Abstract
Anticancer drug resistance demands innovative approaches that boost the activity of drugs against drug- resistant cancers without increasing the systemic toxicity. Here we show the use of enzyme-instructed self-assembly (EIA) to generate intracellular supramolecular assemblies that drastically boost the activity of cisplatin against drug resistant ovarian cancer cells. We design and synthesize the small peptide precursors as the substrates of carboxylesterase (CES). CES cleaves the ester bond pre-installed on the precursors to form the peptides that self-assemble in water to form nanofibers. At the optimal concentrations, the precursors themselves are innocuous to cells, but they double or triple the activity of cisplatin against the drug resistant ovarian cancer cells. This work illustrates a simple, yet fundamental new way to introduce non-cytotoxic components into combination therapy of cisplatin without increasing the systemic burden or side effects.
Keywords: self-assembly, enzyme, combination therapy, cisplatin, drug-resistant
Since its serendipitous discovery five decades ago,[1] cisplatin has become the most successful therapeutic agent for anticancer chemotherapy.[2] Particularly, cisplatin has drastically extended the progression free survival (PFS) of patients with ovarian cancers.[3] However, due to the lack of early detection of ovarian cancer and the almost inevitable relapse in the patients with advanced ovarian cancer, drug resistance remains a major obstacle in treating ovarian cancers.[4] Many approaches have been investigated to address the urgent need of treating drug-resistant ovarian cancers, one of the most explored strategy is combination chemotherapy (i.e., the combination of cisplatin with other therapeutics) because the obvious clinical advantages of cisplatin promise rapid translation from preclinical to clinical. Despite the remarkable clinical success of combination therapy (e.g., the combination of cisplatin and paclitaxel),[3] the 5-year relative survival rate of ovarian cancer hardly improved (45% (2004–2010) vs. 45% (1996–2003)) over the past decade.[4e] Thus, there is an urgent need of innovative approach for cisplatin-based combination therapy.
We have been exploring enzyme-instructed molecular self-assembly[5] inside cells,[6] and our recent study shows that intracellular molecular nanofibers promiscuously interact with cytoskeleton proteins[7] yet selectively inhibit cancer cells.[8] Recently, Maruyama et al.,[9] Pires and Ulijn,[10] Yang et al.,[11] and Wells[12] also reported inhibition of cancer cells by nanofibers formed by the self-assembly of small molecules. The exceptional selectivity[8] and fundamentally new mechanisms[7, 13] of the molecular nanofibers against cancer cells encouraged us to explore the utilization of enzyme-instructed intracellular molecular self-assembly for the combination therapy of cisplatin. Unlike the previous approaches, this work focuses on the use of D-peptides for intracellular self-assembly and is the first demonstration of combining intracellular enzyme-instructed self-assembly with cisplatin. We design and synthesize two enantiomeric pe precursors (L-1 and D-1) that turn into the self-assembling molecules (L-2 and D-2) upon the catalysis of carboxylesterases (CES).[14] Our study confirms that CES are able to convert both L-1 and D-1 to the corresponding molecules of L-2 and D-2, respectively. L-2 or D-2 self-assembles in water to form molecular nanofibers. At the optimal concentration, L-1 or D-1 is innocuous to cells. The co-incubation of L-1 or D-1 at the optimal concentration with cisplatin significantly boosts the activity of cisplatin against SKOV3 and A2780cis, two lines of drug resistant ovarian cancer cells. The efficacy of this simple approach (inhibiting over 80% of SKOV3 by 20 μM of cisplatin and 15 μg/mL of D-1), in fact, is even higher than that of the innovative approach based on the co-delivery of siRNA and cisplatin nanoparticles (80% inhibition of SKOV3 by 75 μM of cisplatin).[15] We choose to work on the enantiomeric precursors L-1 and D-1 to assess the influences of the cell uptake of the precursors and the proteolytic stability of the intracellular nanofibers to the efficacy of combination therapy. These results confirm that enzyme-instructed self-assembly promises a new approach to boost the activity of cisplatin against drug-resistant ovarian cancers without increasing the systemic toxicity.
