the institutional review boards at each institution with the use of a waiver of informed consent for retrospective, deidentified data collection and analysis. Subjects were included if they: (1) were 3 months to 21 years of age; (2) received vancomycin for ≥48 hours; and (3) had normal baseline serum creatinine (SCr) as defined by age at the start of vancomycin therapy (see Supplementary Table 1) [19, 20]. Subjects were excluded if they received dialysis or certain concomitant medications that are associated with nephrotoxicity, including amphotericin B, methotrexate, ifosfamide, cyclosporine, and tacrolimus.
Subject demographic and microbiologic data (eg, cultures, MIC) were collected from the electronic medical records (Epic Systems Corporation at MCH and Chartmaxx at RCHSD) and pharmacy records. Concurrent medications (including all anti-infectives, vasopressors, diuretics, and contrast dye) received 2 days before initiation of vancomycin were recorded. All vancomycin regimens (ie, date, time, dosing regimen, and duration) were documented using flowsheets that pharmacists used to conduct therapeutic drug monitoring. In addition, SCr concentration values were recorded daily from the first day of (or the day before initiation of vancomycin therapy) to 72 hours after therapy completion or until discharge (whichever was sooner). The serum concentrations of vancomycin in all subjects were determined by hospital laboratory assays available at each site [10].
Data Analysis
The methodologies for the population-based PK analyses have been described previously [10]. Using the first-order conditional estimation subroutine and the interaction option in NONMEM 7.2, a 1-compartment model with first-order kinetics was used to estimate Bayesian post hoc values for volume of distribution (Vd), clearance (CL), AUC, and Cmin. The AUC (mg-h/L) was calculated by 24-hour dose (mg/day) ÷ CL (L/h); and steady-state Cmin was determined by the intermittent short infusion model with a 1-h infusion time (Dose = [(Cmin)(CL)(tin)(1−e−kτ)]/[(1−e−ktin)(e−ktmin)], where tin = infusion time, τ = dosing interval, k = elimination rate constant, and tmin = time to Cmin as calculated from the end of infusion).
The primary endpoint was the onset of nephrotoxicity and was defined as an SCr increase of≥ 0.5 mg/dL, or a ≥50% increase in baseline SCr, on at least 2 consecutive days, as has been previously defined in other vancomycin studies [12, 15, 25]. Analyses of patient demographics were summarized by descriptive statistics using SPSS version 21 (IBM, Chicago, IL). For univariate analyses, categorical variables were compared using Pearson χ2 test, and continuous variables were compared using the Student's t test. Initial steady-state (assumed to occur by a timepoint defined as >3.3 half-lives of vancomycin) factors (including Cmin, AUC, and mg/kg per day doses) achieved within the first 72 hours were dichotomized using classification and regression tree (CART). Using some variables significantly associated with nephrotoxicity in the univariate analyses (P < .05) and CART, specific factors were then categorized (ie, Cmin ≤9.9, 10–14.9 mcg/mL, 15–19.9 and ≥20 mcg/mL; AUC <800 and ≥800 mg-h/L; and dose <60 and ≥60 mg/kg per day) and analyzed using multivariate logistic regression modeling. A stepwise approach was used to derive a parsimonious model, and variables remained in the final model if the associated P value was <.05. Adjusted odds ratios (aORs), which were adjusted for pediatric intensive care unit (PICU) stay and concurrent use of nephrotoxic drugs, were computed for variables in the final multivariate model [15]. Stratified Kaplan-Meier analysis was used to examine time to nephrotoxicity. The nonparametric, 2-tailed Spearman's rank correlation coefficient was calculated to determine the statistical dependence between AUC and Cmin.
RESULTS
In total, 1698 subjects were screened and 1018 were excluded (see Supplementary Figure 1). The remaining 680 subjects had a total of 1576 vancomycin serum concentrations used in the population-based PK modeling. Nineteen subjects had multiple vancomycin treatment courses that were accounted for separately.
