Abstract
Background
Patients with advanced squamous-cell non–small-cell lung cancer (NSCLC) who have disease progression during or after first-line chemotherapy have limited treatment options. This randomized, open-label, international, phase 3 study evaluated the efficacy and safety of nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint–inhibitor antibody, as compared with docetaxel in this patient population.
Methods
We randomly assigned 272 patients to receive nivolumab, at a dose of 3 mg per kilogram of body weight every 2 weeks, or docetaxel, at a dose of 75 mg per square meter of body-surface area every 3 weeks. The primary end point was overall survival.
Results
The median overall survival was 9.2 months (95% confidence interval [CI], 7.3 to 13.3) with nivolumab versus 6.0 months (95% CI, 5.1 to 7.3) with docetaxel. The risk of death was 41% lower with nivolumab than with docetaxel (hazard ratio, 0.59; 95% CI, 0.44 to 0.79; P<0.001). At 1 year, the overall survival rate was 42% (95% CI, 34 to 50) with nivolumab versus 24% (95% CI, 17 to 31) with docetaxel. The response rate was 20% with nivolumab versus 9% with docetaxel (P = 0.008). The median progression-free survival was 3.5 months with nivolumab versus 2.8 months with docetaxel (hazard ratio for death or disease progression, 0.62; 95% CI, 0.47 to 0.81; P<0.001). The expression of the PD-1 ligand (PD-L1) was neither prognostic nor predictive of benefit. Treatment-related adverse events of grade 3 or 4 were reported in 7% of the patients in the nivolumab group as compared with 55% of those in the docetaxel group.
Conclusions
Among patients with advanced, previously treated squamous-cell NSCLC, overall survival, response rate, and progression-free survival were significantly better with nivolumab than with docetaxel, regardless of PD-L1 expression level. (Funded by Bristol-Myers Squibb; CheckMate 017 ClinicalTrials.gov number, NCT01642004.)
Squamous-cell carcinoma represents approximately 30% of all cases of non– small-cell lung cancer (NSCLC).1 Treatment for advanced squamous-cell NSCLC remains an unmet need; little therapeutic progress has been made since the approval of docetaxel for second-line treatment in 1999.2-4 Most new agents for the treatment of NSCLC are not indicated for this subtype because of their toxicity or lack of efficacy or because their activity is limited to tumors with specific genetic alterations that are rarely found in squamous-cell NSCLC.5-7 Furthermore, no single-agent therapy has resulted in better survival than that seen with docetaxel.
The programmed death 1 (PD-1) receptor, which is expressed on activated T cells, is engaged by ligands PD-L1 and PD-L2, which are expressed by tumor cells and infiltrating immune cells.8 Tumor PD-L1 expression is prevalent in NSCLC, and the interaction of PD-1 with the PD-L1 and PD-L2 ligands inhibits T-cell activation and promotes tumor immune escape (i.e., the mechanism by which tumor cells escape recognition and elimination by the immune system).8-10 Nivolumab is a fully human IgG4 PD-1 immune-checkpoint– inhibitor antibody that disrupts PD-1–mediated signaling and restores antitumor immunity.11-13 Nivolumab has activity across NSCLCs with various histologic features.11,13-15
In phase 1 and 2 trials, nivolumab was associated with response rates of 15% and approximately 17%, with a median overall survival of 8.2 to 9.2 months and survival rates of 41% at 1 year and 19% at 3 years, among previously treated patients with advanced squamous-cell NSCLC.14,15 We report the results of a randomized, openlabel, international, phase 3 study that compared nivolumab monotherapy with docetaxel monotherapy in patients with advanced squamous-cell NSCLC in whom the disease progressed during or after one prior platinum-containing chemotherapy regimen.
Methods
Patients
Patients with stage IIIB or IV squamous-cell NSCLC who had disease recurrence after one prior platinum-containing regimen were eligible for participation in the study. Eligible patients were 18 years of age or older, had an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1 (on a scale from 0 to 5, with higher scores indicating greater disability; a score of 0 indicates no symptoms, and 1 mild symptoms), and had submitted a pretreatment tumor-tissue specimen for biomarker analyses. Patients with treated, stable brain metastases were eligible. Key exclusion criteria were autoimmune disease, symptomatic interstitial lung disease, systemic immunosuppression, prior therapy with T-cell costimulation or checkpoint-targeted agents, or prior docetaxel therapy. Patients who had received more than one prior systemic therapy for meta-static disease were excluded. Prior maintenance therapy, including an epidermal growth factor receptor tyrosine kinase inhibitor, was allowed. The complete eligibility criteria are provided in the study protocol, available with the full text of this article at NEJM.org.
Study Design and Treatments
From October 2012 through December 2013, we enrolled 352 patients, of whom 272 underwent randomization; 135 patients were randomly assigned to receive nivolumab, at a dose of 3 mg per kilogram of body weight every 2 weeks, and 137 were randomly assigned to received docetaxel, at a dose of 75 mg per square meter of body-surface area every 3 weeks (Fig. S1A in the Supplementary Appendix, available at NEJM.org). Both drugs were administered intravenously. Patients were treated until disease progression or discontinuation of treatment owing to toxic effects or for other reasons (Fig. S1B in the Supplementary Appendix).