The synthesis of 1 and 2 is simple and straightforward. The facile synthetic route (Scheme S1) combines liquid phase synthesis and solid-phase peptide synthesis (SPPS) for making the precursors. For example, starting with loading N-Fmoc-protected phenylalanine (Fmoc-Phe-OH) onto 2-chlorotrityl resin and carrying out SPPS, we obtain Nap-FF[16] for coupling with ethanolamine to produce L-2. After L-2 reacts with succinic anhydride, another step of amide bond formation allows the attachment of taurine to form L-1. After the purification by HPLC, the overall yield of L-1 is about 60%. The same synthetic approach also produces D-1.
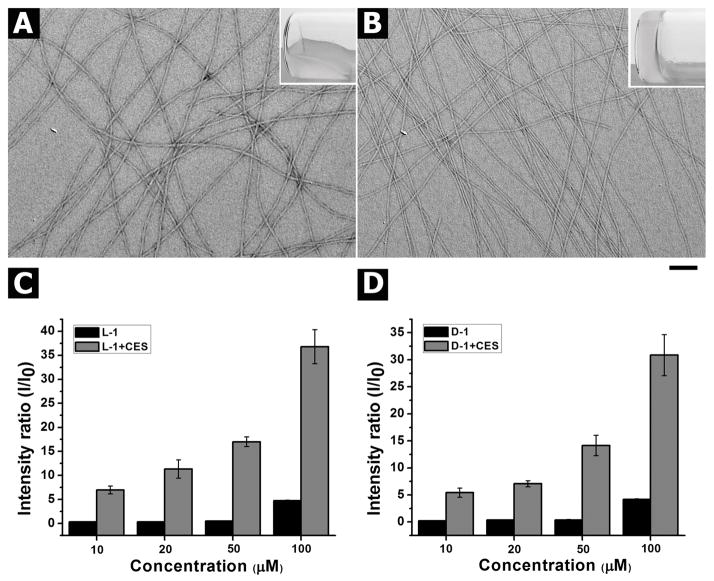
Because enzyme-instructed self-assembly usually leads to the formation of supramolecular hydrogels,[17] we evaluate the hydrogelation resulting from the esterase catalyzed conversion of L-1 and D-1 as a facile method to assay the enzyme-instructed self-assembly. After obtaining the precursors, we test the use of carboxylesterase (i.e., CES) to convert the precursors to the hydrogelators for self-assembly in water to form molecular nanofibers. The addition of L-1 (or D-1) in PBS buffer at pH 7.4 at the concentration of 0.4 wt % (5.5 mM) affords a transparent solution. After the addition of CES (2 U/mL) into the solution of L-1 (or D-1) for 24 h, a translucent hydrogel forms. We also find the minimum gelation concentration (mgc) of L-2 or D-2 is about 0.1 wt % (1.4 mM). While CES efficiently converts both L-1 and D-1 to L-2 and D-2, respectively, the hydrogel of L-2 is apparently weaker than the hydrogel of D-2 (Figure S5). We speculate that this subtle difference might originate from the less interaction between D-2 and CES than between L-2 and CES. As shown in Figure 1A and 1B, the transmission electron microscopy (TEM) images of the resulting hydrogels reveal the formation of uniform nanofibers after the addition of CES. The diameters of the nanofibers of the hydrogel formed by L-2 or D-2 after the addition of CES in the solution of L-1 or D-1 are 10 ± 2 nm or 8 ± 2 nm, respectively.
Figure 1.
TEM images of the hydrogels (inset: optical images) formed by the addition of CES (2 U/mL) to the solution of (A) L-1 or (B) D-1 at the concentration of 0.4 wt % in PBS buffer (Scale bar = 100 nm). The signal intensity ratio of static light scattering (SLS) of the solution of (C) L-1 or (D) D-1 at concentrations from 10 to 100 μM before (black bar) and after (red bar) being treated CES (2 U/mL) for three hours.