Demographic variables, including median age 6.7 years, weight 23 kg, and baseline SCr 0.38 mg/dL, were not statistically significant between subjects with (n = 45) and without (n = 635) nephrotoxicity (Table 1). However, there was a statistically significant increase in the proportion of subjects who experienced nephrotoxicity and either (1) received concurrent nephrotoxic agents (ie, loop diuretics, vasopressors, aminoglycosides, contrast dye, and intravenous acyclovir) (P = .001) or (2) stayed in the PICU (P < .001) (Table 1). In addition, the median number of days of vancomycin therapy was significantly higher in those with toxicity compared with those who did not demonstrate toxicity (8 vs 4 days, respectively; P < .001).
Table 1.
Univariate Analysis of Demographics Overall and Between Subjects With or Without Nephrotoxicity
| Overall N = 680 |
With Nephrotoxicity n = 45 |
Without Nephrotoxicity n = 635 |
P Value | |
|---|---|---|---|---|
| Median age (IQR), years | 6.7 (2.2–13.7) | 5.1 (1.5–14.3) | 6.8 (2.4–13.6) | .605 |
| <1, no. (%) | 88 (12.9) | 10 (22.2) | 78 (12.3) | – |
| 1 to <2, no. (%) | 65 (9.6) | 1 (2.2) | 64 (10.1) | – |
| 2 to <12, no. (%) | 305 (44.9) | 19 (42.2) | 286 (45.0) | – |
| ≥12, no. (%) | 222 (32.6) | 15 (33.3) | 207 (32.6) | – |
| Median weight (IQR), kg | 23.1 (12.8–46.7) | 23.4 (12.9–42.4) | 23 (12.8–47.3) | .573 |
| Median ideal body weight (IQR), kg | 23.4 (12.8–38.6) | 21.2 (12.0–37.1) | 23.8 (12.8–38.6) | .508 |
| Median body surface area (IQR), m2 | 0.9 (0.6–1.4) | 0.9 (0.6–1.3) | 0.9 (0.6–1.4) | .525 |
| Median body mass index (IQR), kg/m2 | 17.6 (15.6–21.0) | 17.4 (15.6–22.0) | 17.6 (15.6–20.9) | .932 |
| Race/Ethnicity | .579 | |||
| Hispanic, no. (%) | 247 (36.3) | 16 (35.6) | 231 (36.3) | – |
| White, no. (%) | 90 (13.2) | 6 (13.3) | 84 (13.2) | – |
| African-American, no. (%) | 42 (6.2) | 2 (4.4) | 40 (6.3) | – |
| Asian, no. (%) | 14 (2.1) | 2 (4.4) | 12 (1.9) | – |
| Other/Unknown, no. (%) | 287 (42.2) | 19 (42.2) | 268 (42.2) | – |
| Male gender, no. (%) | 361 (53.1) | 20 (44.4) | 341 (53.7) | .229 |
| Intensive care unit stay, no. (%) | 271 (39.9) | 32 (71.1) | 239 (37.6) | < .001 |
| Concurrent use of nephrotoxic agents, no. (%) | 264 (38.8) | 28 (62.2) | 236 (37.2) | .001 |
| Median baseline serum creatinine (IQR), mg/dL | 0.4 (0.3–0.5) | 0.3 (0.2–0.6) | 0.4 (0.3–0.5) | .380 |
| Mean empiric vancomycin dose ± SD (IQR), mg/kg per day | 46.7 ± 11.6 (39.8–57.2) | 50.2 ± 11.8 (43.5–60.2) | 46.4 ± 11.6 (39.7–59.6) | .033 |
| Every 6 h, no. (%) | 281 (41.3) | 18 (40.0) | 263 (41.4) | – |
| Every 8 h, no. (%) | 343 (50.4) | 26 (57.8) | 317 (49.9) | – |
| Every 12 h, no. (%) | 50 (7.4) | 0 (0) | 50 (7.9) | – |
| Every 16–24 h, no. (%) | 6 (0.9) | 1 (2.2) | 5 (0.8) | – |
| Median duration of vancomycin therapy (IQR), days | 4 (3–7) | 8 (6–11) | 4 (3–7) | < .001 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Nine models were created to characterize Vd and CL (see Supplementary Table 2). Only age, SCr, and weight were independent covariates with respect to CL and weight was an independent covariate with respect to Vd in both univariate and multivariate analyses. These covariates were used in the final model to produce post hoc Bayesian estimates for CL, Vd, Cmin, and AUC (see Supplementary Table 3). In the final model, minimization and the covariance step were successful. The median post hoc Bayesian estimates of Vd and CL on the first day of vancomycin therapy were 0.63 L/kg and 0.12 L/kg per hour respectively, for our study population, and both were not statistically significant between subjects with and without nephrotoxicity. The post hoc Bayesian estimates for Vd and CL were similar to the median bootstrap analysis values and were within the 95% confidence intervals (CIs) obtained from the bootstrap analysis (see Supplementary Table 4).