Randomization was stratified according to prior use of paclitaxel therapy (yes vs. no) and geographic region (United States or Canada vs. Europe vs. rest of the world [Argentina, Australia, Chile, Mexico, and Peru]). For patients in the nivolumab group, treatment after initial disease progression was permitted at the investigator's discretion according to criteria specified in the protocol. Requirements for treatment delay or discontinuation because of treatment-related adverse events were specified in the protocol, as were requirements regarding reductions in the docetaxel dose owing to toxic effects, which conformed with the prescribing information on the product label. Reductions in the nivolumab dose were not permitted.
End Points and Assessments
The primary end point was overall survival. Patients were followed for survival continuously while they were receiving the study drugs and then every 3 months after discontinuation of treatment. Initially, confirmed objective response rate was also a primary end point, but on the basis of mature data regarding the objective response rate in an expanded cohort of patients with NSCLC who had been treated in the phase 1b study MDX-1106-03 (ClinicalTrials.gov number, NCT00730639),13 the current trial was amended before the planned interim analysis to make overall survival the sole primary end point. The rate of investigator-assessed confirmed objective response was modified to be the first secondary end point. Additional end points included progression-free survival, patient-reported outcomes, efficacy according to tumor PD-L1 expression, and safety.
Tumor response was assessed with the use of the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1,16 at week 9 and every 6 weeks thereafter. Patient-reported outcomes regarding disease-related symptoms and health status were assessed with the use of the Lung Cancer Symptom Scale and the European Quality of Life–5 Dimensions questionnaire. Outcome measures included the proportion of patients who had clinically meaningful improvement in the average Lung Cancer Symptom Scale score by week 12. Analyses of patient-reported outcomes are ongoing.
Safety was assessed by means of evaluations of the incidence of adverse events, which were graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Select adverse events (those with potential immunologic causes) were grouped according to prespecified categories.
PD-L1 Biomarker Analysis
PD-L1 protein expression was evaluated retrospectively in pretreatment (archival or recent) tumor-biopsy specimens with the use of a validated automated immunohistochemical assay (Dako North America) that used a rabbit monoclonal antihuman PD-L1 antibody (clone 28–8, Epitomics). Samples were categorized as positive when staining of the tumor-cell membrane (at any intensity) was observed at prespecified expression levels of 1%, 5%, or 10% of cells in a section that included at least 100 tumor cells that could be evaluated.
Study Oversight
The study was designed by the academic authors in collaboration with the sponsor (Bristol-Myers Squibb); the sponsor also worked jointly with investigators to collect and analyze the data. The study protocol was approved by an institutional review board at each participating institution. The study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines as defined by the International Conference on Harmonisation. Written informed consent was obtained from all the patients before enrollment.
An independent data and safety monitoring committee provided oversight of safety and efficacy. On January 10, 2015, the committee recommended early termination of the study on the basis of a prespecified interim analysis that showed that overall survival among patients receiving nivolumab was superior to that among those receiving docetaxel. Planned enrollment was complete before the study was stopped. We report the results of the interim analysis here, which are based on a December 15, 2014, database lock.
All the authors attest that the study was conducted in accordance with the protocol and vouch for the accuracy and completeness of the data and analyses. The first draft of this manuscript was written by the first and last authors; all the authors contributed to subsequent drafts and made the decision to submit the manuscript for publication. All the authors signed a confidentiality agreement with the sponsor. Medical-writing support, funded by the sponsor, was provided by StemScientific.
Statistical Analysis
Overall survival and progression-free survival were analyzed with the use of a two-sided log-rank test stratified according to prior or no prior paclitaxel use and geographic region. Hazard ratios and corresponding confidence intervals were estimated with the use of a stratified Cox proportional-hazards model, with randomized group as a single covariate. Survival curves for each treatment group were estimated with the use of the Kaplan–Meier method. Survival rates were derived from the Kaplan–Meier estimates. Objective response rates were compared with the use of a two-sided, stratified Cochran–Mantel– Haenszel test, with exact 95% confidence intervals calculated with the use of the Clopper–Pearson method. Nonconventional benefit (i.e., a reduction in the size or number [or both] of target lesions with the simultaneous appearance of new lesions, initial disease progression followed by tumor reduction, or no further progression for at least two tumor assessments) in patients treated beyond initial progression was not included in response-based analyses (objective response rate or progression-free survival). Prespecified analyses were performed to evaluate the prognostic and predictive roles of prestudy status with respect to PD-L1 expression.
Demographic and efficacy analyses included all the patients who underwent randomization (intention-to-treat population). Safety analyses included all the treated patients (those who received at least one dose of study drug). At the time of the interim database lock, 199 of the 272 patients who had undergone randomization had died (86% of the 231 deaths required for the final analysis). The boundary for declaring superiority for overall survival at the interim analysis was a P value of less than 0.03, which was based on an O'Brien–Fleming alpha-spending function. If the P value for overall survival indicated statistical significance, then the key secondary end points of response rate and progression-free survival were tested hierarchically at the 5% alpha level.
Results
Patients and Treatment
A total of 96% of the patients who underwent randomization (260 of 272 patients) received treatment with a study drug: 131 with nivolumab and 129 with docetaxel. The minimum follow-up was approximately 11 months.