Our preliminary test of the cytotoxicity of L-1 and D-1 indicates that L-1 and D-1 show significant cytotoxicity to SKOV3 cells at the concentration below the mgc (Figure S4). Thus, we use static light scattering (SLS) to help verify the existence of nanoscale assemblies (e.g., nanofibers or nanoparticles) in the solution of L-1 (or D-1) at the concentrations lower than the mgc and after the addition of CES (2 U/mL). We choose the concentrations from 10 μM to 100 μM to analyze whether there are differences in self-assembly of the molecules before and after the addition of CES. As shown in Figure 1C and 1D, before being treated with CES, the signal intensity ratios of the solution of L-1 (or D-1) at concentrations from 10 μM to 50 μM are close to zero, indicating that there are hardly any assemblies of L-1 (or D-1) in the solution. When the concentration of the solution of L-1 (or D-1) increases to 100 μM, there is a slight increase of intensity ratio, suggesting that small amounts assemblies of L-1 (or D-1) exist in the solution. In contrast, the addition of CES to the solution of L-1 (or D-1) at concentrations from 10 μM to 100 μM results in a significant increase of the signal intensity ratios, especially when the concentration of L-1 (or D-1) is at or above 50 μM. For example, the signal intensity ratio of the solution of L-1 (or D-1) at concentration of 50 μM drastically increases from about zero (before the addition of CES) to about 17 (after the addition of CES), which reveals the formation of assemblies of L-1 (or D-1). Moreover, the solution of L-1 at concentration of 100 μM shows a 9-fold increase of the signal intensity ratio after the addition of CES, indicating the formation of a larger amount of assemblies after enzymatically converting the precursors to the hydrogelators (as shown in Figure S6). Similarly, the signal intensity ratio of the solution of 100 μM of D-1 increases significantly after the addition of CES, which agrees with that CES converts D-1 to D-2 for self-assembling in water to form nanoscale assemblies of D-2.
After confirming that CES converts the precursor L-1 (or D-1) to the hydrogelator L-2 (or D-2), we determine the stability of the precursors (L-1 or D-1) when incubating them with the ovarian cancer cells. After culturing the precursors with SKOV3 or A2780cis cells at 37 °C for 4 h, we collect the cell lysates and culture medium for liquid chromatography–mass spectrometry (LC-MS) analysis and determine the intracellular concentrations of the precursors, the hydrogelators, and the relevant proteolytic products. As shown in Table 1, after incubation with SKOV3 or A2780cis cells for 4h, more than 85% of the precursors (L-1 or D-1) turns into the corresponding hydrogelators (L-2 or D-2). Moreover, the intracellular concentrations of the hydrogelators all are above 100 μM, which warrants the intracellular self-assembly of the hydrogelators. In addition, the cumulative intracellular concentration of L-1 and L-2 is about 10 times higher than the incubation concentration of L-1, and the cumulative intracellular concentration of D-1 and D-2 is about 5 times higher than the incubation concentration of D-1. These results not only indicate that the cellular uptake of L-1 is more efficient than that of D-1, but also confirm that the selective retention of hydrogelators inside the cells originates from ester bond cleavage catalyzed by CES. A fluorescent dye of esterase, 6-CFDA[18], also confirms that high esterase activity in SKOV3 cells (Figure S7). We also analyze the culture medium containing L-1 (or D-1), which is incubated with SKOV3 cells or A2780cis cells. As listed in Table S1, after 4 h incubation with SKOV3 cells, about 19% of L-1 in the medium turns into L-2, and the concentration of L-1 in the medium decreases from 50 μM to 39 μM; about 15% of D-1 becomes D-2, and the concentration of D-1 in the medium decreases from 20 μM to 16 μM. A similar trend is also observed in A2780cis cells. The results further validate that intracellular enzymatic conversion of the precursors catalyzed by CES results in the intracellular self-assembly of the hydrogelators.
Table 1.
The intracellular concentrations of the precursors and hydrogelators in SKOV3 and A2780cis cells.
| Compd. | Precursor (1) (μM) | Hydrogelator (2) (μM) | Ratio[a] |
|---|---|---|---|
| L-1[b] | 62 | 431 | 6.95 |
| D-1[b] | 16 | 108 | 6.75 |
| D-1[c] | 69 | 582 | 8.43 |
ratio of hydrogelator to precursor after 4 h.
The cell lysates of SKOV3 cells are collected after 4 h incubation with 20 μM (15 μg/mL) of D-1 or with 50 μM (37 μg/mL) of L-1 at 37 °C.
The cell lysates of A2780cis cells are collected after 4 h incubation with 100 μM (73 μg/mL) of D-1 at 37 °C.
