Abstract
Background
Cardiovascular diseases presently rank high as leading causes of death globally. The increasing acceptability of phytomedicine is due to the increasing inefficacy of many modern drugs used for the control of many diseases. The aim of this study was to investigate the ameliorative effects of β-sitosterol (BSS) in comparison with lisinopril, a standard antihypertensive drug, on certain biochemical hypertensive parameters in rats.
Methods
Hypertension was induced with cadmium chloride and biochemical analysis of serum was carried out following treatment with BSS and lisinopril. Serum urea, creatinine and electrolytes were assayed using standard kit as tests for renal function, while alkaline phosphatase (ALP), aspartate transaminase (AST) and alanine aminotransferase (ALT) served as enzyme indices of the liver function. The effect on the serum lipid profile was assessed and histological examination performed on tissues of the liver and kidney.
Results
The rats treated with BSS showed a significant decrease (p<0.05) in the serum creatinine concentration when compared with the hypertensive rats. Treatment with lisinopril showed a significant increase (p<0.05) in the activity of AST and ALP when compared with the normal rats. There were slight variations in the concentration of serum electrolytes of rats treated with BSS and lisinopril respectively when compared with normal and hypertensive rats. BSS significantly reduced calcium levels when compared with the hypertensive group. The histopathological examination of the liver and kidney of animals treated with BSS was not different from the control which showed normal histological structure, while the liver of the hypertensive animals showed scanty inflamed cells.
Conclusion
The study shows that BSS is effective in restoring basal liver and kidney functions in hypertensive rats.
Keywords: β-sitosterol, lisinopril, biochemical indices, hypertension
Introduction
Cardiovascular diseases (CVDs) are collectively responsible for 29% of global deaths [1]. Research into the etiology, pathology, and clinical treatment of the two major types of CVD: strokes and coronary heart disease (CHD) revealed that several risk factors including smoking, lack of physical activity, high blood pressure and dyslipidemia are associated with these conditions [2, 3].
Hypertension a major pathological etiology of CVD is defined as the consistent elevation of systemic arterial blood pressure. Despite the important role of hypertension as a cause of disease, its pathogenesis remains largely unknown [4]. Substantial effort has been devoted to defining the pathogenesis of blood pressure variation. Epidemiologic studies have documented the impact of a variety of factors, including age, gender, and body mass index [5]. Diet has also been implicated, with salt, potassium, and calcium suggested as important factors [6]. How these factors influence physiology to alter blood pressure has been the subject of extensive investigation. Genetic predisposition to hypertension is thought to be polygenic, that is, caused by several different genes that together can produce a wide range of phenotypic variations.
Hypertension could largely be classified by etiology as primary or essential hypertension and secondary hypertension. Essential hypertension is the most prevalent hypertension type, affecting 90–95% of hypertensive patients [7]. Hypertension is not only a major risk factor for stroke and heart failure (HF), but more importantly for coronary heart disease (CHD). Evidence suggests that CHD is the most common outcome of hypertension [8]; however, other major risk factors for CHD include smoking and blood cholesterol and they interact in a multiplicative fashion. CHD remains the leading cause of death and disability in the developed countries [9] and is projected to be the leading cause of death in the developing world by 2020 [10].
Phytosterols, plant natural products found in quite a number of plants, are cholesterol homologues with similar chemical structure and biological functions as cholesterol. The most abundant plant sterols are sitosterol, campesterol and stigmasterol [11]. Blood levels of plant sterols in humans are only 0.1 – 0.14% of cholesterol levels [12]. Their lipid-lowering effect is mediated by competitive inhibition of cholesterol absorption and by transcriptional induction of genes implicated in cholesterol metabolism in both enterocytes and hepatocytes [13]. The cholesterol-lowering properties of phytosterols have been extensively studied and exploited in the preparation of functional foods [14]. Recently, there has been renewed interest in other possible biological activities of these compounds in addition to their cholesterol lowering effect. For instance, they have been shown to exhibit anti-cancer activity [15]. It has been demonstrated [16] that β-sitosterol caused a 24% decrease in cell growth and a four-fold increase in apoptosis; antiatherosclerosis activities have also been investigated [17]. The mechanisms by which plant sterols display their anti-inflammatory activity are thought to include inhibition of secretion of inflammatory mediators such as interleukin-6, and tumor necrosis factor-α by monocytes [16].