The median age of the patients was 63 years. Most patients were men, had an ECOG performance-status score of 1, had stage IV cancer, and were current or former smokers (Table 1, and Table S1 in the Supplementary Appendix). All the patients had received platinum-based therapy previously; 34% had received paclitaxel previously. The demographic and clinical characteristics of the patients were generally well balanced between the groups, with slight between-group imbalances in the percentages of female patients, patients 75 years of age or older, and patients with an ECOG performance-status score of 1.
Table 1. Baseline Characteristics, Stratification Factors, and Prior Therapy*.
| Characteristic | Nivolumab (N = 135) | Docetaxel (N = 137) | Total (N = 272) |
|---|---|---|---|
| Age — yr | |||
| Median | 62 | 64 | 63 |
| Range | 39–85 | 42–84 | 39–85 |
| Age category — no. (%) | |||
| <65 yr | 79 (59) | 73 (53) | 152 (56) |
| ≥65 to <75 yr | 45 (33) | 46 (34) | 91 (33) |
| ≥75 yr | 11 (8) | 18 (13) | 29 (11) |
| Sex — no. (%) | |||
| Male | 111 (82) | 97 (71) | 208 (76) |
| Female | 24 (18) | 40 (29) | 64 (24) |
| Race — no. (%)† | |||
| White | 122 (90) | 130 (95) | 252 (93) |
| Black | 6 (4) | 2 (1) | 8 (3) |
| Asian | 4 (3) | 2 (1) | 6 (2) |
| Other | 1 (1) | 2 (1) | 3 (1) |
| Not reported | 2 (1) | 1 (1) | 3 (1) |
| Disease stage — no. (%) | |||
| IIIB | 29 (21) | 24 (18) | 53 (19) |
| IV | 105 (78) | 112 (82) | 217 (80) |
| Not reported | 1 (1) | 1 (1) | 2 (1) |
| ECOG performance-status score — no. (%)‡ | |||
| 0 | 27 (20) | 37 (27) | 64 (24) |
| 1 | 106 (79) | 100 (73) | 206 (76) |
| Not reported | 2 (1) | 0 | 2 (1) |
| Central nervous system metastasis — no. (%) | |||
| Yes | 9 (7) | 8 (6) | 17 (6) |
| No | 126 (93) | 129 (94) | 255 (94) |
| Smoking status — no. (%) | |||
| Current or former smoker | 121 (90) | 129 (94) | 250 (92) |
| Never smoked | 10 (7) | 7 (5) | 17 (6) |
| Unknown | 4 (3) | 1 (1) | 5 (2) |
| Geographic region — no. (%) | |||
| United States or Canada | 43 (32) | 43 (31) | 86 (32) |
| Europe | 77 (57) | 78 (57) | 155 (57) |
| Rest of world§ | 15 (11) | 16 (12) | 31 (11) |
| Other systemic cancer therapy — no. (%)¶ | |||
| Bevacizumab | 1 (1) | 1 (1) | 2 (1) |
| Cetuximab | 0 | 2 (1) | 2 (1) |
| Etoposide | 17 (13) | 11 (8) | 28 (10) |
| Fluorouracil | 1 (1) | 0 | 1 (<1) |
| Gemcitabine | 60 (44) | 71 (52) | 131 (48) |
| Paclitaxel | 46 (34) | 46 (34) | 92 (34) |
| Pemetrexed | 3 (2) | 3 (2) | 6 (2) |
| Vinorelbine | 20 (15) | 24 (18) | 44 (16) |
| Best response to most recent prior systemic regimen, according to the investigator — no. (%)‖ | |||
| Complete or partial response | 48 (36) | 43 (31) | 91 (33) |
| Stable disease | 33 (24) | 47 (34) | 80 (29) |
| Progressive disease | 44 (33) | 41 (30) | 85 (31) |
| Unknown or not reported | 10 (7) | 6 (4) | 16 (6) |
| Time from completion of most recent prior systemic regimen — no. (%) | |||
| <3 mo | 64 (47) | 59 (43) | 123 (45) |
| 3–6 mo | 35 (26) | 40 (29) | 75 (28) |
| >6 mo | 35 (26) | 37 (27) | 72 (27) |
| Unknown | 1 (1) | 1 (1) | 2 (1) |
Data from all patients who underwent randomization are included. There were no significant differences between the study groups at baseline.
Race was self-reported.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher numbers indicating greater disability; a score of 0 indicates no symptoms, and 1 mild symptoms.
The countries in the rest-of-the-world geographic region were Argentina, Australia, Chile, Mexico, and Peru.
Other systemic cancer therapy includes chemotherapy as part of prior first-line therapy.
All but one patient received only one line of prior cancer therapy, which could include multiple agents or a switch of agents within the first-line regimen.
A median of 8 doses (range, 1 to 48) of nivolumab and 3 doses (range, 1 to 29) of docetaxel were administered. Among the patients in the nivolumab group, 85% received at least 90% of their planned dose intensity. Among the patients in the docetaxel group, 69% received at least 90% of their planned dose intensity, a finding that is consistent with docetaxel dose reductions (which occurred in 27% of patients). At least one dose delay occurred in 37% of the patients in the nivolumab group and in 31% of those in the docetaxel group. The majority of patients in each group had only one dose delay, and the majority of dose delays were from 4 to 7 days in duration (in 61% of the total cycles delayed in the nivolumab group and 71% of those in the docetaxel group). Most delays of nivolumab therapy were attributable to personal or administrative reasons, disease progression, or the administration of radiotherapy; most delays of docetaxel therapy were due to adverse events.