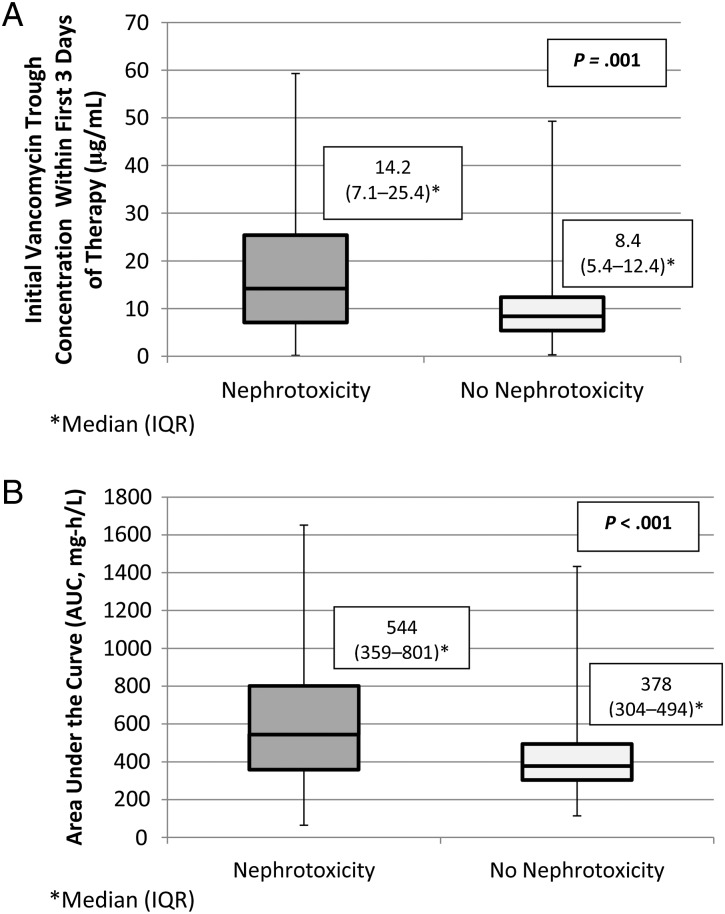
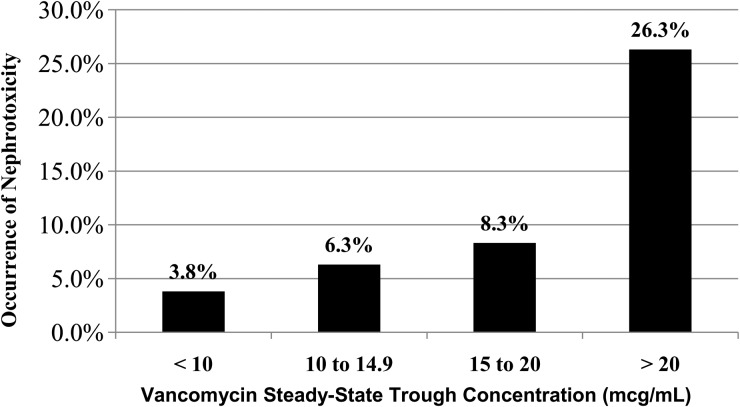
Using univariate analyses, we noted that the median Cmin and AUC were significantly higher in the nephrotoxic vs nonnephrotoxic groups (14.2 vs 8.4 mcg/mL [P = .001] and 544 vs 378 mg-h/L [P < .001], respectively) (Figure 1, A and B). There was a significant positive correlation between nephrotoxicity and high initial steady-state vancomycin Cmin (P < .001; Figure 2). We also noted a significant positive correlation between doses ≥60 mg/kg per day compared with doses <60 mg/kg per day (14.4% vs 5.3%, respectively; P = .001). The incidence of nephrotoxicity in those with steady-state vancomycin Cmin ≥15 mcg/mL and doses ≥60 mg/kg per day were 17% (n = 117) and 14.4% (n = 97), respectively. Using univariate logistic regression, we noted that the mean steady-state vancomycin trough concentration, AUC, and dose were significantly higher among patients who experienced nephrotoxicity compared with those who did not (Table 2). It is also noteworthy that 10%, 33%, and 57% of subjects who achieved AUC ≥400, 800, and 1000 mg-h/L, respectively, experienced nephrotoxicity. The AUC was significantly correlated to Cmin (Spearman's coefficient = 0.963; P < .001).