Another possible effect of plant sterols is their antioxidant activity [18]. Under in-vitro conditions, sitosterol, and sitosterol glucoside have been found to decrease lipid peroxidation of platelet membranes in the presence of iron [19] and in healthy human subjects a 2 and 3g dose of stanol ester reduced oxidized LDL-C levels [20]. While literature is replete with information on the various biological activities mediated by phytosterols, there is insufficient information on their antihypertensive activities, though various preparations of plant extracts have been investigated which suggest that phytosterols may have antihypertensive activities. Thus this study sought to investigate how beta-sitosterol, a very abundant plant phytosterol might modulate some indices of hypertension in Wistar albino rats.
Materials and methods
The leaves of Ficus asperifolia were collected from the premises of the University of Ibadan, Ibadan, Nigeria. The plant was authenticated at the Department of Botany, University of Ibadan where a specimen voucher was deposited. The leaves were air-dried, finely powdered and extracted three times consecutively with ethyl acetate and 80% ethanol. The extracted solutions were concentrated in vacuo (Buchi Rotavapor R-200, Tokyo Rikakikai Co. Ltd.) to obtain crude extracts. Thin layer chromatography (TLC), column chromatography, and high-performance liquid chromatography (HPLC) were used to fractionate the extracts and to isolate the bioactive compounds. Spectroscopic analyses (1H-NMR, 13C-NMR, LC-MS, EI-MS, IR, and UV) were employed to determine the chemical structure.
Albino rats of Wistar strain (weighing 120–160g) were procured from the International Institute of Tropical Agriculture (IITA), Ibadan and housed under standard conditions (room temperature 25 ± 1C, relative air humidity 50 ± 2%). All animals were maintained on 12-hour light and dark cycle, allowed free access to clean drinking water and fed on standard feed throughout the period of study. The animals were divided into six different groups of five animals each, according to their weight, as follows: Group 1- control (distilled water); Group 2- cadmium chloride-treated rats; Group 3- cadmium chloride and lisinopril (1.3mg/kg/day); Group 4- cadmium chloride and lisinopril (2.3mg/kg/day); Group 5- cadmium chloride and β-sitosterol (1.3mg/kg/day); Group 6- cadmium chloride and β-sitosterol (2.3mg/kg/day). The animals in the first group served as the ‘positive control’ and were fed on standard feed with distilled water throughout the study, while the animals in the second group served as a ‘negative control’. The animals in groups 2 to 6 were given Cadmium Chloride (CdCl2) orally for two weeks at 1mg/kg body weight/day to induce hypertension [21]. The animals in the last four groups were placed on treatment: two groups were placed on a standard drug, lisinopril at different concentrations (1.3mg/kg/day, 2.3mg/kg/day) and β-sitosterol, at two different concentrations, same as that of lisinopril. At the end of the fourth week, the rats were fasted for 24hours and sacrificed by cervical dislocation. Blood samples were collected via ocular puncture using capillary tubes and transferred into sterile, labeled 5ml serum bottles. The blood samples were centrifuged at 3,000 rpm for 10 minutes and the serum (supernatant) collected with a syringe and needle, transferred into sample bottles and then stored at 4°C to maintain the enzyme activity.
The serum cholesterol was measured according to the method described by [22] (Randox CH 200 kit was used for the quantitative in vitro determination of cholesterol in serum). Serum triglyceride level was determined according to the method described by [23] (Randox TR 210 kit was used for the quantitative in vitro determination of triglyceride in serum).
High density lipoprotein-cholesterol was determined in the serum by the method of [24] and [25]. The Randox HDL-cholesterol precipitant kit was used. Urea concentration was determined in the serum according to the method of [26]. The Randox Urea kit was used. Serum creatinine was determined according to the method of [27]. ALT and AST levels were determined by the principle described by [28]. The determination of bicarbonate ion concentration in the serum was carried out using back titration method.
All values are expressed as mean ± S.E.M (standard error of mean) and comparisons were made using one-way ANOVA with Dunnett Multiple Comparison Test. p < 0.05 indicated statistical significance in all cases.
Results
During the study period, thirty albino rats of wistar strain (weighing 120–160g) were used and grouped into six different groups. In hypertensive rats induced with cadmium chloride, there was no significant difference (p>0.05) in the electrolyte concentration except for calcium ion which gave a 56% increase when compared with normal rats (Table 1). Both lisinopril (2.3mg/kg) and BSS (1.3mg/kg) caused significant decrease (p<0.05) in the levels of serum sodium and chloride in treated rats when compared with the normal rats.
Table 1.