At the time of the database lock, 16% of the patients in the nivolumab group and 2% of those in the docetaxel group were continuing treatment (Table S2 in the Supplementary Appendix). After discontinuation of treatment, 36% of the patients in the nivolumab group and 30% of those in the docetaxel group received subsequent systemic cancer therapy. In the nivolumab group, 24% of the patients received subsequent docetaxel, reflecting the open-label nature of the study; 2% of the patients in the docetaxel group received subsequent immunotherapy (Table S3 in the Supplementary Appendix).
Efficacy
The median overall survival was 9.2 months (95% confidence interval [CI], 7.3 to 13.3) in the nivolumab group as compared with 6.0 months (95% CI, 5.1 to 7.3) in the docetaxel group. Overall survival was significantly longer with nivolumab than with docetaxel (Fig. 1), with the risk of death 41% lower with nivolumab (hazard ratio, 0.59; 95% CI, 0.44 to 0.79; P<0.001). The overall survival rate at 1 year was 42% (95% CI, 34 to 50) in the nivolumab group versus 24% (95% CI, 17 to 31) in the docetaxel group. The hazard ratios for death in the analysis of overall survival favored nivolumab across all prespecified subgroups, except for the subgroups of patients in the rest-of-world geographic region (Argentina, Australia, Chile, Mexico, and Peru) and those who were 75 years of age or older (Fig. S2 in the Supplementary Appendix).
Figure 1. Kaplan–Meier Curves for Overall Survival.
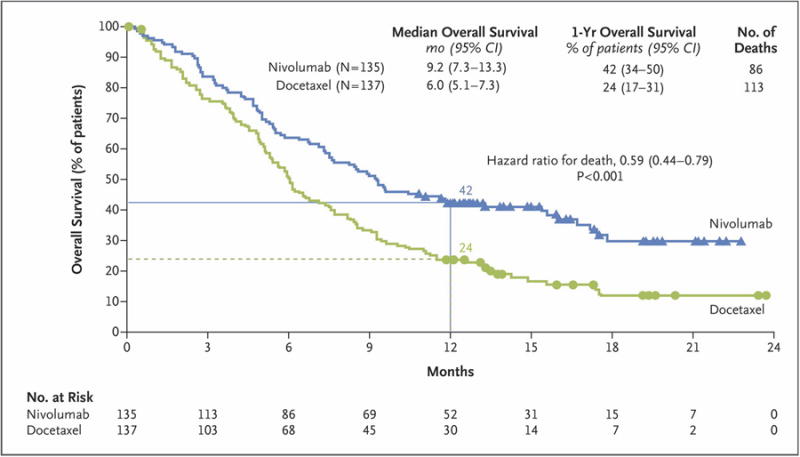
The analysis included all the patients who underwent randomization. Symbols indicate censored observations, and horizontal lines the rates of overall survival at 1 year.
The rate of confirmed objective response was significantly higher with nivolumab than with docetaxel (20% [95% CI, 14 to 28] vs. 9% [95% CI, 5 to 15]; P = 0.008) (Table 2, and Fig. S3 in the Supplementary Appendix). The median time to response was 2.2 months (range, 1.6 to 11.8) in the nivolumab group and 2.1 months (range, 1.8 to 9.5) in the docetaxel group (Fig. 2A). The median duration of response was not reached in the nivolumab group (range, 2.9 to 20.5+ months, with + indicating an ongoing response at the time of analysis), as compared with 8.4 months in the docetaxel group (range, 1.4+ [with the + indicating censored data because the patient received subsequent therapy] to 15.2+ [with the + indicating an ongoing response]).
Table 2. Clinical Activity of Nivolumab versus Docetaxel in Patients with Advanced Squamous-Cell Non–Small-Cell Lung Cancer*.
| Variable | Nivolumab (N = 135) | Docetaxel (N = 137) |
|---|---|---|
| Objective response† | ||
| No. of patients | 27 | 12 |
| % of patients (95% CI) | 20 (14–28) | 9 (5–15) |
| Estimated odds ratio (95% CI) | 2.6 (1.3–5.5) | |
| P value | 0.008 | |
| Best overall response — no. (%) | ||
| Complete response | 1 (1) | 0 |
| Partial response | 26 (19) | 12 (9) |
| Stable disease | 39 (29) | 47 (34) |
| Progressive disease | 56 (41) | 48 (35) |
| Could not be determined | 13 (10) | 30 (22) |
| Time to response — mo‡§ | ||
| Median | 2.2 | 2.1 |
| Range | 1.6–11.8 | 1.8–9.5 |
| Duration of response — mo‡¶ | ||
| Median | NR | 8.4 |
| Range | 2.9 to 20.5+ | 1.4+ to 15.2+ |
NR denotes not reached.