Figure 1.
(A) Univariate analysis for steady-state vancomycin trough concentration within the first 3 days of therapy (µg/mL). (B) Univariate analysis for steady-state, area under the curve over 24 hours.
Figure 2.
Relationship between steady-state vancomycin trough concentration and the occurrence of nephrotoxicity.
Table 2.
Univariate Analysis of Vancomycin Exposure Profile and Nephrotoxicitya
| Antibiotic Exposure Profile | Patients With Nephrotoxicity (n = 45) |
Patients Without Nephrotoxicity (n = 635) | P |
|---|---|---|---|
| Steady-state trough concentration, median (IQR) in mg/L | 14 (7–25) | 8 (5–12) | .001 |
| ≥15 mcg/mLb | 20 (17) | 97 (83) | < .001 |
| <15 mcg/mLb | 25 (4) | 538 (96) | |
| Steady-state AUC over 24 h, median (IQR) in mg-h/L | 544 (359–801) | 378 (304–494) | < .001 |
| ≥400 mg-h/Lb | 31 (10) | 276 (90) | .001 |
| <400 mg-h/Lb | 14 (4) | 359 (96) | |
| ≥800 mg-h/Lb | 11 (33) | 22 (67) | < .001 |
| <800 mg-h/Lb | 34 (5) | 613 (95) | |
| ≥1000 mg-h/Lb | 8 (57) | 6 (43) | < .001 |
| <1000 mg-h/Lb | 37 (6) | 629 (94) | |
| Initial dose, median (IQR) in mg/kg per day | 47.8 (44.4–60.0) | 45.0 (39.7–56.2) | .04 |
| ≥60 mg/kg per dayb | 13 (14) | 78 (86) | .002 |
| <60 mg/kg per dayb | 32 (5) | 557 (95) |
Abbreviations: AUC, area under the curve; IQR, interquartile range.
aData are no. (%) of subjects, unless otherwise noted.
bPercentages are based on total subjects within the specified AUC targets.
Classification and regression tree analysis was used to identify 2 subgroups in the initial population of 680 patients (see Supplementary Figure 2). The first dichotimization was steady-state AUC >1063 mg-h/L, which had a significantly higher incidence of nephrotoxicity (P = .013). For subjects with AUC >1063 mg-h/L, the second dichotomization was steady-state dosing of ≥60 mg/kg per day, in which subjects experienced nephrotoxicity significantly more than those who received steady-state dosing of <60 mg/kg per day (P = .005). According to our CART analysis, AUC seems to be a factor predictive of nephrotoxicity, as do, to a lesser degree, initial daily doses. Furthermore, significantly more subjects with nephrotoxicity, compared with those without, received concurrent use of nephrotoxic agents (62.2% vs 37.2%; P = .001) and stayed in the intensive care unit (71.1% vs 37.6%; P < .001). After adjusting for PICU stay and concurrent use of nephrotoxic drugs, we discovered that steady-state vancomycin Cmin≥15 mcg/mL (aOR, 2.5; 95% CI, 1.1–5.8; P = .028) and AUC ≥800 mg-h/L (aOR, 3.7; 95% CI, 1.2–11.0; P = .018) were associated with increased risk of developing nephrotoxicity (Table 3). Because most hospital microbiology laboratories report MIC results as 1, 2, or 4 mg/L, we chose an AUC of 800 (to correlate with an MIC of 2) when calculating the aOR, rather than using an AUC of 1000 identified from CART. In addition, no significant interaction between PICU stay and vancomycin exposure using both Cmin ≥15 mcg/mL (P = .477) and AUC ≥800 mg-h/L (P = .106) was observed in multivariate regression analysis.