Effect of BSS on serum electrolytes
| Sodium * | Potassium* | Calcium* | Chloride* | Bicarbonate* | |
|---|---|---|---|---|---|
| Group 1 | 145.00±1.00 | 6.77±0.25 | 7.97±0.31 | 113.00±1.73 | 18.67±0.58 |
| Group 2 | 146.75±2.63 | 6.43±0.57 | 8.53±0.38a | 112.75±1.89 | 19.75±1.50 |
| Group 3 | 141.75±1.71 | 5.70±0.77a | 8.85±0.38 | 108.75±2.06 | 19.50±1.29 |
| Group 4 | 142.60±4.93b | 6.18±0.65 | 8.52±0.26a | 110.80±4.60ab | 18.00±1.22 |
| Group 5 | 139.67±4.62b | 6.70±0.36 | 8.23±0.35c | 106.67±2.08 | 19.00±1.73 |
| Group 6 | 143.67±3.51 | 6.33±0.85 | 8.23±0.40c | 111.33±3.21 | 19.33±1.53 |
Values expressed in mmol/l are mean ± S.E of rats in each group
The values with the superscript a,b,c shows significant difference from other groups at 0.05 level.
The induction of hypertension led to 16.3% increase in ALP activity and a 32.7% increase in AST activity (Table 2). Treatment with BSS (1.3mg/kg) gave significant decrease (p> 0.05) in the activity of ALT when compared to the control. The effect of BSS on serum lipid profile is shown in Table 3. The results indicate that there was no significant difference (p>0.05) in the lipid profile of treated rats and the control group.
Table 2.
Effect of BSS on ALP, AST and ALT
| ALT* | AST* | ALP* | |
|---|---|---|---|
| Group 1 | 113.11±5.59 | 116.49±19.73 | 66.67±13.61 |
| Group 2 | 122.12±1.53 | 173.08±24.74a | 79.67±31.34a |
| Group 3 | 114.58±2.89 | 155.83±19.93a | 80.25±43.09a |
| Group 4 | 111.67±3.32a | 146.27±19.16 | 76.50±30.26ab |
| Group 5 | 104.67±6.37 | 135.69±7.93b | 79.00±14.14b |
| Group 6 | 113.66±7.23e | 148.78±11.79 | 67.67±16.29a |
Values expressed in mmol/l are mean ± S.E of rats in each group
The values with the superscript a,b,c shows significant difference from other groups at 0.05 level.
Table 3.
Effect of BSS on serum lipid profile
| Total cholesterol* | Triglyceride* | HDL* | LDL* | |
|---|---|---|---|---|
| Group 1 | 52.67±24.91 | 79.33 ±43.66 | 25.67±4.04 | 11.13±8.95 |
| Group 2 | 60.50±9.33 | 112.00±30.46 | 32.00±6.38 | 4.70 ±3.53 |
| Group 3 | 49.75±12.82 | 101.75±47.20 | 28.50±4.65 | 3.80 ±1.41 |
| Group 4 | 59.80±7.05c | 102.80±29.17a | 33.00±5.03 | 7.65 ±3.44 |
| Group 5 | 68.00±7.00c | 143.67±38.76 | 32.33±4.51 | 6.93 ±5.30 |
| Group 6 | 52.33±10.01 | 62.00 ±30.81e | 29.50±2.12 | 13.30±2.40 |
Values expressed in mmol/l are mean ± S.E of rats in each group
The values with the superscript a,b,c shows significant difference from other groups at 0.05 level.
The results in Table 4 revealed that the elevated serum creatinine level following cadmium administration was restored to normal upon treatment with BSS while the lower dose (1.3mg/kg/day) reduced urea concentration significantly when compared to normal and hypertensive rats. Also, lisinopril at the lower dose (1.3mg/kg/day) significantly reduced (p<0.05) creatinine levels while the higher dose (2.3mg/kg/day) significantly increased (p<0.05) serum creatinine when compared to hypertensive and normal rats. Histopathological examination revealed that the liver and kidney of BSS treated rats did not show any difference from the control with normal histological structure. Hypertensive animals showed scanty inflamed cells. Examination of the liver of the lisinopril treated group showed that the portal triad is mildly infiltrated with inflamed cells, while that of kidney showed normal histology (Fig. 1 to 12).
Table 4.
Effect of BSS on serum urea and creatinine
| Urea * | Creatinine * | |
|---|---|---|
| Group 1 | 50.67±19.55 | 1.57±0.25 |
| Group 2 | 33.50±6.86 | 1.68±0.05 |
| Group 3 | 36.33±11.24 | 1.35±0.13bd |
| Group 4 | 72.67±31.82bc | 1.84±0.29c |
| Group 5 | 38.00±3.61d | 1.57±0.21 |
| Group 6 | 64.00±20.22b | 1.59±0.25d |
Values expressed in mmol/l are mean ± S.E of rats in each group
The values with the superscript a,b,c,d shows significant difference from other groups at 0.05 level.
Fig. 1.
GROUP 1 KIDNEY*
* Stained by H and E technique X 400
Fig. 12.