Confirmed complete and partial responses were assessed by the investigator according to the Response Evaluation Criteria in Solid Tumors, version 1.1. The confidence interval is based on the Clopper–Pearson method. The analysis was stratified according to geographic region (United States or Canada vs. Europe vs. rest of the world) and prior paclitaxel therapy (yes vs. no). The strata-adjusted odds ratio (nivolumab vs. docetaxel) and the two-sided P value were calculated with the use of the stratified Cochran–Mantel–Haenszel method.
The analysis was performed with data from all the patients who had a response (27 patients in the nivolumab group and 12 in the docetaxel group).
The time to response was defined as the time from randomization to the date of first documented complete or partial response.
Results were calculated with the use of the Kaplan–Meier method. The duration of response was defined as the time from the date of first response to the date of first documented disease progression, death, or last tumor assessment that could be evaluated. The + symbol indicates a censored value. The value of 1.4 was censored owing to the start of subsequent therapy in one patient, and the other values were censored because the response was ongoing at the time of the analysis.
Figure 2. Efficacy of Nivolumab versus Docetaxel in Patients with Advanced Squamous-Cell Non–Small-Cell Lung Cancer.
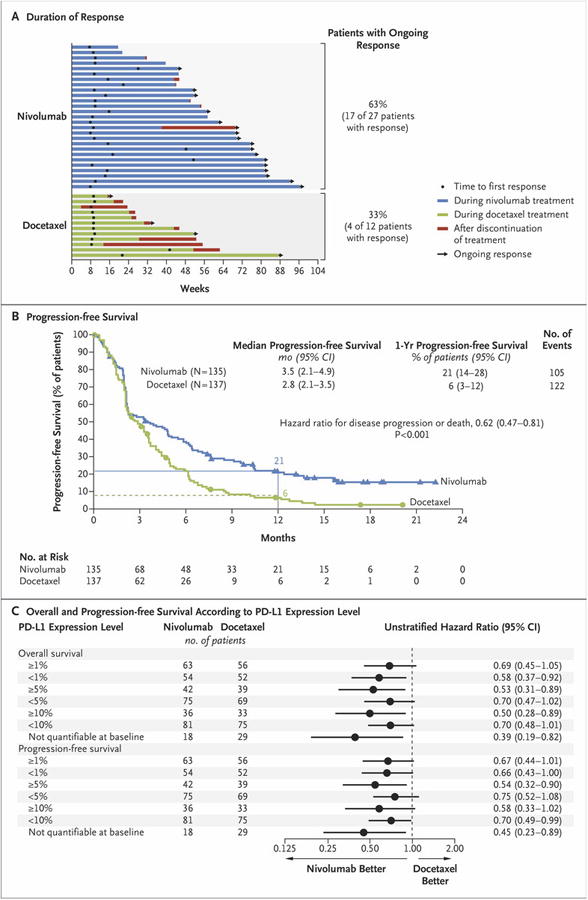
Panel A shows the characteristics of response and disease progression as assessed by the investigator, according to the Response Evaluation Criteria in Solid Tumors, version 1.1. Bars indicate the duration of response. Arrows indicate ongoing response at the time of data censoring. Panel B shows the Kaplan–Meier curves for progression-free survival, defined as the time from randomization to the date of the first documented event of tumor progression, death, or last tumor assessment that could be evaluated (data-censoring date). The analysis included all the patients who underwent randomization. Symbols indicate censored observations, and the horizontal lines the rates of progression-free survival at 1 year. Panel C shows the plot of hazard ratios for death (in the analysis of overall survival) and death or disease progression (in the analysis of progression-free survival), according to the level of expression of the ligand for programmed death 1 (PD-L1) at baseline. The prespecified expression levels for the PD-L1 biomarker analysis were 1%, 5%, and 10% of cells in a section with at least 100 tumor cells that could be evaluated.
The median progression-free survival was 3.5 months (95% CI, 2.1 to 4.9) in the nivolumab group and 2.8 months (95% CI, 2.1 to 3.5) in the docetaxel group (hazard ratio for death or disease progression, 0.62; 95% CI, 0.47 to 0.81; P<0.001) (Fig. 2B, and Fig. S4 in the Supplementary Appendix). The rate of progression-free survival at 1 year was 21% (95% CI, 14 to 28) in the nivolumab group and 6% (95% CI, 3 to 12) in the docetaxel group.
A total of 28 patients were treated with nivolumab after initial progression as defined by RECIST, version 1.1, with 9 patients having a nonconventional pattern of benefit. The characteristics of the patients who were treated after progression, including the change in tumor burden over time, are provided in Figure S5 and Table S4 in the Supplementary Appendix.
A total of 83% of the patients who underwent randomization (225 of 272 patients) had quantifiable PD-L1 expression. Rates of PD-L1 positivity were balanced between the two treatment groups (Table S5 in the Supplementary Appendix). Across the prespecified expression levels (1%, 5%, and 10%), PD-L1 expression was neither prognostic nor predictive of any of the efficacy end points (Fig. 2C, and Table S6 in the Supplementary Appendix). The rates of overall survival and progression-free survival in the PD-L1 subgroups favored nivolumab and were similar to those in the primary population. Similar rates of objective response were observed among patients with PD-L1–positive tumors and those with PD-L1–negative tumors and were consistently higher in the nivolumab group than in the docetaxel group (Table S5 in the Supplementary Appendix). Survival outcomes according to PD-L1 expression across all the prespecified expression levels are provided in Figures S6 and S7 in the Supplementary Appendix.