Table 3.
Multivariate Logistic Regression Model for the Occurrence of Vancomycin-Associated Nephrotoxicitya,b
| Parameter | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|
| AUC over 24 hc, ≥800 mg-h/L | 3.7 (1.2–11.0) | .018 |
| Trough concentration, ≥15 mcg/mL | 2.5 (1.1–5.8) | .028 |
Abbreviations: AUC, area under the curve; CI, confidence interval; PICU, pediatric intensive care unit.
aAdjusted for stay in the intensive care unit and use of concurrent nephrotoxic medications.
bThe interaction between PICU stay and vancomycin exposure using both Cmin ≥15 mcg/mL (P = .477) and AUC ≥800 mg-h/L (P = .106) was not significant.
cThe adjusted odds ratio (95% CI) for AUC ≥1000 mg-h/L was 8.7 (2.3–32.8), P = .001.
The mean time to nephrotoxicity was 3.6 ± 2.9 days (median 3, range 1–15 days), but there was no significant difference in the time to nephrotoxicity using Kaplan-Meier analysis stratified by the vancomycin Cmin, AUC, or dose ( ≤9.9, 10–14.9, 15–19.9, and ≥20 mcg/mL, P = .225; <10 and ≥10 mcg/mL, P = .652; <800 and ≥800 mg-h/L, P = .573; <60 and ≥60 mg/kg per day, P = .756, respectively). However, subjects who were hospitalized in the PICU had a higher probability of developing nephrotoxicity compared with those who were hospitalized on the wards. The predicted probability of nephrotoxicity was approximately 15% for subjects in the PICU with steady-state vancomycin Cmin ≥15 mcg/mL, compared with a probability of approximately 5% for those hospitalized on the wards (P = .001) (see Supplementary Figure 3). For those hospitalized on the wards, nephrotoxicity was observed in approximately 15% of those with a higher steady-state Cmin of ≥25 mcg/mL (see Supplementary Figure 3).
DISCUSSION
There are adequate studies evaluating the PK of vancomycin-associated nephrotoxicity in adults using PD dosing targets, but very few studies exist in children. In a meta-analysis of 14 adult studies and 1 pediatric study, vancomycin Cmin ≥15 mcg/mL was associated with a 2.67-fold increase in nephrotoxicity, which occurred between 4.3 and 17 days after initiation of vancomycin therapy [16]. The time to nephrotoxicity in our study ranged from 1 to 15 days, implicating the challenge in predicting the time to toxicity for an individual patient. Studies show an incremental risk of nephrotoxicity associated with vancomycin doses >4 g/day and exposure determined by Cmin ≥15 mcg/mL. The risk also increases with longer duration of vancomycin use, concomitant use of other nephrotoxic agents, and in patients who are critically ill or have compromised renal function [12–16].
This is the first study in children uniquely utilizing population-based PK/PD modeling with Bayesian estimation to evaluate significant PD indices of vancomycin-associated nephrotoxicity. With Bayesian estimation, descriptions of PK data in specific populations and plausible explanations for PK/PD variability for drug exposure and toxicity in these populations can be quantified. These defined parameters can then be used to better predict outcomes from analytical models constructed for the population, with implications for the individual patient. Furthermore, Bayesian analysis allows for more accurate quantification of parameters of interest (ie, Vd and CL that can then be used to estimate Cmin and AUC) than standard statistical analysis of PK data, and predictive statements can be more easily derived [21]. Our study also distinctively incorporated an age-based definition for normal baseline SCr values, which is important because renal function changes with age.