GROUP 6 LIVER*
* Stained by H and E technique X 400
Discussion
In this study, we have examined the effect of oral administration of BSS on basic biochemical parameters implicated in hypertension in rats. The electrolyte concentration of hypertensive rats was not significantly different (p>0.05) from those of control. A similar observation was made by [29] who reported that there was no detectable change in serum electrolyte even after four weeks of cadmium exposure at the same concentration. Cadmium mimics calcium which often leads to increase in calcium concentration in the extracellular fluid. According to [30], excess calcium in the extracellular fluid may increase the amplitude of action potential and enhance smooth muscle contractility. Although there was no significant change in many electrolyte concentrations following hypertension induction, the reduction of calcium concentration at lower and higher doses of BSS suggests that BSS could reduce the accumulation of calcium in hypertensive conditions.
The observed decrease in ALT activity and increase in the activities of ALP and AST suggests that oral administration of BSS (1.3mg/kg) leads to restoration of basal liver function after cadmium induced hepatotoxicity. However, BSS does not show this activity at the higher dose (2.3mg/kg); this suggests that the lower dose may be more effective in this context than the higher dose.
The lipid profile of the treated and control rats were not significantly different (p>0.05). This is probably because the mechanism of hypertension induction by cadmium does not involve modulation of lipids; rather, it majorly act as an antagonist of atrial natriuretic hormone receptor [31] thereby inhibiting the natriuretic, diuretic and smooth muscle relaxing activity of Atrial Natriuretic Peptide (ANP).
It was observed that the elevated serum creatinine level following cadmium administration was restored to normal upon treatment with BSS while the lower dose (1.3mg/kg/day) reduced urea concentration significantly when compared to normal and hypertensive rats. This suggests that oral administration of BSS may be beneficial for the restoration of basal kidney function rate and in the improvement of serum creatinine in hypertensive rats. This fact is corroborated by the normal histology pattern of the kidney section. In comparison with normal rats, the lower dose (1.3mg/kg/day) of lisinopril was found to significantly reduce (p<0.05) creatinine level while the higher dose (2.3mg/kg/day) gave an increase. This suggests that lisinopril may be therapeutic at the lower dose but nephrotoxic at the higher dose.
Conclusion
Hypertension remains a major health challenge worldwide. This study has demonstrated the possible ameliorative effects of BSS on some biochemical indices of hypertension in wistar albino rats. BSS was effective in restoring basal liver and kidney functions in hypertensive animals. This important phytosterol therefore holds promise in the therapeutic management of CVDs.
Fig. 2.
GROUP 2 KIDNEY*
* Stained by H and E technique X 400
Fig. 3.
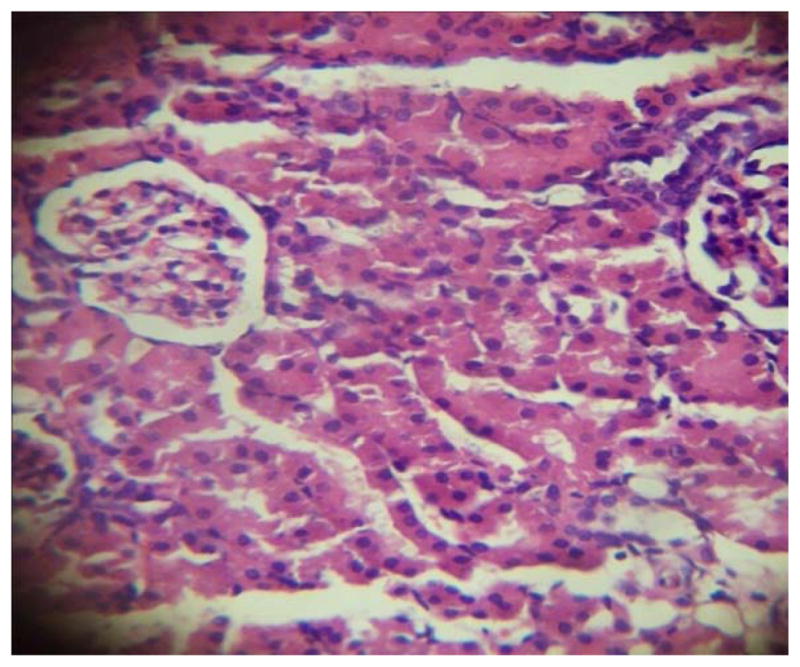
GROUP 3 KIDNEY*
* Stained by H and E technique X 400
Fig. 4.
GROUP 4 KIDNEY*
* Stained by H and E technique X 400
Fig. 5.