Safety
Treatment-related adverse events, including both hematologic and nonhematologic toxic events, occurred less frequently with nivolumab than with docetaxel. In the nivolumab group, 58% of the patients had events of any grade, 7% had events of grade 3 or 4, and none had grade 5 events; in the docetaxel group, 86% of the patients had events of any grade, 55% had events of grade 3 or 4, and 2% had events of grade 5 (Table 3, and Table S7 in the Supplementary Appendix). The most frequently reported treatment-related adverse events with nivolumab were fatigue (in 16% of the patients), decreased appetite (in 11%), and asthenia (in 10%); docetaxel-treated patients most frequently had neutropenia (33%), fatigue (33%), alopecia (22%), and nausea (23%).
Table 3. Treatment-Related Adverse Events Reported in at Least 5% of Patients*.
| Event | Nivolumab (N = 131) | Docetaxel (N = 129) | ||
|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| number of patients with an event (percent) | ||||
| Any event | 76 (58) | 9 (7) | 111 (86) | 71 (55) |
| Fatigue | 21 (16) | 1 (1) | 42 (33) | 10 (8) |
| Decreased appetite | 14 (11) | 1 (1) | 25 (19) | 1 (1) |
| Asthenia | 13 (10) | 0 | 18 (14) | 5 (4) |
| Nausea | 12 (9) | 0 | 30 (23) | 2 (2) |
| Diarrhea | 10 (8) | 0 | 26 (20) | 3 (2) |
| Arthralgia | 7 (5) | 0 | 9 (7) | 0 |
| Pyrexia | 6 (5) | 0 | 10 (8) | 1 (1) |
| Pneumonitis | 6 (5) | 0 | 0 | 0 |
| Rash | 5 (4) | 0 | 8 (6) | 2 (2) |
| Mucosal inflammation | 3 (2) | 0 | 12 (9) | 0 |
| Myalgia | 2 (2) | 0 | 13 (10) | 0 |
| Anemia | 2 (2) | 0 | 28 (22) | 4 (3) |
| Peripheral neuropathy | 1 (1) | 0 | 15 (12) | 3 (2) |
| Leukopenia | 1 (1) | 1 (1) | 8 (6) | 5 (4) |
| Neutropenia | 1 (1) | 0 | 42 (33) | 38 (30) |
| Febrile neutropenia | 0 | 0 | 14 (11) | 13 (10) |
| Alopecia | 0 | 0 | 29 (22) | 1 (1) |
Safety analyses included all the patients who received at least one dose of study drug. No treatment-related deaths occurred in patients treated with nivolumab. Treatment-related deaths were reported in three patients treated with docetaxel (one death each from interstitial lung disease, pulmonary hemorrhage, and sepsis).
Treatment-related serious adverse events occurred less frequently with nivolumab than with docetaxel. In the nivolumab group, 7% of the patients had serious events of any grade, 2% had serious events of grade 3 or 4, and none had grade 5 serious events; in the docetaxel group, 24% of patients had serious events of any grade, 19% had serious events of grade 3 or 4, and 2% had serious events of grade 5 (Table S8 in the Supplementary Appendix). The higher rates of treatment-related serious adverse events of grade 3 or 4 with docetaxel than with nivolumab were attributable mainly to hematologic toxic events and infections.
The most frequently reported (in ≥3% of patients) treatment-related select adverse events of any grade were hypothyroidism (4% with nivolumab vs. 0% with docetaxel), diarrhea (8% vs. 20%), pneumonitis (5% vs. 0%), increased blood creatinine level (3% vs. 2%), and rash (4% vs. 6%) (Table S9 in the Supplementary Appendix). Three treatment-related select adverse events of grade 3 were reported in the nivolumab group, with one case each of tubulointerstitial nephritis, colitis, and pneumonitis; no grade 4 events were reported. The median times to the onset of treatment-related select adverse events in the nivolumab group ranged from 0.3 to 17.6 weeks across categories (Table S10 in the Supplementary Appendix).
Immune-modulating medications, most often systemic glucocorticoids, were administered for the management of a percentage (18 to 83%) of treatment-related adverse events in each category. Topical preparations were also used for the management of skin-related events. The median times to resolution of treatment-related select adverse events ranged from 0.3 to 5.0 weeks in the nivolumab group (Table S10 in the Supplementary Appendix). The median time to onset of treatment-related pulmonary events was 15.1 weeks (range, 2.6 to 85.1). All but one patient with pulmonary events received glucocorticoids, and all cases resolved, with a median time to resolution of 5.0 weeks (range, 0.6 to 12.1). Among the patients with resolved cases, one patient had a subsequent recurrence of pneumonitis, which was managed appropriately with glucocorticoid treatment.