Three published pediatric studies have associated vancomycin use with the development of nephrotoxicity, albeit in the presence of other risk factors, such as ICU stay and concurrent nephrotoxic agents [15, 17, 22, 23]. Consistent with multiple adult studies, 1 study reported a 28% incidence of nephrotoxicity in children with vancomycin Cmin ≥15 mcg/mL, which is a 3-fold increase compared with those with Cmin <15 mcg/mL [12–15, 24, 25]. Another study reported a 19% incidence of nephrotoxicity in children with an initial vancomycin Cmin ≥15 mcg/mL and ICU admission as independent predictors of nephrotoxicity that doubled the risk of acute kidney injury [17]. However, in 1 of the studies of children, vancomycin was not associated with nephrotoxicity, probably attributable to the low vancomycin exposure with Cmin <5–10 mcg/mL [22]. Our study demonstrated that vancomycin Cmin ≥15 mcg/mL and AUC ≥800 mg-h/L increased the risk of nephrotoxicity by more than 2.5-fold and 3.7-fold, respectively.
In our CART analysis, we found that elevated AUC >1063 mg-h/L was an important predictor of nephrotoxicity. The median AUC for children who developed nephrotoxicity was 544 (interquartile range [IQR], 359–801) mg-h/L. In addition, 10%, 33%, and 57% of subjects who achieved AUC ≥400, 800, and 1000 mg-h/L, respectively, experienced nephrotoxicity, suggesting a strong exposure-toxicity relationship with high exposure resulting in increased toxicity. Although our CART analysis demonstrated AUC >1063 mg-h/L as the natural “breakpoint” above which nephrotoxicity was more likely to occur with the highest statistical probability, we examined the odds of nephrotoxicity using AUC ≥800 mg-h/L in our multivariate analysis because it permits the relatively safe treatment of organisms with MICs of 2 mg/L, associated with a target AUC of 800 mg-h/L. Clinical and microbiologic efficacy associated with this target AUC is likely to be achieved for organisms with MICs of 2 mg/L, suggesting that, out of concerns for toxicity, an alternative anti-MRSA agent is preferred for pathogens with MICs exceeding 2 mg/L. It is interesting to note that AUC ≥700 mg-h/L has been associated with increased risks of nephrotoxicity in adults [12, 26, 27].
In this study, both Cmin ≥15 mcg/mL and AUC ≥800 mg-h/L were associated with increased risk of nephrotoxicity after controlling PICU stay and concomitant nephrotoxic drugs. Although there may exist some correlation between Cmin and AUC (ie, higher doses leads to higher Cmin and AUC), AUC is unaffected by changes in dosing interval provided that total daily doses remain the same (ie, 3000 mg/day every 6 or 8 hours produce the same AUC, but Cmin will vary).
Large vancomycin doses have been correlated with nephrotoxicity. In 1 retrospective study of 246 adults, a significant difference in nephrotoxicity was distinguished between subjects who received vancomycin ≥4 g/day, <4 g/day, and linezolid (34.6%, 10.9%, and 6.7%, respectively; P = .001) [11]. In fact, vancomycin ≥4 g/day was associated with an increased risk of nephrotoxicity (aOR, 4.4; 95% CI, 1.7–11.8; P = .003) [11]. When normalized to the average adult weight of 70 kg, vancomycin 4 g/day is equivalent to approximately 60 mg/kg per day in an average child. This finding is consistent with our CART analysis showing that vancomycin doses ≥ 60 mg/kg per day, albeit to a lesser degree than AUC, was an important predictor of nephrotoxicity.
Stay in the PICU was associated with nephrotoxicity in the Kaplan-Meier analysis (see Supplementary Figure 3). This finding is similar to published adult and pediatric studies [12, 15, 28]. In 1 adult study, the probability of nephrotoxicity was 10%–15% higher in ICU patients compared to those not in the ICU [28]. Patients in the PICU are at an increased risk for developing nephrotoxicity potentially due to underlying comorbid conditions that significantly decrease renal blood flow (eg, shock) and use of concurrent medications that may compromise renal function. The addition of vancomycin, which is essential for many children in the PICU, only aggravates this risk of renal injury. After adjusting for stay in the PICU and concurrent nephrotoxic agents in our multivariate logistic regression, vancomycin AUC ≥800 mg-h/L and Cmin ≥15 mcg/mL remained as independent predictors of nephrotoxicity.