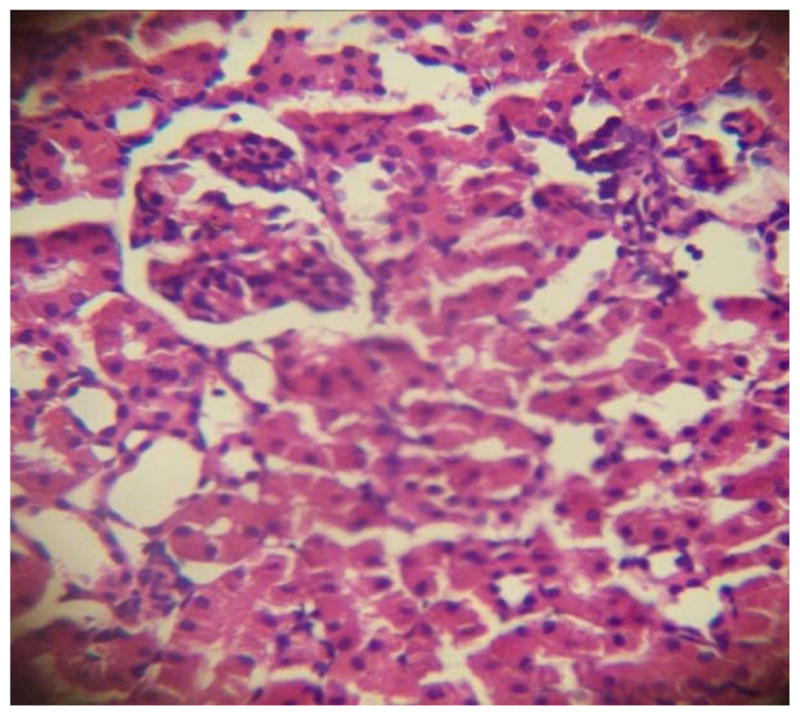
GROUP 5 KIDNEY*
* Stained by H and E technique X 400
Fig. 6.
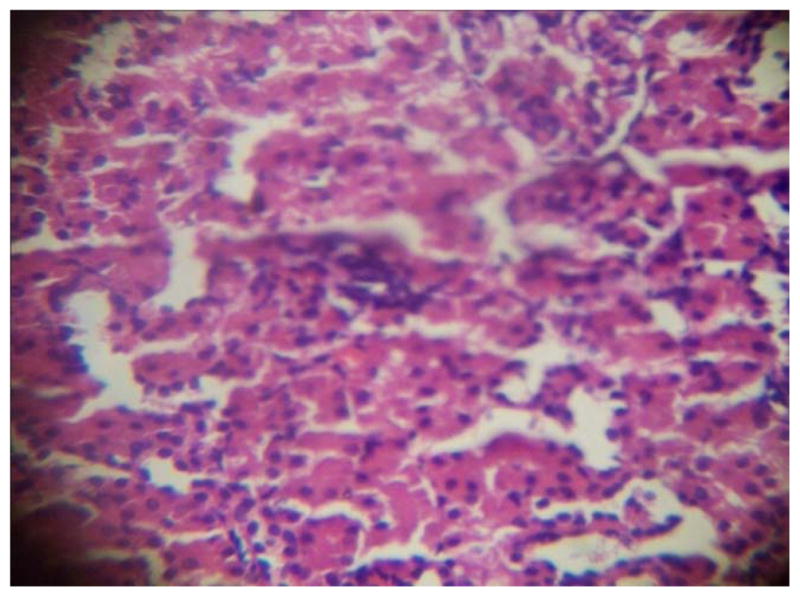
GROUP 6 KIDNEY*
* Stained by H and E technique X 400
Fig. 7.
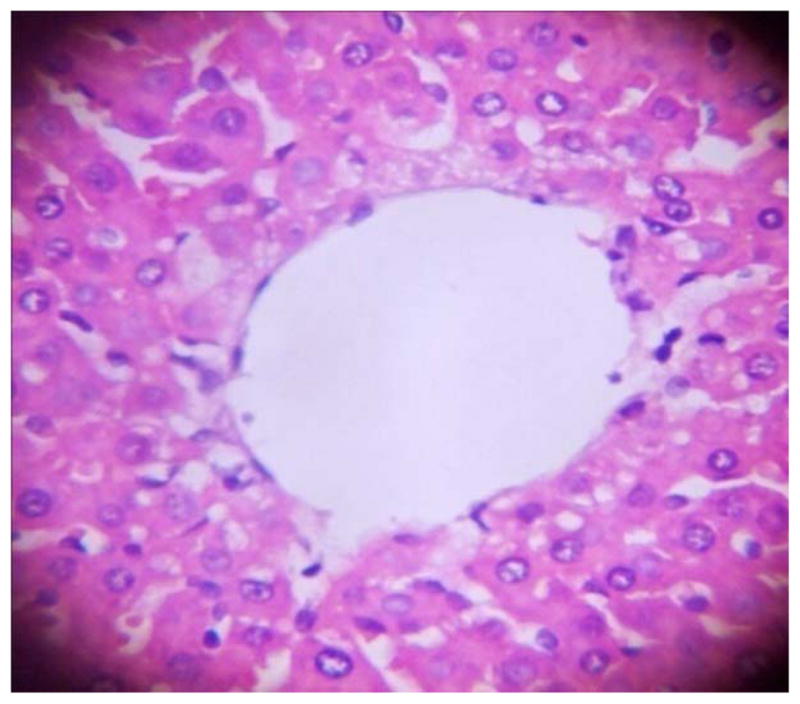
GROUP 1 LIVER*
* Stained by H and E technique X 400
Fig. 8.