Treatment-related adverse events led to treatment discontinuation less frequently in the nivolumab group than in the docetaxel group (in 3% vs. 10% of the patients) (Tables S11 and S12 in the Supplementary Appendix). The most common (in ≥1% of patients) treatment-related events leading to discontinuation were pneumonitis in the nivolumab group (in 2%) and peripheral neuropathy and fatigue in the docetaxel group (in 3% and 2%, respectively). Two additional patients in the nivolumab group discontinued treatment owing to pneumonitis (one for whom the relationship was changed from not treatment-related to treatment-related after database lock, and one who discontinued >30 days after the most recent dose). Details of the four patients who discontinued owing to pneumonitis are provided in the Supplementary Appendix. No deaths were attributed to nivolumab, as compared with three deaths that were attributed to docetaxel (one each from interstitial lung disease, pulmonary hemorrhage, and sepsis).
Discussion
Previously treated squamous-cell NSCLC represents an area of unmet need, with little progress made since the approval of docetaxel in 1999. A retrospective review of recent U.S. Medicare data indicates that survival remains poor among patients receiving second-line treatment for squamous-cell NSCLC, with a median overall survival of 6.4 months and survival rates of 22% at 1 year and 5% at 2 years.17 Here we report results of an international, prospective, randomized, phase 3 trial that showed superior survival and an improved safety profile with nivolumab versus standard-of-care docetaxel in patients with advanced, previously treated squamous-cell NSCLC.
A phase 3 trial comparing docetaxel with docetaxel plus ramucirumab showed a significant but modest improvement in overall survival (hazard ratio for death, 0.86; 95% CI, 0.75 to 0.98; P = 0.02) when ramucirumab was added to docetaxel for use in patients with previously treated squamous-cell and non–squamous-cell cancers, but the addition of ramucirumab was associated with added toxicity.18,19 In contrast, in our trial, nivolumab monotherapy, as compared with docetaxel, was associated with a 41% lower risk of death, a 3.2-month longer median survival, and nearly twice the 1-year survival rate. Despite the confounding effects of comparisons with historical data, the outcomes in the docetaxel group in the current trial were consistent with expectations that were based on previous studies in which subgroup data were available for patients with squamous-cell tumors.7, 1 8 Early separation of the Kaplan–Meier estimates favoring nivolumab indicates an early survival benefit with nivolumab.
Consistent with the finding of superior overall survival, nivolumab was associated with a significant improvement across secondary efficacy end points. The rate of confirmed objective response with nivolumab was more than double that with docetaxel. Most patients in the nivolumab group had a response by the time the first scan was obtained, and responses were durable. The durability of benefit was reflected further in the significantly longer progression-free survival than that seen with docetaxel (38% lower risk of progression). The observed efficacy of nivolumab was similar to that observed in a phase 2, single-group trial (CheckMate 063) of nivolumab in the context of third-line therapy and beyond for squamous-cell NSCLC.15 In that study, nivolumab was associated with a response rate of 15%, a median overall survival of 8.2 months, and a 1-year survival rate of 41%.15 Together, these trials formed the basis for the March 2015 approval of nivolumab by the Food and Drug Administration for the treatment of patients with metastatic squamous-cell NSCLC who had disease progression during or after platinum-based chemotherapy.20
A consistent treatment effect favoring nivolumab was observed in prespecified subgroups, except in the group of patients 75 years of age or older and the group in the rest-of-the-world geographic region. This result was probably attributable to small sample sizes, a lack of adjustment of type I error for multiple comparisons, and an imbalance in ECOG performance-status score that favored the docetaxel group in the subgroup of patients who were 75 years of age or older (in this subgroup, an ECOG performance-status score of 1 was assessed in 91% of the patients in the nivolumab group, vs. 61% of those in the docetaxel group). Further studies that are focused on a larger elderly population than was included in our trial may more fully characterize the degree of benefit with nivolumab in this subgroup.
Early-stage trials have suggested that PD-L1 expression on tumor cells or tumor-infiltrating lymphocytes (or both) may increase the likelihood of response to PD-1–directed or PD-L1–directed therapies.21,22 Depending on the chosen assay and expression levels, response rates as high as 83% have been reported.23 However, responses are consistently seen in patients with tumors or tumor-infiltrating lymphocytes that are not positive for PD-L1.
In this study, the efficacy of nivolumab, including a survival benefit, was observed regardless of tumor PD-L1 expression levels, with results showing that PD-L1 expression was neither prognostic nor predictive of efficacy in the population of patients with squamous-cell NSCLC. Limitations of these analyses were that PD-L1 expression was assessed in archival tumor tissue, which may not have reflected tumor PD-L1 status at the time of treatment, and that only 83% of the patients who underwent randomization had quantifiable PD-L1 expression. We think that the lack of an association between PD-L1 expression and efficacy is probably not related to the performance of the PD-L1 assay but is rather a function of complex interactions between tumors and the immune system.
At least two groups of investigators have suggested that mutational burden or combinations of immune markers might predict which patients would be more likely to benefit from PD-1 checkpoint inhibition.22,24 Data from the current study indicate that PD-L1 testing is not required in order to inform treatment decisions regarding the use of nivolumab in second-line therapy of squamous-cell NSCLC and that patients may have a survival benefit that is independent of PD-L1 expression level.