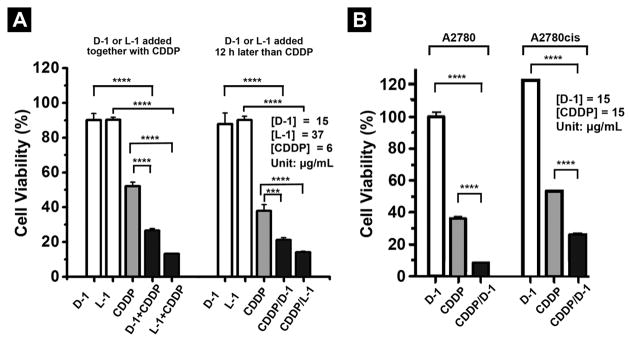
To evaluate the effect of intracellular self-assembly of L-2 or D-2 for cisplatin-based combination therapy, we test the cell viability of three ovarian cancer cell lines by incubating them with the mixture of precursors and cisplatin (CDDP). As shown in Figure 2A, after 72 h, the mixture of CDDP (6 μg/mL) with D-1 (15 μg/mL) or L-1 (37 μg/mL) inhibits about 74% or 87%, respectively, of SKOV3 cells, while D-1 (15 μg/mL) or L-1 (37 μg/mL) alone is almost innocuous to the cells and CDDP (6 μg/mL) alone inhibits only 48% SKOV3 cells. We also use another method to treat the SKOV3 cells, in which the addition of D-1 (or L-1) follows the addition of CDDP to SKOV3 cells, 12 h after. As shown in Figure 2A, 72h after the addition of D-1 (15 μg/mL) or L-1 (37 μg/mL) following the addition of CDDP (6 μg/mL), the inhibition of SKOV3 is about 80% or 86%, respectively. The higher efficacy exhibited by L-1 agrees with the higher uptake and incubation concentration of L-1.
Figure 2.
Cell viability of ovarian cancer cells incubated with the precursors with and without cisplatin (CDDP). (A) The cell viability of SKOV3 cells incubated with the precursors D-1 or L-1 alone, or in combination with CDDP for 72 h. (B) The cell viability of A2780 cells and A2780cis cells incubated with the precursors D-1 alone, or in combination with CDDP for 72 h (*** = p≤0.001, **** = p≤0.0001).
We also test the combination of CDDP and D-1 for treating A2780cis (cisplatin resistant) and A2780 (cisplatin sensitive) cells. As shown in Figure 2B, D-1 (15 μg/mL) alone hardly exhibits any cytotoxicity to A2780cis cells. The combination of D-1 and CDDP inhibit 70% of A2780cis cells, which doubles the activity of CDDP. The combination of D-1 and CDDP significantly inhibits A2780 cell and decreases the cell viability of A2780 from about 38% (without adding D-1) to 9%. Since SKOV3 and A2780cis are two drug resistant ovarian cell lines, CDDP shows lower inhibition ability against these two cell lines comparing with it on A2780 cells. These results, undoubtedly, confirm that the addition of the precursors of self-assembling small molecules in the combination therapy of cisplatin drastically boosts the activity of cisplatin against drug resistant ovarian cancer cells. As shown in Table S2, the IC50 values of L-1 and D-1 against the ovarian cancer cells are 62–94 μM and 48–69 μM, respectively, but their concentrations for the combination therapy can be lower than IC50 values because EIA accumulates the hydrogelators intracellularly. In addition, the intracellularly formed nanofibers (of D-1) are about seven times more effective against HeLa cells than the nanofibers of the dipeptides reported previously (Nap-FF[8], Table S3).
To verify the critical role of enzyme-instructed self-assembly, we design and synthesize a control compound (3), which replaces the ester bond in D-1 by an amide bond (Scheme S2). This change (-COO- to –CONH-) renders 3 to resist CES. As shown in Figure S8A and S9A, 3 (500 μM) alone hardly inhibits SKOV3 cells after 72 h incubation. After 72 h incubation with SKOV3 cells, while CDDP (6 μg/mL) alone causes about 40% cell death, the mixture of 3 (15 μg/mL) and CDDP (6 μg/mL) inhibits only about 32% of SKOV3 cells. The cell compatibility exhibited by 3 also excludes the possibility that L-1 or D-1 acts as a surfactant to inhibit cells. A similar trend is observed in A2780cis cells (Figure S8B, S9B). These results further confirm that enzyme-instructed self-assembly inside cells is the main cause for boosting the efficacy of CDDP in the combination therapy of CDDP with the precursors (i.e., D-1 and L-1). Some of the cell viabilities slightly exceed 100% (e.g., Fig. 2B) because MTT assay measures the activity of mitochondrial reductase and it is not unusual for treated group to have higher enzyme activity than the control group.