In one study in which pediatric patients in the ICU were evaluated, nephrotoxicity was not linked to high Cmin of 15–20 mcg/mL when compared with Cmin of 5–15 mcg/mL [23]. Our results differ from that study, perhaps owing to the younger age of subjects in that study (ie, median age 2 years vs 6.7 years). In addition, nephrotoxicity was defined by Cies and Shankar [23] as an absolute increase in SCr of 0.3 mg/dL, or a 50% increase from baseline value in that study, which differed from our study. It is noteworthy that subjects who achieved the high vancomycin Cmin were compared with historical control subjects with low Cmin rather than comparing both groups during the same time interval.
Multiple studies in adults have reported that an AUC/MIC ≥400 is associated with improved clinical outcome [7, 13, 29]. Although the Infectious Diseases Society of America guidelines recommend vancomycin Cmin of 15–20 mcg/mL in adults to target this target AUC/MIC for complicated MRSA infections (eg, endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia), our previous study in children correlated lower Cmin of 8–9 mcg/mL to an AUC ∼400 mg-h/L [1, 7, 10]. Although we acknowledge the need for rapid achievement of therapeutic concentrations, this correlation infers that high Cmin of 15–20 mcg/mL may not be necessary in children, especially in light of the increased risk of nephrotoxicity with Cmin ≥15 mcg/mL, and assuming that one targets an AUC of ∼400 mg-h/L for pathogens with an MIC of ≤1 mcg/mL [10, 15]. One adult study by Neely et al [27], in which non-parametric population PK modeling was used, reported that 60% of 5000 simulated subjects had Cmins <15 mcg/mL despite achieving therapeutic AUC/MIC ≥ 400 mg-h/L.
There were several study limitations to our analysis. Vancomycin concentrations were analyzed at 2 separate clinical laboratories using different assays [10]. We were unable to determine how dose adjustment, which is often necessary in children, contributes to decreased nephrotoxicity. Although our multivariate analysis validated our overall conclusions when adjusting for confounders, our concern is the degree, if any, in which frequently coadministered medications (with a risk of nephrotoxicity also) contribute to nephrotoxicity. We did not use the pediatric risk, injury, failure, loss and end-stage renal disease criteria to define nephrotoxicity, because most children receiving vancomycin only receive them for a defined course, with early indications of renal injury being less concerning given the benefits of vancomycin, and end-stage renal failure with vancomycin being exceedingly rare [30].
For children infected by strains of MRSA with MICs of ≥2 mg/L, future prospective, controlled studies should focus on subjects receiving vancomycin at doses ≥60 mg/kg per day, compared with subjects receiving an antibiotic not known to cause nephrotoxicity (such as linezolid or clindamycin), to better ascertain the high-dose vancomycin-attributable nephrotoxicity in this population.
CONCLUSIONS
Vancomycin exposure using AUC ≥800 mg-h/L and Cmin ≥15 mcg/mL are strong predictors for nephrotoxicity in children. When aggressive vancomycin regimens are initiated empirically in children, clinicians should be vigilant in assessing signs of nephrotoxicity, especially in the presence of concurrent use of nephrotoxic medications or for children in the PICU. For children infected by strains of MRSA demonstrating an MIC >2 mg/L, an alternative anti-MRSA antibiotic should be considered because the risk of nephrotoxicity associated with vancomycin significantly increases when AUC ≥800 mg-h/L and Cmin ≥15 mcg/mL.
Supplementary Data
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases Grant Number K23AI089978 (to J. J. Le.) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant Number U54HD071600 (to J. B. and E. C.) and T35 (to R. M.).