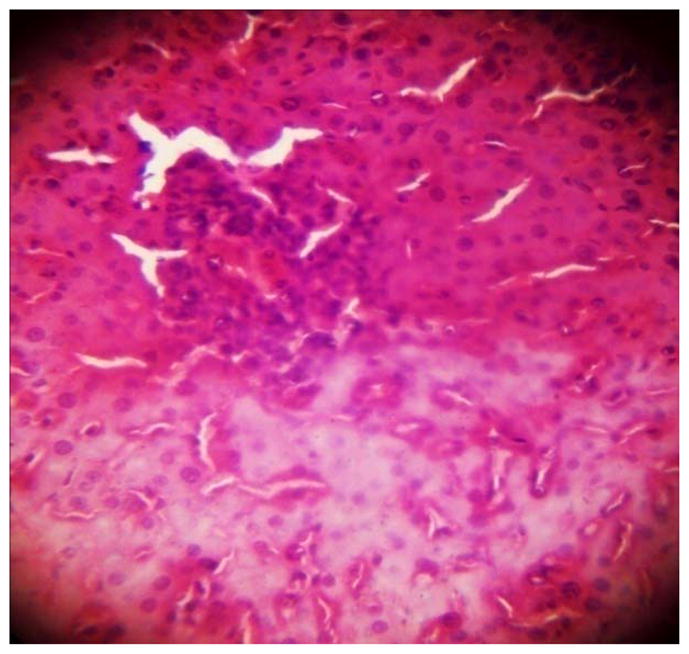
GROUP 2 LIVER*
* Stained by H and E technique X 400
Fig. 9.
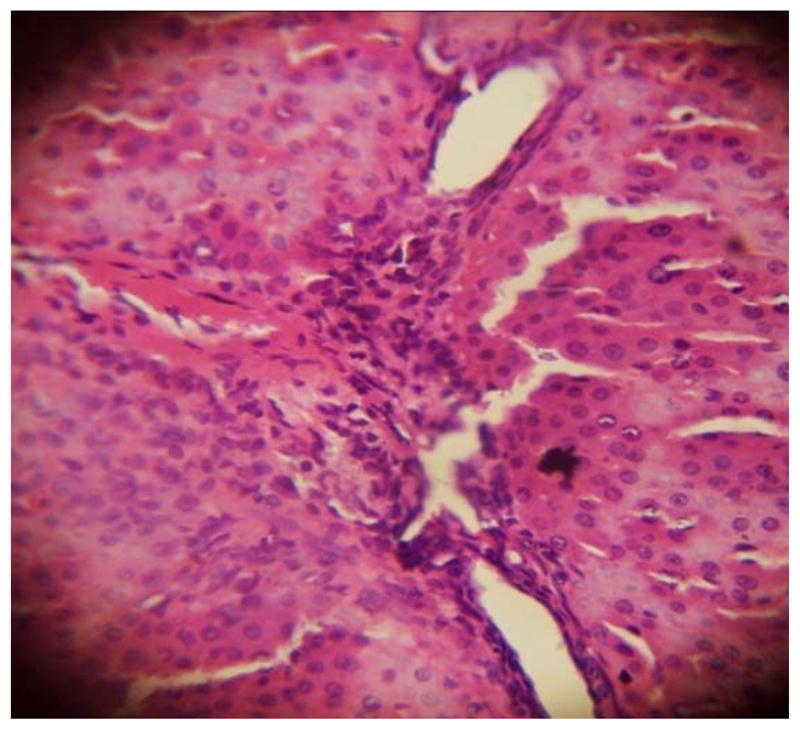
GROUP 3 LIVER*
* Stained by H and E technique X 400
Fig. 10.