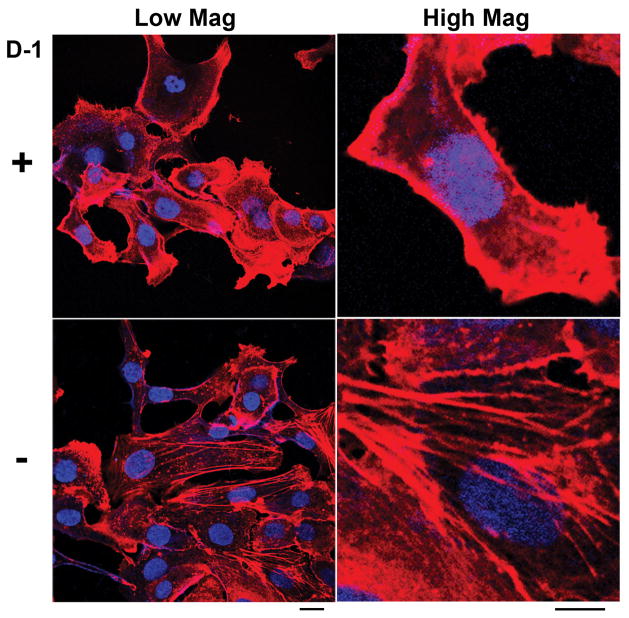
To gain insight on the action of intracellular self-assembly to cells, we examine the change of the actin filaments inside cells. As shown in Figure 3, the SKOV3 cells treated by D-1 (20 μM (15 μg/mL), 20h) exhibit much less well-defined, long actin filaments than that in the control SKOV3 cells (without the treatment of D-1). This trend becomes more pronounced after the increase of the concentration from 20 to 100 μM (Figure S11), as evidenced by the amount of the actin filaments in the cells (Figure S19). This observation agrees with that the intracellular nanofibers of small peptides can interact with actins.[7] To verify the reversibility of the assembly of D-2 inside cells, we treat the SKOV3 cells with D-1 at the concentrations of 20, 50, and 100 μM respectively for 20 h and then we replace the medium containing D-1 with fresh medium for the treatment of an additional 20 hrs. As shown in Figure S12, actin filaments recover after being treated with fresh medium for 20 h when the concentrations of D-1 are 20 and 50 μM. The incomplete recovery of actin filaments, when [D-1] = 100 μM, agrees with that D-1 starts to self-assemble at 100 μM. Being incubated with L-1, SKOV3 cells exhibit similar behavior (Figure S13, S14): after 20 h, cells incubated with L-1 (50 μM) exhibit fewer well-defined actin filaments comparing with the cells without the treatment of L-1. However, with the replacement of the culture medium to a fresh one without L-1 for 20 h, the morphology of actin filaments become normal. These results suggest that intracellular nanofibers formed by enzyme-instructed self-assembly exhibit transient cytotoxicity that should help minimize long-term systemic burden in the combination therapy. Dissociation likely reduces the long term cytotoxicity after the apoptosis of cells so that the precursors and nanofibers cause minimal systemic toxicity.
Figure 3.
The fluorescence images of SKOV3 cells stained with Alexa Fluor 633 Phalloidin (F-actin) and Hoechst (nuclei) (upper) after treatment of D-1 at concentration of 20 μM for 20 h or (bottom) without the treatment of D-1. Scale bar (left) = 20 μm, scale bar (right) = 10 μm.