Potential conflicts of interest. J. L. has previously received investigator-initiated grants from Pfizer, Astellas, and Cubist and served on the speaker's bureau for Pfizer. E. C. has served as a consultant to Trius, Cerexa, and Abbott Pharmaceuticals. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52:285–92. [DOI] [PubMed] [Google Scholar]
- 2.Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest 2010; 138:1356–62. [DOI] [PubMed] [Google Scholar]
- 3.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52:3315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S . aureus (MRSA) blood isolates from 2001-05. J Antimicrob Chemother 2007; 60:788–94. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44:3883–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moise PA, Forrest A, Bhavnani SM, et al. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am J Health Syst Pharm 2000; 57(Suppl 2):S4–9. [DOI] [PubMed] [Google Scholar]
- 7.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–42. [DOI] [PubMed] [Google Scholar]
- 8.Jeffres MN, Isakow W, Doherty JA, et al. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest 2006; 130:947–55. [DOI] [PubMed] [Google Scholar]
- 9.Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant staphylococcal infections. Pediatr Infect Dis J 2013; 32:1077–9. [DOI] [PubMed] [Google Scholar]
- 10.Le J, Bradley JS, Murray W, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J 2013; 32:e155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52:1330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodise TP, Patel N, Lomaestro BM, et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009; 49:507–14. [DOI] [PubMed] [Google Scholar]
- 13.Hidayat LK, Hsu DI, Quist R, et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–44. [DOI] [PubMed] [Google Scholar]
- 14.Jeffres MN, Isakow W, Doherty JA, et al. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther 2007; 29:1107–15. [DOI] [PubMed] [Google Scholar]
- 15.McKamy S, Hernandez E, Jahng M, et al. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr 2011; 158:422–6. [DOI] [PubMed] [Google Scholar]
- 16.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013; 57:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoderer CA, Nichols KR, Lyon KC, Veverka MM, Wilson AC. Are elevated vancomycin serum trough concentrations achieved within the first 7 days of therapy associated with acute kidney injury in children? J Ped Infect Dis 2014; 3:127–31. [DOI] [PubMed] [Google Scholar]
- 18.Gordon CL, Thompson C, Carapetis JR, et al. Trough concentrations of vancomycin: adult therapeutic targets are not appropriate for children. Pediatr Infect Dis J 2012; 31:1269–71. [DOI] [PubMed] [Google Scholar]
- 19.Pottel H, Vrydags N, Mahieu B, et al. Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta 2008; 396:49–55. [DOI] [PubMed] [Google Scholar]
- 20.Pottel H, Mottaghy FM, Zaman Z, Martens F. On the relationship between glomerular filtration rate and serum creatinine in children. Pediatr Nephrol 2010; 25:927–34. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Bayesian methods in health technology assessment: a review. Health Technol Assess 2000; 4:1–130. [PubMed] [Google Scholar]
- 22.Totapally BR, Machado J, Lee H, et al. Acute kidney injury during vancomycin therapy in critically ill children. Pharmacotherapy 2013; 33:598–602. [DOI] [PubMed] [Google Scholar]
- 23.Cies JJ, Shankar V. Nephrotoxicity in patients with vancomycin trough concentrations of 15–20 mug/ml in a pediatric intensive care unit. Pharmacotherapy 2013; 33:392–400. [DOI] [PubMed] [Google Scholar]
- 24.Kralovicova K, Spanik S, Halko J, et al. Do vancomycin serum levels predict failures of vancomycin therapy or nephrotoxicity in cancer patients? J Chemother 1997; 9:420–6. [DOI] [PubMed] [Google Scholar]
- 25.Bosso JA, Nappi J, Rudisill C, et al. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother 2011; 55:5475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki Y, Kawasaki K, Sato Y, et al. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 2012; 58:308–12. [DOI] [PubMed] [Google Scholar]
- 27.Neely MN, Youn G, Jones B, et al. Are vancomycin troughs adequate for optimal dosing? Antimicrob Agents Chemother 2014; 58:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel N, Pai MP, Rodvold KA, et al. Vancomycin: we can't get there from here. Clin Infect Dis 2011; 52:969–74. [DOI] [PubMed] [Google Scholar]
- 29.Kullar R, Davis SL, Taylor TN, et al. Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy 2012; 32:195–201. [DOI] [PubMed] [Google Scholar]
- 30.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007; 71:1028–35. [DOI] [PubMed] [Google Scholar]