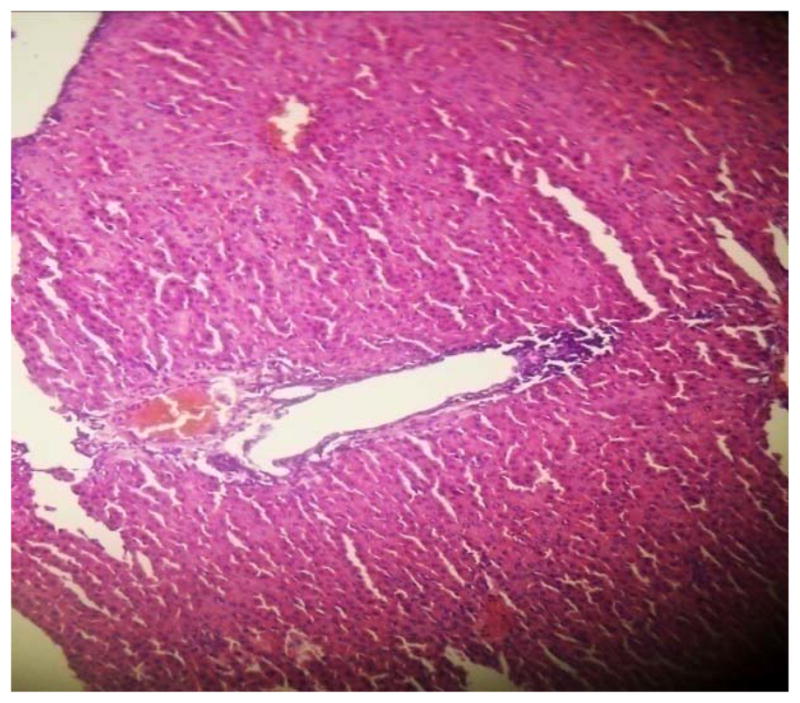
GROUP 4 LIVER*
* Stained by H and E technique X 400
Fig.11.
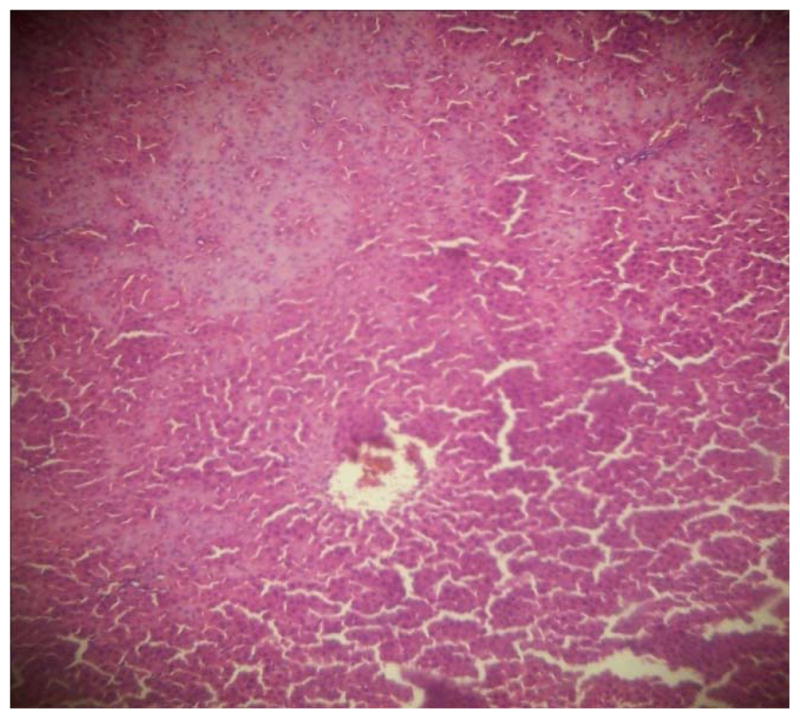
GROUP 5 LIVER*
* Stained by H and E technique X 400
Acknowledgments
Data analysis and writing of this paper was supported by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by Fogarty International Centre, the Office of AIDS Research, and the National Human Genome Research Institute of the National Institute of Health, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under Award Number R24TW008878.