The safety profile of nivolumab was more favorable than that of docetaxel, with infrequently reported toxic effects that are expected with cytotoxic chemotherapies used as second-line therapies. The frequencies of both hematologic and nonhematologic adverse events, including severe toxic events, were substantially less with nivolumab than with docetaxel, as were adverse events leading to discontinuation. No new safety concerns were identified, and no deaths were attributed to nivolumab. Immune-mediated adverse events with immunotherapies such as nivolumab differ from those seen with traditional cytotoxic therapies, and particular attention should be given to rapid evaluation and initiation of treatment. These adverse events, including pneumonitis, were infrequent and of low severity in this study and were managed with the use of established guidelines.
In conclusion, nivolumab is a PD-1 checkpoint inhibitor that showed a clinically meaningful survival benefit, with an improved safety profile, over that seen with the current standard of care in patients with advanced, previously treated squamous-cell NSCLC. The benefit was observed regardless of prestudy PD-L1 expression level. Further research is needed to identify relevant biomarkers that have sufficient sensitivity and specificity to predict which patients are most likely to benefit.
Supplementary Material
Acknowledgments
Supported by Bristol-Myers Squibb.
We thank the patients and their families, as well as the study teams involved in the trial, for making this study possible; the staff of Dako North America for collaborative development of the automated immunohistochemical assay for assessing the programmed death 1 ligand; Haolan Lu, Ph.D., for serving as lung-indication biostatistics lead; Konstantina Gialelis, B.S., for serving as the protocol manager; Rosaria Gallucci, B.Sc., for serving as the study coordinator at Fondazione IRCCS Istituto Nazionale dei Tumori, Milan; and Michel van den Heuvel, M.D., Ph.D., of Netherlands Cancer Institute, Amsterdam, for contributions to the conduct of the study. Earlier versions of the manuscript were prepared with medical-writing and editorial assistance provided by Elyse Mallimo, Ph.D., and Lisa Sullivan, M.A., of StemScientific, with funding from Bristol-Myers Squibb.
Appendix
The authors' affiliations are as follows: the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore (J.B.); the City of Hope Comprehensive Cancer Center, Duarte, CA (K.L.R.); the Netherlands Cancer Institute and Antoni van Leeuwenhoek Hospital, Amsterdam (P.B.), Erasmus MC Cancer Institute, Rotterdam (J.G.A.), and Ziekenhuis Amphia, Breda (J.G.A.) — all in the Netherlands; the University Hospital of Perugia, Perugia (L.C.), and the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan (M.C.G.) — both in Italy; the Department of Medical Oncology, West German Cancer Center, Universitätsklinikum Essen, and the Ruhrlandklinik, Universität Duisburg–Essen, Essen (W.E.E.E.), the Thoraxklinik, Heidelberg University Hospital, Heidelberg (M.S.), and the LungenClinic Grosshansdorf, Grosshansdorf (M.R.) — all in Germany; the N.N. Blokhin Russian Cancer Research Center, Moscow (E.P.); the H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL (S.A.); the Centrum Onkologii-Instytut im. Marii Skłodowskiej-Curie, Warsaw, Poland (A.P.); the University of Chicago Medicine and Biological Sciences, Chicago (E.E.V.); the Hospital Madrid Norte Sanchinarro (E.H.), the Hospital Universitario Fundación Jiménez Díaz, Madrid (M.D.), and the Hospital Universitario Virgen Del Rocío, Seville (L.P.-A.) — all in Spain; Oncology Hematology Care, Cincinnati (D.W.); the Duke University Medical Center, Durham, NC (N.R.); Massachusetts General Hospital, Boston (J.G.); Centro Internacional de Estudios Clinicos, Santiago, Chile (O.A.F.); Nemocnice Na Bulovce, Prague, Czech Republic (L.H.); Bristol-Myers Squibb, Princeton, NJ (C.B., C.T.H., B.L.); and the Sarah Cannon Research Institute and Tennessee Oncology, Nashville (D.R.S.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–92. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens: the TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 4.Taxotere (docetaxel) U S prescribing information. Bridgewater, NJ: Sanofi-Aventis; May 2010, http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdf. [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) Non-small cell lung cancer guidelines, v4. 2015 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl.
- 6.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 7.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 8.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–5. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 10.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–16. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–56. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-PD-1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015 Apr 20; doi: 10.1200/JCO.2014.58.3708. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Penrod JR, Korytowsky B, Petrilla A, et al. Survival of U.S. Medicare patients with advanced non small cell lung cancer by line of therapy. J Clin Oncol. 2014;32:15. Suppl. abstract. [Google Scholar]
- 18.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 19.Cyramza (ramucirumab) U S prescribing information. Indianapolis: Eli Lilly; Dec 2014, http://pi.lilly.com/us/cyramza-pi.pdf. [Google Scholar]
- 20.FDA expands approved use of Opdivo to treat lung cancer. Press release of the Food and Drug Administration; Bethesda, MD: Mar 4, 2015. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm436534.htm. [Google Scholar]
- 21.Garon EB, Gandhi L, Rizvi N, et al. Antitumor activity of pembrolizumab (Pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients (pts) with advanced non-small cell lung carcinoma (NSCLC) Ann Oncol. 2014;25 Suppl 5. abstract. [Google Scholar]
- 22.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soria JC, Cruz C, Bahleda R, et al. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1) Eur J Cancer. 2013;49 Suppl 2. abstract. [Google Scholar]
- 24.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