In conclusion, we demonstrate that enzyme-instructed intracellular self-assembly of small molecules as a new approach to boost the activity of CDDP against two drug-resistant ovarian cancer cell lines. Moreover, at the optimal concentrations, 20 μM (D-1), 50 μM (L-1) used for boosting the activities of the cisplatin, L-1 and D-1 hardly inhibit HS-5 and PC-12 cells (Figure S15, S17), despite cisplatin significantly inhibits HS-5 (Figure S16) and PC-12 cells[19]. Intravenous injection of L-1 or D-1 hardly affect the weight and organ index of mice (Figure S18), confirming the low systemic toxicity of the precursors. The genome analysis according to The Cancer Genome Atlas (TCGA) indicates the amplification of CES in certain tumors (e.g., breast and ovarian cancer) (Figure S23), which not only supports the observation in this work, but also provides useful guidance for treating other cancers based on this approach. This work, together with other emerging evidence,[6–10, 12] indicates that enzyme-instructed self-assembly promises a new way for developing combination therapy for cancer treatment. Other than cisplatin, carboplatin has been used as the preferred platinum-based drug[20] and we will use carboplatin for our future work.
Supplementary Material
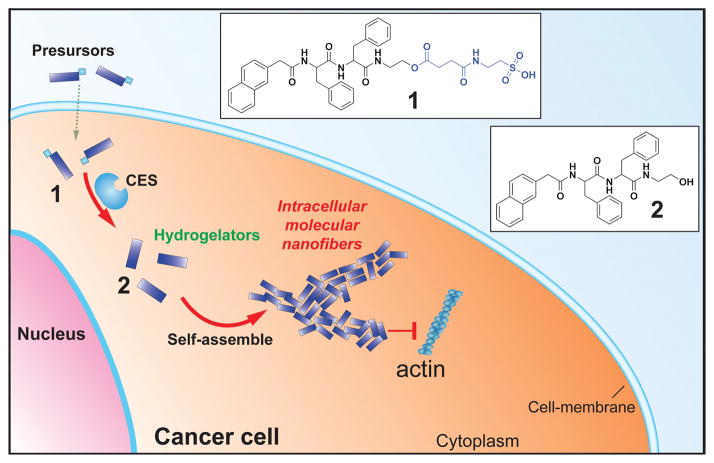
Scheme 1.
The illustration of enzymatic transformation of the precursor as a substrate of carboxylesterase (CES) to the corresponding hydrogelator for intracellular self-assembly.
Acknowledgments
This work was partially supported by NIH (R01CA142746), DOD OCRP (W81XWH-10-1-0263, W81XWH-14-1-0092, and W81XWH-14-1-0205), International S&T Cooperation Program of China (ISTCP, 2015DFA50310) and NSFC (81471727).
Footnotes
Supporting information for this article is available on the WWW under http://
Contributor Information
Jie Li, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA).
Yi Kuang, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA).
Junfeng Shi, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA).
Jie Zhou, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA).
Jamie E. Medina, Department of Pathology, Brigham and Women’s hospital, Harvard Medical School, Boston, MA 02115 (USA)
Rong Zhou, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA).
Dan Yuan, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA).
Cuihong Yang, Chinese Academy of Medical Science & Peking Union Medical College, Tianjin 300192, P. R. China.
Huaimin Wang, State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, Tianjin 300071, China.
Prof. Zhimou Yang, State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, Tianjin 300071, China
Prof. Jianfeng Liu, Chinese Academy of Medical Science & Peking Union Medical College, Tianjin 300192, P. R. China
Prof. Dr. Daniela M. Dinulescu, Email: ddinulescu@rics.bwh.harvard.edu, Department of Pathology, Brigham and Women’s hospital, Harvard Medical School, Boston, MA 02115 (USA)
Prof. Dr. Bing Xu, Email: bxu@brandeis.edu, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
References