We also wish to acknowledge the support of Academy of Sciences for the Developing World (TWAS), Italy for research support through the award of fellowship FR number: 3240223519 to COO.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
References
- 1.W.H.O; Organization WH, editor. Fact sheet no 317. Sep, 2009. Cardiovascular diseases. [Google Scholar]
- 2.NCEP. Final Report: Circulation. Vol. 106. Philadelphia: 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) pp. 3143–3421.pp. 554–556. [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 4.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton JL, Braitman LE, Riley AM, Jr, Khoo CS, Smith JL. Demographic, dietary, life style, and anthropometric correlates of blood pressure. Hypertension. 1982;4:135–142. doi: 10.1161/01.hyp.4.5_pt_2.iii135. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1999;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 7.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101 (3):329–35. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, He J, Muntner P. Prevalence, awareness, treatment and control of hypertension in North America, North Africa and Asia. Journal of Human Hypertension. 2004;18:545–551. doi: 10.1038/sj.jhh.1001701. [DOI] [PubMed] [Google Scholar]
- 9.AHA. ACC/AHA guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology. 2007 [Google Scholar]
- 10.Murray CJ, Lopez AD. Global Burden of Disease and Injury Series 1996. Vol. 1. Harvard School of Public Health; Cambridge, Mass, USA: 1996. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2020. [Google Scholar]
- 11.Moreau R, Whitaker B, Hicks K. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res. 2002;41:457. doi: 10.1016/s0163-7827(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 13.Calpe-Berdiel L, Escola-Gil JC, Blanco-Vaca F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 2009;203:18–31. doi: 10.1016/j.atherosclerosis.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Brendsel J, Green SJ. Regarding the potential perils of phytosterols. Atherosclerosis. 2007;192:227–229. doi: 10.1016/j.atherosclerosis.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Awad AB, Roy R, Fink CS. Beta-sitosterol, a plant sterol, induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol Rep. 2003;10:497–500. [PubMed] [Google Scholar]
- 16.Bouic PJ. The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Curr Opin Clin Nutr Metab Care. 2001;4:471–475. doi: 10.1097/00075197-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Moghadasian MH, McManus BM, Godin DV, Rodrigues B, Frohlich JJ. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: possible mechanisms of action. Circulation. 1999;99:1733–1739. doi: 10.1161/01.cir.99.13.1733. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Hicks KB, Moreau R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J Am Oil Chem Soc. 2002;79:1201–1206. [Google Scholar]
- 19.van Rensburg SJ, Daniels WM, van Zyl JM, Taljaard JJ. A comparative study of the effects of cholesterol, beta-sitosterol, betasitosterol glucoside, dehydroepiandrosterone sulphate and melatonin on in vitro lipid peroxidation. Metab Brain Dis. 2000;15:257–265. doi: 10.1023/a:1011167023695. [DOI] [PubMed] [Google Scholar]
- 20.Homma Y, Ikeda I, Ishikawa T, Tateno M, Sugano M, Nakamura H. A randomized, placebo-controlled trial: Decrease in plasma low-density lipoprotein cholesterol, apolipoprotein B, cholesteryl ester transfer protein, and oxidized low-density lipoprotein by plant stanol ester-containing spread. Nutrition. 2003;19:369–374. doi: 10.1016/s0899-9007(02)00926-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen KS. Effect of high calcium diet on cadmium-induced hypertension in rat. The Kaohsiung J Med Sci. 1992;8(4):189–94. [PubMed] [Google Scholar]
- 22.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19:1350–1356. [PubMed] [Google Scholar]
- 23.Tietz NW. Clinical guide to laboratory tests. 2. Sunders, W.B. company; 1990. [Google Scholar]
- 24.Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 25.Jacobs DS, Kashen BL, De Mott WR, Wolfson WL. Lexi-com Inc, editor. In laboratory and test handbook. Hudson (Cleveland): 1990. p. 219. [Google Scholar]
- 26.Fawcett JK, Scott JF. A new semi-micro method for determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels H, Bohmer M, Heierli C. Serum creatinine determination without protein precipitation. Clinica Chemica Acta. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 28.Reitman S, Frankel S. A Colorimetric Method of Determination of Serum Glutamate Oxaloacetate and Pyruvate Transaminases. Am J Clin Path. 1957;28:56– 61. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Lall SB, Das N, Rama R, Peshin SS, Khattar S, Gulati K, Seth SD. Cadmium induced nephrotoxicity in rats. Indian J Exp Biol. 1997;35(2):151–154. [PubMed] [Google Scholar]
- 30.Agada PO, Braide VB. Effect of dietary Garcinia Kola seed on selected serum electrolytes and trace metals in male albino rats. Nig. J. Physiol. Sci. 2009;24:53–57. doi: 10.4314/njps.v24i1.46381. [DOI] [PubMed] [Google Scholar]
- 31.Giridhar J, Rathinavelu A, Isom GE. Interaction of Cadmium with Atrial Natriuretic peptide receptors: implications for toxicity. Toxicology. 1992;75 (2):133–43. doi: 10.1016/0300-483x(92)90152-5. [DOI] [PubMed] [Google Scholar]