- 1.Rosenber B, Vancamp L, Krigas T. Nature. 1965;205:698. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 2.a) Rosenber B, Vancamp L, Trosko JE, Mansour VH. Nature. 1969;222:385. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]; b) Rosenberg B. Cancer. 1985;55:2303–2316. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA G. Gynecologic Oncology. New Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 4.a) Yap TA, Carden CP, Kaye SB. Nat Rev Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]; b) Parkin DM. Lancet Oncol. 2001;2:596–596. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]; c) Greenlee RT, Hill-Harmon MB, Murray T, Thun M. CA-Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]; d) Rabik CA, Dolan ME. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Siegel RL, Miller KD, Jemal A. Ca-cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 5.a) Yang ZM, Gu HW, Fu DG, Gao P, Lam JK, Xu B. Advanced Materials. 2004;16:1440. [Google Scholar]; b) Pappas CG, Sasselli IR, Ulijn RV. Angewandte Chemie International Edition. 2015;54:8119–8123. doi: 10.1002/anie.201500867. [DOI] [PubMed] [Google Scholar]
- 6.a) Yang Z, Liang G, Xu B. Accounts Chem Res. 2008;41:315–326. doi: 10.1021/ar7001914. [DOI] [PubMed] [Google Scholar]; b) Gao Y, Kuang Y, Du X, Zhou J, Chandran P, Horkay F, Xu B. Langmuir. 2013;29:15191–15200. doi: 10.1021/la403457c. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gao Y, Berciu C, Kuang Y, Shi J, Nicastro D, Xu B. ACS Nano. 2013;7:9055–9063. doi: 10.1021/nn403664n. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Gao Y, Shi J, Yuan D, Xu B. Nat Commun. 2012;3:1033. doi: 10.1038/ncomms2040. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Yang ZM, Xu KM, Guo ZF, Guo ZH, Xu B. Advanced Materials. 2007;17:3152–3156. [Google Scholar]
- 7.Kuang Y, Long MJC, Zhou J, Shi J, Gao Y, Xu C, Hedstrom L, Xu B. J Biol Chem. 2014;289:29208–29218. doi: 10.1074/jbc.M114.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuang Y, Xu B. Angew Chem Int Ed. 2013;52:6944–6948. doi: 10.1002/anie.201302658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka A, Fukuoka Y, Morimoto Y, Honjo T, Koda D, Goto M, Maruyama T. J Am Chem Soc. 2014;137:770–775. doi: 10.1021/ja510156v. [DOI] [PubMed] [Google Scholar]
- 10.Pires RA, Abul-Haija YM, Costa DS, Novoa-Carballal R, Reis RL, Ulijn RV, Pashkuleva I. J Am Chem Soc. 2014;137:576–579. doi: 10.1021/ja5111893. [DOI] [PubMed] [Google Scholar]
- 11.a) Yang C, Bian M, Yang Z. Biomater Sci. 2014;2:651–654. doi: 10.1039/c3bm60252d. [DOI] [PubMed] [Google Scholar]; b) Wang H, Yang Z. Soft Matter. 2012;8:2344–2347. [Google Scholar]
- 12.a) Zorn JA, Wille H, Wolan DW, Wells JA. J Am Chem Soc. 2011;133:19630–19633. doi: 10.1021/ja208350u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. J Am Chem Soc. 2002;124:15030–15037. doi: 10.1021/ja027993g. [DOI] [PubMed] [Google Scholar]; c) Newcomb CJ, Sur S, Ortony JH, Lee OS, Matson JB, Boekhoven J, Yu JM, Schatz GC, Stupp SI. Nature Communications. 2014;5 doi: 10.1038/ncomms4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang Y, Shi J, Li J, Yuan D, Alberti KA, Xu Q, Xu B. Angew Chem Int Ed. 2014;53:8104–8107. doi: 10.1002/anie.201402216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Guichard S, Terret C, Hennebelle I, Lochon I, Chevreau P, Fretigny E, Selves J, Chatelut E, Bugat R, Canal P. Brit J Cancer. 1999;80:364–370. doi: 10.1038/sj.bjc.6690364. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sanghani SP, Quinney SK, Fredenburg TB, Sun ZJ, Davis WI, Murry DJ, Cummings OW, Seitz DE, Bosron WF. Clin Cancer Res. 2003;9:4983–4991. [PubMed] [Google Scholar]; c) Xu Y, Villalona-Calero MA. Ann Oncol. 2002;13:1841–1851. doi: 10.1093/annonc/mdf337. [DOI] [PubMed] [Google Scholar]; d) Ohtsuka K, Inoue S, Kameyama M, Kanetoshi A, Fujimoto T, Takaoka K, Araya Y, Shida A. Lung Cancer. 2003;41:187–198. doi: 10.1016/s0169-5002(03)00223-x. [DOI] [PubMed] [Google Scholar]; e) Stella VJ, Borchardt RT, Hageman MJ, Oliyai R, Maag H, Tilley JW. Prodrugs: Challenges and Rewards. Springer; New York: 2007. [Google Scholar]
- 15.He CB, Lu KD, Liu DM, Lin WB. J Am Chem Soc. 2014;136:5181–5184. doi: 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Kuang Y, Gao YA, Xu B. Langmuir. 2011;27:529–537. doi: 10.1021/la1020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Boekhoven J, Koot M, Wezendonk TA, Eelkema R, van Esch JH. J Am Chem Soc. 2012;134:12908–12911. doi: 10.1021/ja3051876. [DOI] [PubMed] [Google Scholar]; b) Yamanaka M, Kawaharada M, Nito Y, Takaya H, Kobayashi K. J Am Chem Soc. 2011;133:16650–16656. doi: 10.1021/ja207092c. [DOI] [PubMed] [Google Scholar]; c) He M, Li J, Tan S, Wang R, Zhang Y. J Am Chem Soc. 2013;135:18718–18721. doi: 10.1021/ja409000b. [DOI] [PubMed] [Google Scholar]; d) Cheetham AG, Zhang P, Lin Y-a, Lock LL, Cui H. J Am Chem Soc. 2013;135:2907–2910. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Rosenzweig BA, Ross NT, Adler MJ, Hamilton AD. J Am Chem Soc. 2010;132:6749–6754. doi: 10.1021/ja100485n. [DOI] [PubMed] [Google Scholar]; f) Pochan DJ, Schneider JP, Kretsinger J, Ozbas B, Rajagopal K, Haines L. J Am Chem Soc. 2003;125:11802–11803. doi: 10.1021/ja0353154. [DOI] [PubMed] [Google Scholar]; g) Nagy KJ, Giano MC, Jin A, Pochan DJ, Schneider JP. J Am Chem Soc. 2011;133:14975–14977. doi: 10.1021/ja206742m. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Yoshii T, Mizusawa K, Takaoka Y, Hamachi I. J Am Chem Soc. 2014;136:16635–16642. doi: 10.1021/ja508955y. [DOI] [PubMed] [Google Scholar]; i) Woodward RT, Stevens LA, Dawson R, Vijayaraghavan M, Hasell T, Silverwood IP, Ewing AV, Ratvijitvech T, Exley JD, Chong SY, Blanc F, Adams DJ, Kazarian SG, Snape CE, Drage TC, Cooper AI. J Am Chem Soc. 2014;136:9028–9035. doi: 10.1021/ja5031968. [DOI] [PubMed] [Google Scholar]; j) Deng W, Yamaguchi H, Takashima Y, Harada A. Angew Chem Int Edit. 2007;46:5144–5147. doi: 10.1002/anie.200701272. [DOI] [PubMed] [Google Scholar]; k) Shen Z, Wang T, Liu M. Angew Chem Int Edit. 2014;53:13424–13428. doi: 10.1002/anie.201407223. [DOI] [PubMed] [Google Scholar]; l) Singh S, Topuz F, Hahn K, Albrecht K, Groll J. Angew Chem Int Edit. 2013;52:3000–3003. doi: 10.1002/anie.201206266. [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Maity C, Hendriksen WE, van Esch JH, Eelkema R. Angew Chem Int Edit. 2015;54:998–1001. doi: 10.1002/anie.201409198. [DOI] [PubMed] [Google Scholar]; n) Tamesue S, Takashima Y, Yamaguchi H, Shinkai S, Harada A. Angew Chem Int Edit. 2010;49:7461–7464. doi: 10.1002/anie.201003567. [DOI] [PubMed] [Google Scholar]; o) Liu TW, MacDonald TD, Shi J, Wilson BC, Zheng G. Angew Chem Int Edit. 2012;51:13128–13131. doi: 10.1002/anie.201206939. [DOI] [PubMed] [Google Scholar]; p) Carter KK, Rycenga HB, McNeil AJ. Langmuir. 2014;30:3522–3527. doi: 10.1021/la404567b. [DOI] [PubMed] [Google Scholar]
- 18.Riordan HD, Riordan NH, Meng XL, Zhong J, Jackson JA. Anticancer Res. 1994;14:927–931. [PubMed] [Google Scholar]
- 19.Mendonca LM, dos Santos GC, Antonucci GA, dos Santos AC, Pires Bianchi MdL, Greggi Antunes LM. Mutat Res-Genet Toxicol Environ Mutag. 2009;675:29–34. doi: 10.1016/j.mrgentox.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.